Abstract
Humans encode seven APOBEC3 (A3A-A3H) cytidine deaminase proteins that differ in their expression profiles, preferred nucleotide recognition sequence and capacity for restriction of RNA and DNA viruses. We identified APOBEC3 hotspots in numerous herpesvirus genomes. To determine the impact of host APOBEC3 on herpesvirus biology in vivo, we examined whether murine APOBEC3 (mA3) restricts murine gammaherpesvirus 68 (MHV68). Viral replication was impaired by several human APOBEC3 proteins, but not mA3, upon transfection of the viral genome. The restriction was abrogated upon mutation of the A3A and A3B active sites. Interestingly, virus restriction by A3A, A3B, A3C, and A3DE was lost if the infectious DNA was delivered by the virion. MHV68 pathogenesis, including lung replication and splenic latency, was not altered in mice lacking mA3. We infer that mA3 does not restrict wild type MHV68 and restriction by human A3s may be limited in the herpesvirus replication process.
Keywords: herpesvirus, murine gammaherpesvirus, pathogenesis, replication, latency, host restriction, APOBEC3, cytidine deaminase
INTRODUCTION
Apolipoprotein B mRNA editing enzyme catalytic polypeptide-like editing complex 3 (APOBEC3) proteins belong to a family of host cytidine deaminases with a wide array of biological functions, subcellular localization and tissue expression profiles (Bransteitter et al., 2009; Smith et al., 2012). Eleven members of this enzyme family have been discovered in humans; APOBEC1, APOBEC2, APOBEC3, APOBEC4 and activation induced deaminase (AID). APOBEC1 (A1), the founding member of this family, is an mRNA editing enzyme that deaminates Apolipoprotein B mRNA to produce two isoforms of apolipoproteins that are required for low-density lipid formation(Driscoll and Zhang, 1994; Mehta and Driscoll, 1998). APOBEC2 (A2) is a more recently discovered member detected predominantly in the heart and skeletal muscle (Mikl et al., 2005). Mice transgenic for human A2 develop lung and liver tumors (Okuyama et al., 2012). APOBEC4 (A4) was discovered by computational searches for genes homologous to A1 and, like A2, has no ascribed function. The most widely studied members are AID, which is critical for generating antibody diversity through ssDNA deamination of the immunoglobulin locus, and APOBEC3 (A3) proteins, which act as innate immune barriers to the replication of viruses and endogenous retroelements (Cullen, 2006; Refsland and Harris, 2013).
APOBEC3 genes have undergone duplication events in the course of mammalian evolution. Murid rodents encode the single APOBEC3, mA3, while felines encode four APOBEC3s (Munk et al., 2008) and the equine genome encodes six (Bogerd et al., 2008). Primates have an expanded repertoire of seven APOBEC3 proteins; A3A, A3B, A3C, A3DE, A3F, A3G and A3H with each having slightly different target sequences. A3s have been widely studied since the identification of human A3G (hA3G) as a host restriction factor of viral infectivity factor (Vif)-deficient HIV (Sheehy et al., 2002). In Vif-deficient HIV infections, hA3G is packaged into viral cores through interactions with both the HIV nucleopcapsid protein and the viral genomic RNA. A3G deaminates cytosine residues to uracils in the newly synthesized strand. Vif enhances HIV replication by binding to hA3G and either targeting it for degradation or preventing packaging into the HIV core (Goila-Gaur and Strebel, 2008; Zhang et al., 2003).
Retroviruses, retroelements and more recently DNA viruses have emerged as targets of hA3 proteins (Bishop et al., 2004; Delebecque et al., 2006; Mahieux et al., 2005; Mangeat et al., 2003; Narvaiza et al., 2009; Stenglein et al., 2010; Suspene et al., 2005a; Suspene et al., 2005b; Suspene et al., 2004; Vartanian et al., 2008). Human A3A and A3C have been reported to restrict herpes simplex virus type 1 (HSV-1) replication in transient expression studies (Suspene et al., 2011). Additionally, Suspene et al. reported the detection of virus variants consistent with cytidine deamination in clinical specimens positive for either HSV-1 or Epstein-Barr virus (EBV) (Suspene et al., 2011). However, in most cases the impact of hA3 activity on the in vivo pathogenesis of a given virus in the host has not been extensively studied. The murine APOBEC3 exhibits antiviral activity against multiple murine and human retroviral targets, (Langlois et al., 2009; Low et al., 2009; Okeoma et al., 2007; Takeda et al., 2008) implying that mA3 retains the retroviral targeting function of the human A3. However, the antiviral function of mA3 against herpesviruses is not known. In this report, we examined the ability of mA3 to restrict murine gammaherpesvirus 68 (MHV68), a natural pathogen of murid rodents that has strong genetic colinearity with, and biological similarities to, the human gammaherpesviruses, Kaposi’s sarcoma-associated herpesvirus (KSHV/HHV-8) and Epstein Barr Virus (EBV). We determined the distribution of A3 hotspots in the MHV68 genome, queried the effect of A3 overexpression on viral replication and the finally ascertained the impact of loss of A3 on in vivo pathogenesis. Although we observed restriction of MHV68 replication with co-expression of several hA3s, the murine APOBEC3 did not alter MHV68 replication and pathogenesis.
MATERIALS AND METHODS
Viruses, cells and mice
The recombinant marking virus, MHV68-H2bYFP was used the for cell-culture experiments. This virus encodes a histone 2b (H2b)-enhanced yellow fluorescent protein (eYFP) fusion protein that tethers the YFP protein to nucleosomes and eliminates the passive diffusion of the fluorescent YFP signal out of the nucleus to enhance infected cell imaging by microscopy (Collins and Speck, 2012). Mice were infected with MHV68 WUMS (ATCC VR1465) as the wild-type virus. Virus passage and titer determination were performed as previously described (Weck et al., 1996; Weck et al., 1999a).
Human epithelial kidney cells (HEK 293T) were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum, 1% penicillin, and streptomycin at 37°C in 5% CO2. Murine fibroblast cells (NIH 3T12) were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 8% fetal calf serum, 100 U of penicillin per ml and 100 mg of streptomycin per ml at 37°C in 5% CO2
Murine APOBEC3 knock-out (mA3−/−) mice were generated by blastocyst injection of 129P2/OlaHsd embryonic stem cell clone XN450 (Baygenomics, San Francisco) that contained an 8 kb galactosidase-neomycin fusion (β-geo) gene trap insertion retroviral vector between exons 4 and 5 of mA3. The mice were crossed for several generations onto a C57/Bl6 background and genotyped by PCR using primers spanning the insertion site. The WT genotype was amplified with primer 21 (5′ CTGTAACCTGGTATCTCCCGTC 3′) and primer 22 (5′ GGAAAAACTGCTTGCCAGGCTC 3′). The mA3−/− genotype was amplified with primer 21 (5′ CTGTAACCTGGTATCTCCCGTC 3′) and primer 23 (5′ CACAAGGTTCATATGGTGCCGT 3′). The mA3−/− mice and their WT C57BL6 counterparts were maintained at the Stony Brook University Division of Laboratory Animal Research (DLAR) facility in accordance with protocols approved by the Institutional Animal Care and Use Committee of Stony Brook. Eight- to twelve-week old WT and mA3−/− mice were infected intranasally with 1000 PFU of MHV68 under isoflurane anesthesia. At the indicated times post infection, organs were harvested and processed as described below.
Transfections
For transient co-transfections, 4 × 105 HEK 293T cells were seeded per well of a 12-well tissue culture plate one day prior to transfection with 2.5 μg of APOBEC3 expression plasmids and 1.55 μg of MHV68 BAC DNA. All transient co-transfections were performed using TransIT-LT1 Transfection Reagent according to the manufacturer’s instructions (Mirus, Madison WI). Forty-eight hrs later, the cells were subjected to multiple freeze-thaw cycles to release infectious virus that was then titered by plaque assay on a NIH 3T12 cell monolayer.
For the transfection followed by infection experiments, HEK 293T cells were transfected with the desired human and mouse APOBEC3 proteins as outlined above. Twenty-four hrs post-transfection, cells were infected with MHV68-H2bYFP virus at an MOI of 0.01. Forty-eight hrs after the infection virus output was quantified by a plaque assay.
Fluorescence microscopy
Virus infection with MHV68-H2bYFP was visualized by fluorescence microscopy. Imaging was performed with a Zeiss Axiovert S100 inverted microscope (Carl Zeiss Microscopy GmbH, Jena Germany) equipped with a Luminera INFINITY 3-1UR 1.4 megapixel low light CCD digital camera (Lumenera, Ottawa ON Canada). Images were analyzed using Axiovision Software (Axiovision LE Rel.4.3, Carl Zeiss Microscopy GmbH, Jena Germany).
Plaque assay
1.8 × 105 NIH 3T12 cells were seeded per well in a 6-well tissue culture plate one day prior to infection. The next day, the NIH 3T12 cells were infected with serial dilutions of cell homogenate and overlayed with 1.5% methylcellulose in DMEM containing 5% FBS. One week later, the methylcellulose was removed and cells were washed twice with PBS prior to methanol fixation and staining with a 0.1% crystal violet solution in 10% methanol.
Immunoblot
Total protein lysate was harvested in lysis buffer (150 mM sodium chloride, 1.0% IGEPAL CA-630, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, 50 mM Tris pH 8.0) supplemented with a protease inhibitor cocktail (Sigma, St. Louis MO). Fifty micrograms of each lysate were separated in a 12% SDS PAGE gel and transferred onto polyvinylidene fluoride (PVDF) membrane. APOBEC3 proteins were detected using a mouse monoclonal antibody specific for the HA-epitope (Cell signaling, Danvers MA) on mA3, A3B, A3C, A3F, and A3G constructs, a V5 epitope (Santa Cruz biotechnology, Dallas Texas) for A3DE and a rabbit monoclonal specific for human A3A (Hakata and Landau, 2006). A rabbit monoclonal antibody to human glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as a loading control. HRP-conjugated secondary antibodies were detected using an enhanced chemiluminescence reagent (ECL, Thermo Scientific, Waltham MA).
Quantitative PCR
Total cell DNA from the co-transfection experiments was column-purified (Qiagen, Limburg, Netherlands) and digested with 20 units of Dpn1 (New England Biolabs, Ipswich MA) for 12 hours at 37°C to remove input MHV68 BAC DNA. One ng of DpnI-digested DNA was input into a quantitative PCR reaction (Quanta Biosceinces- Perfecta SYBR GREEN, Gaithersburg MD) using primers specific to a region of MHV68 ORF12 (forward primer, 5′ GTCTACAACAGGATCTGCATTT 3′, reverse primer 5′ AAAACTCTACCGTGACTGTGAA 3′) and primers for human GAPDH (forward primer, 5′ GTATGACTGGGGGTGTTGGG 3′, reverse primer 5′ GCGCCCAATAGGACCAAATC 3′). The relative level of viral genome copy number was determined by ΔΔCt.
Sequencing
Total cell DNA from the co-transfection experiments was extracted as outlined above. The DNA was digested with 20 units of Dpn1 (New England Biolabs, Ipswich MA) for 12 hours at 37°C to remove input MHV68 BAC DNA. Two regions in the MHV68 genome (bp 69,873– 70,761 and bp 88,884 to 89,613) were amplified with Dynazyme II DNA polymerase (Thermo Scientific, Waltham MA), cloned into the TOPO TA cloning vector (Life Technologies, Carlsbad, CA) and sequenced to identify mutations that arose during infection.
Pathogenesis
For acute titers, the right and left lungs of mice were removed 4 and 9 days post infection and disrupted with a Mini-Beadbeater (Biospec, Bartlesville OK). The homogenates were titered by plaque assay. Spleens were harvested to determine viral latency establishment and reactivation from latency 16 dpi.
To determine the frequency of cells harboring the viral genome, a single cell suspension of splenocytes was prepared and analyzed by limiting dilution PCR. Six, three-fold serial dilutions of splenocytes were plated in a 96-well PCR plate in a background of NIH 3T12 cells and lysed overnight at 56°C with Proteinase K. The plate was then subjected to an 80-cycle nested PCR with primers specific for MHV68 ORF50. Twelve replicates were analyzed at each serial dilution and plasmid DNA at 0.1,1 and 10 copies was included to verify the sensitivity of the assay.
To determine the frequency of cells harboring latent virus capable of reactivation upon explant, single cell splenocytes were prepared from mice 16 dpi, resuspended in DMEM containing 10% fetal bovine serum and plated in twelve serial two-fold dilutions onto a monolayer of mouse embryonic fibroblast (MEF) cells prepared from C57BL6J mice in 96-well tissue culture plates. Twenty-four replicates were plated per serial dilution. The wells were scored for cytopathic effect (CPE) two to three weeks after plating. To differentiate between pre-formed infectious virus and virus spontaneously reactivating upon cell explant, parallel samples were mechanically disrupted using a Mini-Beadbeater prior to plating on the monolayer of MEFs to release preformed virus that is scored as CPE.
Bioinformatic analysis
To measure under- and over-representation of APOBEC3 hotspots, we compared the observed frequency of each APOBEC consensus motif (TC for hA3A, hA3B, hA3F and hA3H, TTC for hA3C, CCC for hA3G and TCC for mA3) to a random expectation null model. To assess the effects throughout the viral genomes we used sliding 1 kilobase windows to scan each genome at intervals of 100 nucleotides (i.e. first window from position 1–1000, second window from 101–1100, and so on). The null model was generated using 1,000 randomly shuffled versions of each genome. The number of occurrences of the motif (TC, TTC, etc) in each randomly shuffled sequence was used to build a null model distribution for that window. If the true frequency that was observed in the unshuffled sequence was within the lowest 5th percentile of this distribution, we labeled that window as being underrepresented. Conversely, if the true frequency was in the highest 5th percentile, the window was labeled as overrepresented for that motif. The NCBI accession numbers for the genomes analyzed are: Human papilloma virus type 16(NC_001526), Herpes simplex virus 1(NC_001806), Herpes simplex virus 2(NC_001798), Varicella-zoster virus (NC_001348), Epstein-Barr virus (NC_007605), Human cytomegalovirus (NC_006273), Human herpesvirus 6A (NC_001664), Human herpesvirus 6B (NC_000898), Human herpesvirus 7(NC_001716), Kaposi’s sarcoma- associated herpesvirus (NC_009333) and Murine gammaherpesvirus 68 (NC_001826).
Statistical analyses
Data was analyzed using Graphpad Prism Software (Prism 5, La Jolla CA). The statistical significance of differences between groups was tested using a non-paired two-tailed t test. Under Poisson distribution analysis, the frequencies of latency establishment and reactivation from latency were determined by the intersection of nonlinear regression curves with the line at 63.2%.
RESULTS
APOBEC3 hotspots are prevalent in herpesvirus genomes
In a single report outlining the impact of APOBEC3 cytidine deaminase on herpesviruses, HSV-1 replication was reduced 4–10 fold upon transient expression of hA3C. In addition, hyperedited HSV-1 and EBV sequences consistent with hA3 deamination were detected in patient samples using a sensitive, but non-quantitative PCR detection method (Suspene et al., 2011). However, the natural occurrence of APOBEC3-mediated cytidine deamination and the consequence of this selective pressure are not known. We utilized an in silico approach to predict the distribution of APOBEC3 recognition sites in herpesvirus genomes. The ‘hotspot’ or consensus recognition sequence is TC (where the underlined C represents the targeted cytidine) for hA3A, hA3B, hA3F and hA3H, (Hultquist et al., 2011; Liddament et al., 2004; Senavirathne et al., 2012; Yu et al., 2004; Zheng et al., 2004) TTC for hA3C (Taylor et al., 2013), CCC for hA3G (Jern et al., 2009), and TCC for mA3 (MacMillan et al., 2013). To determine whether particular A3 hotspot motifs were over- or under-represented throughout the viral genomes we measured the frequency of each A3 hotspot motif (TC, TTC, CCC and TCC) within a 1 kb sliding window across each genome at intervals of 100 nt. The observed frequencies in each window were then compared to a null model based on randomly shuffled versions of that genome sequence, allowing us to statistically evaluate overrepresentation and underrepresentation.
Viruses that are susceptible to various A3 enzymes would likely have evolved to limit hotspot motifs in their genomes. Viruses that do not encounter certain A3 proteins or those with a viral countermeasure might have an overrepresentation of motifs in their genomes, especially if they have evolved under pressures to avoid a different A3 protein. For example. under-representation of TTC (the motif for hA3C) will increase the relative frequency of other motifs such as CCC (the motif for hA3G) assuming that there are no additional selective pressures against the other motifs. We evaluated the predictive ability of our model by examining the APOBEC3 hotspot distribution in human papilloma virus 16 (HPV16). HPV genomes are targets for deamination upon overexpression of hA3A, hA3C, and hA3H, and mutation signatures that are consistent with deamination at TC hotspots are detected by a sensitive PCR assay in clinical wart specimens (Vartanian et al., 2008). Human A3A, hA3B and hA3H are expressed in dermal epithelial cells, the preferred site of infection and viral replication of HPV (Madsen et al., 1999; OhAinle et al., 2006; Quinlan et al., 1984; Trivedi et al., 2006). Taken together, HPV is subjected to the selective pressures of hA3s with TC hotspots (Vartanian et al., 2008). In agreement, our computational analysis determined that TC hotspots were largely underrepresented in the HPV genome (Figure 1A). This depletion of TC hotspots in the genome is consistent with evasion from the antiviral pressures of hA3A, hA3B, hA3F or hA3H (A3A/A3B/A3F/A3H). Moreover, the HPV genome had an underrepresentation of TTC hotspots favored by the ubiquitously expressed hA3C. In contrast, the HPV genome was largely neutral with some enrichment for CCC hotspots recognized by hA3G, which is expressed predominantly in lymphocytes, a cell type not known as a reservoir for HPV. Finally, the HPV genome was predominately neutral with some occurrences of mA3 hotspot-enriched regions. This is consistent with a human pathogen that would not have been under selective pressure to limit mA3 sites in the genome.
Figure 1. APOBEC3 hotspots in herpesvirus genomes.
A. Percentage of 1 kb windows neutral, overrepresented or underrepresented for human and mouse APOBEC3 hotspots in the indicated viral genome. B. Heatmap of A3 hotspots in herpesvirus genomes. Scale indicates the percentage of 1 kb windows of the viral genome with overrepresentation or underrepresentation of hotspots as described in A. C. Distribution of 1 kb regions of the MHV68 genome overrepresented (red bars) or underrepresented (blue bars) for the indicated A3 hotspots. The boundary of each bar corresponds to the midpoint of the first and last 1 kb window represented within the bar. The gray trace represents the distribution of the hotspot occurrence in a region compared to the occurrence in 1,000 randomly shuffled 1 kb genomic regions. The scale of the trace is from −0.5 to 0.5, the numbers on the y-axis indicate the distance from the mean of each distribution (y=0). Genomic regions are significantly overrepresented (red bars) or underrepresented (blue bars) if they are above 0.45 or below −0.45 respectively.
We next applied this analysis to multiple human herpesviruses and MHV68. As depicted in Figure 1A, we found that the genomes of the alphaherpesviruses HSV-1 and VZV were enriched for A3G hotspots. These genomes were largely neutral or even underrepresented for A3A/A3B/A3F/A3H hotspots. Depletion of hotspots for A3A/A3B/A3H expressed at high levels in epithelial cells is consistent with selective pressure to limit recognition of virus that replicates in the dermal epithelium, yet a finding of enrichment for A3G in the VZV genome was unexpected given its T lymphocyte-associated viremia (Zerboni et al., 2014). Within the betaherpesviruses, the HCMV genome had regions overrepresented with A3A/A3B/A3F/A3H and A3C hotspots, but was largely underrepresented for A3G. The HHV-6A genome was also enriched for A3A/A3B/A3F/A3H and A3C hotspots. While the betaherpesviruses were largely depleted or neutral for A3G, the genomes of the gammaherpesviruses that are also lymphotropic were enriched for A3G hotspots. The EBV and KSHV gammaherpesvirus genomes were underrepresented for A3A/A3B/A3F/A3H hotspots. The MHV68 genome was largely neutral for APOBEC3 hotspots with a higher representation of hA3G and mA3 hotspots. With the exception of HCMV, mA3 sites were overrepresented in each human herpesvirus genome analyzed. In Figure 1B, the heatmap representation of the percentage of overrepresented regions (left panel) and underrepresented regions (right panel) in the genomes of each human herpesvirus and MHV68 illustrates similar trends in the profile of human and mouse APOBEC3 hotspots among members within the subfamilies. Lastly, we examined the distribution of hotspots in the MHV68 genome. With the exception of the internal repeats at nt positions 26,778–28,191 and 98,981–101,170, the regions of overrepresented hotspots were evenly distributed along the MHV68 genome (Figure 1C). Taken together, our analysis predicts that some herpesvirus genomes are targets of APOBEC3 deamination.
Human APOBEC3 restriction of MHV68 replication
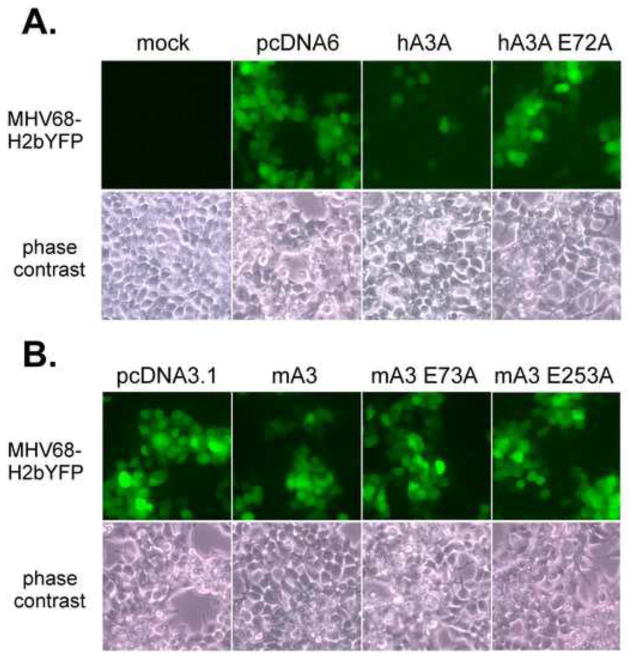
Given the identification of A3 hotspots in the MHV68 genome, we next examined the impact of A3 expression on lytic replication. We co-transfected A3 expression constructs with infectious MHV68+ BAC DNA that encodes the YFP marker to monitor viral replication by fluorescent microscopy. Expression of hA3A limited the infection to single YFP+ cells (Figure 2, top panel). In contrast, co-transfection of the empty vector or the A3A E72A active site mutant resulted in foci of 10–20 YFP+ cells, indicating no impairment of virus spread. Expression of wild type mA3 did not impair the formation of YFP+ foci in the infection (Figure 2, bottom panel).
Figure 2. Restriction of MHV68 spread by human APOBEC3A, but not murine APOBEC3.
HEK 293T cells were cotransfected with MHV68-H2bYFP BAC and the indicated human (hA3) and murine (mA3) APOBEC3 constructs and virus spread was visualized by fluorescence microscopy (upper panels) and bright field microscopy (lower panels) 48 hrs later. HA3A E72A is an active site mutant of human A3A. MA3 E73A and mA3 E253A are active site mutants of mA3. Images shown are cropped fields of pictures taken at 20X magnification. The images of MHV68-H2bYFP+ foci were false-colored green.
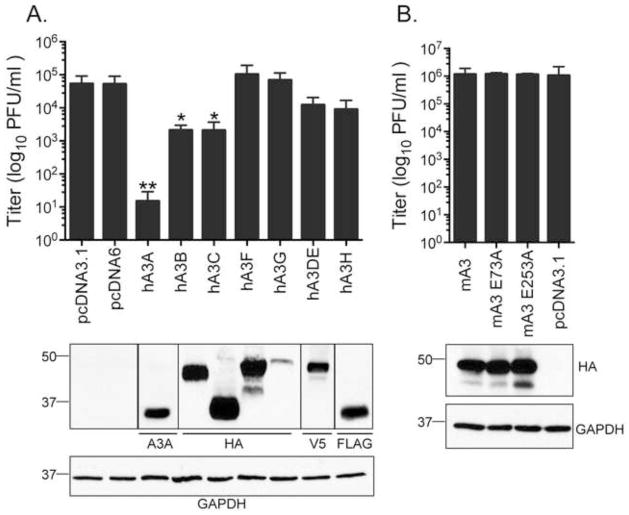
These observations of virus restriction by hA3 but not mA3 were confirmed and extended to other human APOBEC3s upon quantitation of virus yield by plaque assay (Figure 3A). Human A3A inhibited viral replication by nearly 4 logs while co-transfection with hA3B and hA3C proteins resulted in a 2.5 log-fold reduction in replication. Co-transfection of either hA3DE or hA3H resulted in a lesser 5-fold reduction in virus output while hA3F and hA3G did not impair MHV68 replication. In agreement with our previous observation that mA3 overexpression did not impact virus spread in the culture, co-transfection with mA3 did not impact MHV68 yield (Figure 3B). Expression of the human and mouse APOBEC3 proteins was verified by immunoblot (Figure 3A and 3B, respectively). We conclude that hA3A, hA3B and hA3C are restrictive against MHV68 while mA3 is not.
Figure 3. Restriction of MHV68 replication by human APOBEC3.
A. Variable restriction by human APOBEC3 constructs as measured by plaque assay 48 hrs after HEK 293T cells were cotransfected with infectious MHV68-H2bYFP BAC DNA and the indicated APOBEC3 constructs. B. Murine A3 did not significantly impair MHV68 replication in HEK293Ts. Below each bar graph, immunoblots using antibodies against the indicated epitopes were used to validate APOBEC3 expression. Lysates from the empty vectors pcDNA3.1 (control vector for hA3B-hA3H) and pcDNA6 (control vector for hA3A and hA3C) were included in each blot and revealed no specific signal. Bars represent the mean +/− standard deviation of three replicates (A) and five replicates (B); *p≤0.05 and **p≤0.005.
Human APOBEC3 restriction of MHV68 replication is dependent on A3 active sites
Given the observation that several human APOBEC3 proteins impair MHV68 replication, we next examined if APOBEC3 enzymatic activity is required by comparing restriction of MHV68 by the WT and active site mutant forms of hA3A and hA3B. Similar to other APOBEC proteins, A3 enzymes have retained a conserved active site sequence consisting of His-X-Glu-X23-28-Pro-Cys-X2-4-Cys. The histidine and cysteine residues coordinate a Zinc ion while the glutamic acid serves as a proton shuttle in catalysis (Cullen, 2006). Mutation of either the cysteine or the glutamic acid abolishes the cytidine deaminase function of A3 enzymes (Hakata and Landau, 2006; Narvaiza et al., 2009). The hA3A E72A active mutant did not restrict MHV68 yield compared to WT hA3A (Figure 4A). Restriction by hA3B was lost with the N-terminal E68A single active site mutant and the E68A/E255A double mutant. However, the C-terminal single hA3B E255A mutant retained restrictive function by reducing virus yield by 4.5-fold, albeit 56% less than the WT hA3B (Figure 4A). The changes in restriction were not attributed to differences in expression levels of the WT and active site hA3 mutants (Figure 4A, lower panel). Since hA3A reduced MHV68 DNA copy number by greater than 50-fold compared to the empty vector and A3A E72A mutant controls (Figure 4B), we examined two regions of the MHV68 genome for C to T or G to A transition mutations. In samples derived from A3A and A3B cotransfections, the incidence of mutation was extremely low in 18 and 11 respective clones of a GC-rich region of the genome (bp 69,873 to 70,761) and 24 and 23 respective clones of a second region of the genome that is enriched for TC hotspots (bp 88,884 to 89,613) (Table 1). While three transition mutations were observed for region two in A3A-restricted samples, these led to silent or conservative aa changes. We also found a similar number of transition mutations in the viral DNA of samples derived from the empty control vector cotransfections. The mean mutation frequency for MHV68-infected samples restricted by A3A (0.15%) and A3B (0.06%) contrasts sharply with the mutation frequencies reported for HSV-1 and EBV (6–20% by Suspene et al. 2001) and hepatitis B virus (25–50% by Vartanian et al. 2008). Taken together, the active sites of hA3A and hA3B are required for MHV68 restriction, but we found no strong evidence for hA3A or hA3B deamination of the genome.
Figure 4. APOBEC3A and APOBEC3B active sites are required for restriction of viral replication and viral DNA synthesis.
A. Loss of restriction by mutant human APOBEC3A (hA3) and APOBEC3B (hA3B) constructs as measured by plaque assay and quantitative PCR (hA3B) 48 hrs after HEK 293T cells were cotransfected with infectious MHV68-H2bYFP BAC DNA and the indicated APOBEC3 constructs. Below each bar graph, immunoblots using antibodies against the indicated epitopes were used to validate APOBEC3 expression. Lysates from the empty vectors pcDNA6 (control vector for WT and mutant hA3A) and pcDNA3.1 (control vector for WT and mutant hA3B) were included in each blot and revealed no specific signal. Bars represent the mean +/− standard deviation of three replicates; *p≤0.05 and **p≤0.005. B. Quantitation of MHV68 genomes by qPCR analysis of DpnI-digested DNA. DNA levels were normalized to levels upon cotransfection with the empty vector, pCDNA6; *p≤0.05 and **p≤0.005.
Table 1.
Analysis of mutations arising in the viral genome upon cotransfection with human APOBEC3A or APOBEC3B.
| Experimenta | Number of clonesb | Mutation frequencyc | SNPd, genomic coordinatee |
|---|---|---|---|
| Region 1 (bp 69,873–70,761) | |||
| APOBEC3A | 18 | 0.008%, 1/12,492 | T→C 70,265 |
| APOBEC3B | 11 | 0%; 0/5,269 | |
| empty vector 0.5 | 11 | 0%; 0/7,634 | |
| Region 2 (bp 88,884–89,613) | |||
| APOBEC3A replicate 1 | 6 | 0.14%; 6/4,086 | A→G 88,939 T→C 89,050, G→A 89,230 A→G 89,191 A→G 89,283, A→G 89,286 |
| APOBEC3A replicate 2 | 9 | 0.16%; 9/5,526 | C→T 89,033f, A→G 89,178 G→A 89,083f T→C 89,248, T→C 89,270 T→C 89,293 A→G 89,308 T→C 89,407 A→T 89,450 |
| APOBEC3A replicate 3 | 9 | 0.16%; 8/5,148 | A→G 88,985 T→C 89,197 A→G 89,207, T→C 89,417 A→C 89,320 A→G 89,352 A→G 89,357 A→G 89,434 |
| APOBEC3B replicate 1 | 7 | 0.09%; 4/4,417 | A→G 89,103, T→C 89,236 A→G 89,495, T→C 89,507 |
| APOBEC3B replicate 2 | 7 | 0%; 0/4,921 | |
| APOBEC3B replicate 3 | 8 | 0.09%; 5/5,416 | T→C 89,100, A→G 89,106 T→C 89,234 A→G 89,265 T→C 89,286 |
| empty vector replicate 1 | 7 | 0.14%; 7/4893 | T→C 89,050 A→G 89,083, T→C 89,419 T→C 89,432, T→C 89,499, C→T 89,587f T→C 89,500 |
| empty vector replicate 2 | 11 | 0.10%; 7/6699 | T→A 88,908 G→A 88,926f, A→G 89,936, A→G 89,506 G→A89,083f A→G 89,481 T→C 89,498 |
| empty vector replicate 3 | 6 | 0.08%; 3/3774 | A→G 89,127, A→G 89,199, A→G 89,287 |
Cotransfection of MHV68 genomic BAC DNA with the identified construct.
Number of independent colonies sequenced for mutation analysis.
Mutation frequency is calculated as follows: (number of changes/total number of nucleotides sequenced per experiment) x 100.
SNP- Single nucelotide polymorphism.
Position of SNP in the MHV68 genome.
SNPs that reflect transition mutations (G to A or C to T changes)
Human APOBEC3 restriction of MHV68 replication is lost upon de novo infection
Several human APOBEC3 proteins were identified as potent inhibitors of MHV68 replication, the nuclear localized hA3B (Lackey et al., 2012), the predominantly cytoplasmic hA3DE (Kinomoto et al., 2007), and hA3A, hA3C and hA3H, which shuttle between the nucleus and cytoplasm (Bogerd et al., 2006; Kinomoto et al., 2007; Li and Emerman, 2011). The MHV68 genome delivered by transfection would likely be susceptible to APOBEC3s in both the nucleus and cytoplasm. However, we reasoned that the encapsidated genome would be protected from cytoplasmic A3s in a de novo infection with intact particles. As such, we transfected the panel of APOBEC3 constructs one day prior to infection with MHV68 at a multiplicity of infection (MOI) of 0.01. We unexpectedly found that the APOBEC3 proteins with potent restrictive function in the co-transfection experiment were no longer restrictive against infection initiated by virion particles (Figure 5). Thus, the method of viral genome delivery and initiation of lytic infection influences the restrictive capacity of the APOBEC3 enzymes. This suggests that either compartmentalization of the herpesvirus genome or counter-functions imparted by virion-associated factors result in a replicative process that is impervious to antiviral APOBEC3 function.
Figure 5. Loss of APOBEC3 restriction in the context of de novo infection with virus particles.
HEK 293T cells were transfected with the indicated APOBEC3 constructs 24 hrs prior to de novo infection with MHV68-H2bYFP virus. MHV68 replication was measured by plaque assay 48 hpi. Below each bar graph, immunoblots using antibodies against the indicated epitopes were used to validate APOBEC3 expression. Lysates from the empty vectors pcDNA3.1 (control vector for hA3B, hA3DE-hA3H) and pcDNA6 (control vector for hA3A and hA3C) were included in each blot and revealed no specific signal.
Murine APOBEC3 does not impact MHV68 replication in vivo
Murine A3 overexpression in 293T cells did not significantly restrict MHV68 replication. However transient expression studies cannot be relied upon as an absolute indicator of the capacity of an antiviral molecule to restrict a pathogen in the host. Thus, we determined the impact of murine A3 on MHV68 pathogenesis in mice with a disruption in the mA3 gene (Figure 6A). The galactosidase-neomycin fusion (β-geo) stop cassette that is inserted between exons IV and V of mA3 in murine APOBEC3 knock-out mice (mA3−/− mice) was confirmed by PCR and amplimer sequencing (Figure 6B). Additionally, mA3 protein was not detected in the spleens or thymus of mA3−/− mice (Figure 6C). Okeoma et al. (2007) generated mA3−/− mice as described here; a truncated protein in mA3−/− tissues was not observed. Furthermore, a truncated mA3 protein generated from exons 1–4 was devoid of deaminase function upon in vitro expression (Okeoma et al., 2007).
Figure 6. Absence of murine APOBEC3 does not alter MHV68 pathogenesis.

A. Schematic depicting the disruption of murine APOBEC3 (mA3) by the insertion of a β-geo cassette to generate mA3 knockout mice (mA3−/−). B. Genotype PCR analysis of DNA harvested from the splenocytes of infected WT C57BL/6 or mA3−/− mice. The locations of primers for either the WT or mutant mA3 gene are shown in panel A. C. Immunoblot demonstrating expression of mA3 in WT but not mA3−/− mice. D. Acute replication in the lungs of WT or mA3−/− lungs 4 and 7 days post intranasal infection with 1000 PFU of WT MHV68. E. Spleen weights from naïve WT, infected mA3−/− mice or infected WT mice 16 dpi. F. Latency is determined as the frequency of viral genome positive splenocytes determined by limiting dilution PCR. G. Frequency of reactivation from intact splenocytes determined by a limiting dilution explant reactivation assay 16 dpi. Open symbols represent preformed infectious virus from mechanically disrupted cells plated in parallel. The data shown represent three independent experiments with splenocytes pooled from three to five mice per experimental group.
Infection of mice with MHV68 by intranasal inoculation is followed by a period of acute replication in the lung. To examine the influence of mA3 on acute replication, the levels of virus replication in the lungs of infected WT or mA3−/− mice were measured at 4 and 7 dpi. Although there is a slight, non-statistically significant difference between the two groups at 4 dpi, viral titers were indistinguishable between WT and mA3−/− mice at 7 dpi, indicating that the loss of murine APOBEC3 did not enhance MHV68 replication in the lung (Figure 6D).
Active MHV68 replication is typically cleared from the lung within two weeks prior to virus dissemination to the spleen. MHV68 establishes a latent infection primarily in B cells of the spleen with the peak of latency 16–18 dpi, a timepoint closely corresponding to germinal center expansion (Weck et al., 1996; Weck et al., 1999a). We observed a robust two-fold increase in splenomegaly as expected, yet there was no difference between the degree of splenic expansion in the infected WT and mA3−/− mice. To examine whether loss of mA3 would impact latency establishment in the spleen 16 dpi, intact splenocytes were analyzed by a limiting dilution PCR assay to determine the frequency of viral genome positive cells. MHV68 established equivalent levels of latency in the spleens of WT (1/156) and mA3−/− mice (1/128) (Figure 6F). The frequency of intact splenocytes that spontaneously reactivated was examined by the observation of CPE upon explant and coculture with MEFs in a limiting dilution reactivation assay. Loss of mA3 had an insignificant two-fold impact on MHV68 reactivation, with splenocytes from infected WT mice and infected mA3−/− mice reactivating at frequencies of 1/19,074 and 1/10,966 cells, respectively (Figure 6G). Moreover, this slight difference in reactivation at 16 dpi had no effect on the maintenance of the frequency of genome positive cells later during chronic infection at 42 dpi (data not shown). Taken together, the absence of mA3 did not impact any aspect of MHV68 pathogenesis up to six weeks following an intranasal infection.
DISCUSSION
Targets of human APOBEC3 deaminase activity have been recently expanded to include plasmid DNA (Stenglein et al., 2010), small DNA viruses (Chen et al., 2006) and the large human herpesviruses (Suspene et al., 2011). In this study, we set out to determine if the single APOBEC3 protein, mA3, encoded by the mouse had the ability to restrict the rodent herpesvirus pathogen MHV68. Our in silico analysis determined that A3 sites were abundant in MHV68, implying that this genome would be susceptible to A3 mediated deamination. Overexpression of mA3 together with infectious herpesvirus DNA did not significantly impact MHV68 titers compared to a more substantial restriction observed with hA3s. Surprisingly, the hA3 restriction was lost upon de novo infection. WT and mA3−/− C57BL6 mice were indistinguishable in the levels of acute MHV68 replication in the lung, latency establishment in the spleen and reactivation from the spleen. Thus, loss of mA3 had no discernable effect on MHV68 replication in vivo.
In this study we identified several human APOBEC3s as potent restrictors of MHV68 replication. Additionally, we found that the restriction by hA3A and hA3B is dependent on their previously identified active sites (Hakata and Landau, 2006; Stenglein et al., 2010). Interestingly hA3B restriction of MHV68 was absolutely dependent on the N-terminal amino acid, E68 and less dependent on the C-terminal E255 residue, possibly indicating a separation of function between the E68 and E255 active sites. The requirement for these hA3 active sites to restrict MHV68 growth and DNA replication is consistent with deamination of the genome as the basis of restriction. However, we have no direct evidence to support this hypothesis since DNA from A3A or A3B cotransfections contained few C to T or G to A transitions. Although two transitions were present in A3A cotransfected samples, we observed a similar frequency of transitions in DNA from control transfections. The lack of a high mutation frequency in the A3A samples might be attributed to heavily deaminated DNA that is unable to serve as a template for replication or rare events that are below the limit of detection. Alternatively, A3A might impair MHV68 replication using a deamination-independent mechanism that is dependent on the active site. For example, A3A mutants that were deaminase defective but retained other known functions such as DNA binding and localization to the nucleus were found to restrict parvoviruses (Chen et al., 2006; Narvaiza et al., 2009).
We hypothesized that in silico analysis would reveal a hotspot profile for a herpesvirus that reflects tropism for cell types that express high levels of particular host APOBEC3s. Namely, virus genomes under selective pressure by one or more APOBEC3s would be underrepresented or depleted of that particular APOBEC hotspot. The HPV genome is depleted of A3A/A3B/A3H hotspots and neutral or enriched for hA3G, consistent with its epithelial cell tropism. However, the association of cell tropism and hotspot profiles was not as straightforward for the herpesviruses. Human herpesviruses such as VZV, EBV, and KSHV that reside in lymphocytes during their life cycle were not depleted for A3G hotspots. The VZV genome was depleted for A3A/A3B/A3H hotspots, yet HSV-1, another virus that replicates in the epithelium was predominately neutral with overrepresentation in some regions. The overrepresentation of TC and TTC hotspots in the HSV-1 genome is consistent with the restriction of HSV-1 replication by the ectopic expression of hA3A and hA3C reported by Suspene et al., 2011. Interestingly, this study reported a high incidence of edited HSV-1 genomes upon transient transfection of hA3C and a striking frequency of hypereditied HSV-1 and EBV genomes in clinical specimens. These findings relied on the detection of rare deamination events by PCR based amplification at low denaturing temperatures (Suspene et al., 2011). A non-biased quantitative determination of the incidence of herpesvirus deamination in human infections and the impact on pathogenesis warrants further investigation.
At this juncture, the APOBEC3 hotspot analysis has some predictive value and correlates with some aspects of herpesvirus biology, but an interpretation of hotspot frequencies is more complex since a multitude of factors apply evolutionary pressures. The occurrence of a particular dinucleotide or trinucleotide sequence is influenced by codon bias, a wide expanse of protein-DNA interactions, and restrictive pressures by one APOBEC3 deaminase that may in turn bias the incidence of another hotspot. These pressures are further compounded by the fact that each herpesvirus engages multiple cell types in its lifecycle and, as discussed below, these viruses may have evolved countermeasures.
Strikingly, hA3 restriction of MHV68 replication was observed upon transfection of nonencapsidated infectious DNA but was lost upon de novo infection with virion particles. MHV68 virions may deliver a factor that counteracts the hA3 proteins. Tegument proteins from several herpesviruses families function in either the cytoplasm or the nucleus to limit the antiviral response from the host. KSHV ORF45 inhibits type I interferon signaling by impairing the phosphorylation and nuclear translocation of IRF-7 (Sathish et al., 2011; Zhu et al., 2002). HSV1 ICPO, HCMV pp71, HVS ORF3, EBV BNRF1 and MHV68 ORF75C translocate to the nucleus upon virus infection to degrade or relocalize components of the antiviral nuclear domain 10 structures (ND10) (Boutell and Everett, 2013; Full et al., 2012; Ling et al., 2008; Penkert and Kalejta, 2012; Tsai et al., 2011). We did not find significant homology of MHV68 virion components including glycoproteins, tegument and capsid proteins to known A3 antagonists. However, we cannot rule out the existence of a novel mechanism by which the virion structural components or even RNA molecules mediates resistance to APOBEC3 proteins.
The loss of MHV68 restriction might also be explained by the compartmental separation of A3 proteins from the MHV68 genome in a natural course of infection. Several of the hA3 proteins that exhibit restriction of MHV68, namely hA3A, hA3B, hA3C and hA3H are targeted to the nucleus (Bogerd et al., 2006; Kinomoto et al., 2007). We propose that the hA3 proteins might be separated from viral replication centers in the nucleus via either the direct action of a viral factor or indirectly due to viral remodeling of the nucleus. Herpesvirus replication, transcription and the formation of viral capsids transpires in intranuclear structures known as replication centers that are defined by the presence of key viral replication proteins as well as host proteins (de Bruyn Kops and Knipe, 1994; Quinlan et al., 1984; Weller, 2010). Nuclear substructures such as the ND10, as well as nuclear protein localization and abundance are tightly regulated by herpesviruses to maintain optimal viral gene expression and replication (Saffert and Kalejta, 2008). The subcellular localization of A3 proteins and herpesvirus replication compartments could provide mechanistic insight for viral evasion of hA3 restriction.
Several human APOBEC3 proteins exhibit restriction of MHV68 replication, but mA3 exhibited no restrictive capacity in the cotransfection experiments. This lack of restriction with mA3 overexpression is not likely due to the absence of a co-factor in the HEK 293T cells. Murine APOBEC3 partially restricts Moloney murine leukemia virus replication and restricts HIV-1 lacking Vif to similar levels as hA3G in 293T cells (Doehle et al., 2005; Sanchez-Martinez et al., 2012). The absence of restriction with overexpressed mA3 was supported by a lack of enhancement in MHV68 pathogenesis in mA3 knockout mice. Thus, MHV68 might encode a mA3 antagonist that significantly limits antiviral APOBEC3 activity such as HIV Vif (Malim, 2009; Malim and Emerman, 2008; Mariani et al., 2003) or Murine Leukemia Virus (MLV) glyco-gag (Stavrou et al., 2013). Additionally, a recent report identified a significant role for host uracil DNA glycosylase (UNG) in reducing the effect of hA3G-induced hyperediting of duck hepatitis B virus (Kitamura et al., 2013). MHV68, like other herpesviruses encodes a uracil DNA glycosylase (UNG/ORF46). However, we did not observe an increased restriction of MHV68 replication by hA3A, hA3B or mA3 in cells infected with a recombinant MHV68 harboring a stop codon in ORF46 compared to the control repair virus (Minkah, Chavez, and Krug, unpublished results).
Infection of mice lacking mA3 did not lead to an enhancement of MHV68 pathogenesis. This may be attributed to a viral countermeasure as described above, a lack of mA3 deamination function in the context of a herpesvirus infection or a yet undiscovered role in a particular aspect of chronic infection in this pathogenesis model. The intranasal route of inoculation is characterized by high levels of productive replication in the lungs, a tissue with lower mA3 expression levels (Mikl et al., 2005). MA3 protein is expressed at high levels in the spleens, but its absence did not influence latency establishment in the spleen or reactivation from latency. Another route of infection, such as intraperitoneal inoculation might reveal the importance of MHV68 replication in peritoneal exudate cells wherein the primary latency reservoir is macrophages (Weck et al., 1999b). Indeed, the influence of some host proteins such as promyelocytic leukemia nuclear bodies (PML) (Sewatanon et al., 2013) or viral proteins such as M1 and M2 on MHV68 pathogenesis is dependent on the route of infection (Clambey et al., 2000; Jacoby et al., 2002). Finally, it is possible that another APOBEC protein compensates for the loss of mA3 to limit MHV68 replication. A hyperediting signature similar to murine APOBEC1 deamination was reported in a mouse model of hepatitis B virus infection (Petit et al., 2009; Renard et al., 2010). Furthermore, in a rat model of HSV-1-induced encephalitis, APOBEC1 levels were increased upon HSV-1 infection and overexpression of APOBEC1 both enhanced hyperediting of HSV-1 genomes and reduced viral titers (Gee et al., 2011).
MHV68 infection of mice provides a tractable model pathogenesis system to dissect the contribution of virus and host factors that influence chronic gammaherpesvirus infection. Primates have diversified their repertoire of A3 cytidine deaminases under the selective pressure of retroelements and viral pathogens. We sought to examine whether the evolutionary distinct yet ‘primordial’ APOBEC3 encoded by mice shared the restrictive property of the expanded human APOBEC3 molecules against MHV68, with the aim of utilizing mA3−/− mice as a platform for pathogenesis and mechanistic investigations. However, mA3 did not restrict MHV68 replication in cell culture or pathogenesis in mice. The unexpected loss of the hA3 restrictive phenotype when replication was initiated upon de novo infection with virus particles leads us to posit that herpesviruses are largely protected from hA3 restriction. Possible evasion mechanisms to explore include a virion-associated countermeasure such as a tegument protein or a block in APOBEC3 access to the viral genome either due to encapsidation of the viral genome during cytoplasmic transport to the nucleus or compartmentalization of the genome in nuclear replication centers.
Highlights.
We examined APOBEC3 hotspots in herpesvirus genomes.
Human APOBEC3s impaired MHV68 replication upon viral genome transfection.
MHV68 restriction was abrogated upon mutation of the A3A and A3B active sites.
Restriction was lost if the infectious DNA was delivered by the virion.
MHV68 replication and pathogenesis were not altered by murine APOBEC3.
Acknowledgments
Kevin Chavez was supported by an NIH-MARC fellowship grant (grant #5T34GM008655). Parth Shah was partly supported by the Stony Brook University Simons Summer Research Program (2013) and Thomas MacCarthy was supported by Stony Brook University startup funds. Nathaniel Landau was supported by NIH grants (AI058864 and AI074967). Laurie Krug was supported by an American Cancer Society research scholar grant, RSG-1-160-01-MPC and NIH AI097875. Special thanks to Steven Reddy for technical support and members of the Krug laboratory for helpful discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
LITERATURE CITED
- Bishop KN, Holmes RK, Sheehy AM, Malim MH. APOBEC-mediated editing of viral RNA. Science. 2004;305:645. doi: 10.1126/science.1100658. [DOI] [PubMed] [Google Scholar]
- Bogerd HP, Tallmadge RL, Oaks JL, Carpenter S, Cullen BR. Equine infectious anemia virus resists the antiretroviral activity of equine APOBEC3 proteins through a packaging-independent mechanism. Journal of virology. 2008;82:11889–11901. doi: 10.1128/JVI.01537-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogerd HP, Wiegand HL, Hulme AE, Garcia-Perez JL, O’Shea KS, Moran JV, Cullen BR. Cellular inhibitors of long interspersed element 1 and Alu retrotransposition. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:8780–8785. doi: 10.1073/pnas.0603313103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutell C, Everett RD. Regulation of alphaherpesvirus infections by the ICP0 family of proteins. The Journal of general virology. 2013;94:465–481. doi: 10.1099/vir.0.048900-0. [DOI] [PubMed] [Google Scholar]
- Bransteitter R, Prochnow C, Chen XS. The current structural and functional understanding of APOBEC deaminases. Cellular and molecular life sciences: CMLS. 2009;66:3137–3147. doi: 10.1007/s00018-009-0070-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Lilley CE, Yu Q, Lee DV, Chou J, Narvaiza I, Landau NR, Weitzman MD. APOBEC3A is a potent inhibitor of adeno-associated virus and retrotransposons. Current biology: CB. 2006;16:480–485. doi: 10.1016/j.cub.2006.01.031. [DOI] [PubMed] [Google Scholar]
- Clambey ET, Virgin HWt, Speck SH. Disruption of the murine gammaherpesvirus 68 M1 open reading frame leads to enhanced reactivation from latency. Journal of virology. 2000;74:1973–1984. doi: 10.1128/jvi.74.4.1973-1984.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins CM, Speck SH. Tracking murine gammaherpesvirus 68 infection of germinal center B cells in vivo. PloS one. 2012;7:e33230. doi: 10.1371/journal.pone.0033230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen BR. Role and mechanism of action of the APOBEC3 family of antiretroviral resistance factors. Journal of virology. 2006;80:1067–1076. doi: 10.1128/JVI.80.3.1067-1076.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruyn Kops A, Knipe DM. Preexisting nuclear architecture defines the intranuclear location of herpesvirus DNA replication structures. Journal of virology. 1994;68:3512–3526. doi: 10.1128/jvi.68.6.3512-3526.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delebecque F, Suspene R, Calattini S, Casartelli N, Saib A, Froment A, Wain-Hobson S, Gessain A, Vartanian JP, Schwartz O. Restriction of foamy viruses by APOBEC cytidine deaminases. Journal of virology. 2006;80:605–614. doi: 10.1128/JVI.80.2.605-614.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doehle BP, Schafer A, Wiegand HL, Bogerd HP, Cullen BR. Differential sensitivity of murine leukemia virus to APOBEC3-mediated inhibition is governed by virion exclusion. Journal of virology. 2005;79:8201–8207. doi: 10.1128/JVI.79.13.8201-8207.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll DM, Zhang Q. Expression and characterization of p27, the catalytic subunit of the apolipoprotein B mRNA editing enzyme. The Journal of biological chemistry. 1994;269:19843–19847. [PubMed] [Google Scholar]
- Full F, Reuter N, Zielke K, Stamminger T, Ensser A. Herpesvirus saimiri antagonizes nuclear domain 10-instituted intrinsic immunity via an ORF3-mediated selective degradation of cellular protein Sp100. Journal of virology. 2012;86:3541–3553. doi: 10.1128/JVI.06992-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee P, Ando Y, Kitayama H, Yamamoto SP, Kanemura Y, Ebina H, Kawaguchi Y, Koyanagi Y. APOBEC1-mediated editing and attenuation of herpes simplex virus 1 DNA indicate that neurons have an antiviral role during herpes simplex encephalitis. Journal of virology. 2011;85:9726–9736. doi: 10.1128/JVI.05288-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goila-Gaur R, Strebel K. HIV-1 Vif, APOBEC, and intrinsic immunity. Retrovirology. 2008;5:51. doi: 10.1186/1742-4690-5-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakata Y, Landau NR. Reversed functional organization of mouse and human APOBEC3 cytidine deaminase domains. The Journal of biological chemistry. 2006;281:36624–36631. doi: 10.1074/jbc.M604980200. [DOI] [PubMed] [Google Scholar]
- Hultquist JF, Lengyel JA, Refsland EW, LaRue RS, Lackey L, Brown WL, Harris RS. Human and rhesus APOBEC3D, APOBEC3F, APOBEC3G, and APOBEC3H demonstrate a conserved capacity to restrict Vif-deficient HIV-1. Journal of virology. 2011;85:11220–11234. doi: 10.1128/JVI.05238-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby MA, Virgin HWt, Speck SH. Disruption of the M2 gene of murine gammaherpesvirus 68 alters splenic latency following intranasal, but not intraperitoneal, inoculation. Journal of virology. 2002;76:1790–1801. doi: 10.1128/JVI.76.4.1790-1801.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jern P, Russell RA, Pathak VK, Coffin JM. Likely role of APOBEC3G-mediated G-to-A mutations in HIV-1 evolution and drug resistance. PLoS pathogens. 2009;5:e1000367. doi: 10.1371/journal.ppat.1000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinomoto M, Kanno T, Shimura M, Ishizaka Y, Kojima A, Kurata T, Sata T, Tokunaga K. All APOBEC3 family proteins differentially inhibit LINE-1 retrotransposition. Nucleic acids research. 2007;35:2955–2964. doi: 10.1093/nar/gkm181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura K, Wang Z, Chowdhury S, Simadu M, Koura M, Muramatsu M. Uracil DNA glycosylase counteracts APOBEC3G-induced hypermutation of hepatitis B viral genomes: excision repair of covalently closed circular DNA. PLoS pathogens. 2013;9:e1003361. doi: 10.1371/journal.ppat.1003361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackey L, Demorest ZL, Land AM, Hultquist JF, Brown WL, Harris RS. APOBEC3B and AID have similar nuclear import mechanisms. Journal of molecular biology. 2012;419:301–314. doi: 10.1016/j.jmb.2012.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlois MA, Kemmerich K, Rada C, Neuberger MS. The AKV murine leukemia virus is restricted and hypermutated by mouse APOBEC3. Journal of virology. 2009;83:11550–11559. doi: 10.1128/JVI.01430-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MM, Emerman M. Polymorphism in human APOBEC3H affects a phenotype dominant for subcellular localization and antiviral activity. Journal of virology. 2011;85:8197–8207. doi: 10.1128/JVI.00624-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddament MT, Brown WL, Schumacher AJ, Harris RS. APOBEC3F properties and hypermutation preferences indicate activity against HIV-1 in vivo. Current biology: CB. 2004;14:1385–1391. doi: 10.1016/j.cub.2004.06.050. [DOI] [PubMed] [Google Scholar]
- Ling PD, Tan J, Sewatanon J, Peng R. Murine gammaherpesvirus 68 open reading frame 75c tegument protein induces the degradation of PML and is essential for production of infectious virus. Journal of virology. 2008;82:8000–8012. doi: 10.1128/JVI.02752-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low A, Okeoma CM, Lovsin N, de las Heras M, Taylor TH, Peterlin BM, Ross SR, Fan H. Enhanced replication and pathogenesis of Moloney murine leukemia virus in mice defective in the murine APOBEC3 gene. Virology. 2009;385:455–463. doi: 10.1016/j.virol.2008.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMillan AL, Kohli RM, Ross SR. APOBEC3 inhibition of mouse mammary tumor virus infection: the role of cytidine deamination versus inhibition of reverse transcription. Journal of virology. 2013;87:4808–4817. doi: 10.1128/JVI.00112-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen P, Anant S, Rasmussen HH, Gromov P, Vorum H, Dumanski JP, Tommerup N, Collins JE, Wright CL, Dunham I, MacGinnitie AJ, Davidson NO, Celis JE. Psoriasis upregulated phorbolin-1 shares structural but not functional similarity to the mRNA-editing protein apobec-1. The Journal of investigative dermatology. 1999;113:162–169. doi: 10.1046/j.1523-1747.1999.00682.x. [DOI] [PubMed] [Google Scholar]
- Mahieux R, Suspene R, Delebecque F, Henry M, Schwartz O, Wain-Hobson S, Vartanian JP. Extensive editing of a small fraction of human T-cell leukemia virus type 1 genomes by four APOBEC3 cytidine deaminases. The Journal of general virology. 2005;86:2489–2494. doi: 10.1099/vir.0.80973-0. [DOI] [PubMed] [Google Scholar]
- Malim MH. APOBEC proteins and intrinsic resistance to HIV-1 infection. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 2009;364:675–687. doi: 10.1098/rstb.2008.0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malim MH, Emerman M. HIV-1 accessory proteins--ensuring viral survival in a hostile environment. Cell host & microbe. 2008;3:388–398. doi: 10.1016/j.chom.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Mangeat B, Turelli P, Caron G, Friedli M, Perrin L, Trono D. Broad antiretroviral defence by human APOBEC3G through lethal editing of nascent reverse transcripts. Nature. 2003;424:99–103. doi: 10.1038/nature01709. [DOI] [PubMed] [Google Scholar]
- Mariani R, Chen D, Schrofelbauer B, Navarro F, Konig R, Bollman B, Munk C, Nymark-McMahon H, Landau NR. Species-specific exclusion of APOBEC3G from HIV-1 virions by Vif. Cell. 2003;114:21–31. doi: 10.1016/s0092-8674(03)00515-4. [DOI] [PubMed] [Google Scholar]
- Mehta A, Driscoll DM. A sequence-specific RNA-binding protein complements apobec-1 To edit apolipoprotein B mRNA. Molecular and cellular biology. 1998;18:4426–4432. doi: 10.1128/mcb.18.8.4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikl MC, Watt IN, Lu M, Reik W, Davies SL, Neuberger MS, Rada C. Mice deficient in APOBEC2 and APOBEC3. Molecular and cellular biology. 2005;25:7270–7277. doi: 10.1128/MCB.25.16.7270-7277.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munk C, Beck T, Zielonka J, Hotz-Wagenblatt A, Chareza S, Battenberg M, Thielebein J, Cichutek K, Bravo IG, O’Brien SJ, Lochelt M, Yuhki N. Functions, structure, and read-through alternative splicing of feline APOBEC3 genes. Genome biology. 2008;9:R48. doi: 10.1186/gb-2008-9-3-r48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narvaiza I, Linfesty DC, Greener BN, Hakata Y, Pintel DJ, Logue E, Landau NR, Weitzman MD. Deaminase-independent inhibition of parvoviruses by the APOBEC3A cytidine deaminase. PLoS pathogens. 2009;5:e1000439. doi: 10.1371/journal.ppat.1000439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OhAinle M, Kerns JA, Malik HS, Emerman M. Adaptive evolution and antiviral activity of the conserved mammalian cytidine deaminase APOBEC3H. Journal of virology. 2006;80:3853–3862. doi: 10.1128/JVI.80.8.3853-3862.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okeoma CM, Lovsin N, Peterlin BM, Ross SR. APOBEC3 inhibits mouse mammary tumour virus replication in vivo. Nature. 2007;445:927–930. doi: 10.1038/nature05540. [DOI] [PubMed] [Google Scholar]
- Okuyama S, Marusawa H, Matsumoto T, Ueda Y, Matsumoto Y, Endo Y, Takai A, Chiba T. Excessive activity of apolipoprotein B mRNA editing enzyme catalytic polypeptide 2 (APOBEC2) contributes to liver and lung tumorigenesis. International journal of cancer. Journal international du cancer. 2012;130:1294–1301. doi: 10.1002/ijc.26114. [DOI] [PubMed] [Google Scholar]
- Penkert RR, Kalejta RF. Tale of a tegument transactivator: the past, present and future of human CMV pp71. Future virology. 2012;7:855–869. doi: 10.2217/fvl.12.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit V, Guetard D, Renard M, Keriel A, Sitbon M, Wain-Hobson S, Vartanian JP. Murine APOBEC1 is a powerful mutator of retroviral and cellular RNA in vitro and in vivo. Journal of molecular biology. 2009;385:65–78. doi: 10.1016/j.jmb.2008.10.043. [DOI] [PubMed] [Google Scholar]
- Quinlan MP, Chen LB, Knipe DM. The intranuclear location of a herpes simplex virus DNA-binding protein is determined by the status of viral DNA replication. Cell. 1984;36:857–868. doi: 10.1016/0092-8674(84)90035-7. [DOI] [PubMed] [Google Scholar]
- Refsland EW, Harris RS. The APOBEC3 family of retroelement restriction factors. Current topics in microbiology and immunology. 2013;371:1–27. doi: 10.1007/978-3-642-37765-5_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renard M, Henry M, Guetard D, Vartanian JP, Wain-Hobson S. APOBEC1 and APOBEC3 cytidine deaminases as restriction factors for hepadnaviral genomes in non-humans in vivo. Journal of molecular biology. 2010;400:323–334. doi: 10.1016/j.jmb.2010.05.029. [DOI] [PubMed] [Google Scholar]
- Saffert RT, Kalejta RF. Promyelocytic leukemia-nuclear body proteins: herpesvirus enemies, accomplices, or both? Future virology. 2008;3:265–277. doi: 10.2217/17460794.3.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Martinez S, Aloia AL, Harvin D, Mirro J, Gorelick RJ, Jern P, Coffin JM, Rein A. Studies on the restriction of murine leukemia viruses by mouse APOBEC3. PloS one. 2012;7:e38190. doi: 10.1371/journal.pone.0038190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathish N, Yuan Y. Evasion and subversion of interferon-mediated antiviral immunity by Kaposi’s sarcoma-associated herpesvirus: an overview. Journal of virology. 2011;85:10934–10944. doi: 10.1128/JVI.00687-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senavirathne G, Jaszczur M, Auerbach PA, Upton TG, Chelico L, Goodman MF, Rueda D. Single-stranded DNA scanning and deamination by APOBEC3G cytidine deaminase at single molecule resolution. The Journal of biological chemistry. 2012;287:15826–15835. doi: 10.1074/jbc.M112.342790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sewatanon J, Ling PD. Murine gammaherpesvirus 68 ORF75c contains ubiquitin E3 ligase activity and requires PML SUMOylation but not other known cellular PML regulators, CK2 and E6AP, to mediate PML degradation. Virology. 2013;440:140–149. doi: 10.1016/j.virol.2013.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sewatanon J, Liu H, Ling PD. PML protein modulates establishment and maintenance of latent gamma-herpesvirus infection in peritoneal cells. Journal of virology. 2013 doi: 10.1128/JVI.01696-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehy AM, Gaddis NC, Choi JD, Malim MH. Isolation of a human gene that inhibits HIV-1 infection and is suppressed by the viral Vif protein. Nature. 2002;418:646–650. doi: 10.1038/nature00939. [DOI] [PubMed] [Google Scholar]
- Smith HC, Bennett RP, Kizilyer A, McDougall WM, Prohaska KM. Functions and regulation of the APOBEC family of proteins. Seminars in cell & developmental biology. 2012;23:258–268. doi: 10.1016/j.semcdb.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavrou S, Nitta T, Kotla S, Ha D, Nagashima K, Rein AR, Fan H, Ross SR. Murine leukemia virus glycosylated Gag blocks apolipoprotein B editing complex 3 and cytosolic sensor access to the reverse transcription complex. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:9078–9083. doi: 10.1073/pnas.1217399110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenglein MD, Burns MB, Li M, Lengyel J, Harris RS. APOBEC3 proteins mediate the clearance of foreign DNA from human cells. Nature structural & molecular biology. 2010;17:222–229. doi: 10.1038/nsmb.1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suspene R, Aynaud MM, Koch S, Pasdeloup D, Labetoulle M, Gaertner B, Vartanian JP, Meyerhans A, Wain-Hobson S. Genetic editing of herpes simplex virus 1 and Epstein-Barr herpesvirus genomes by human APOBEC3 cytidine deaminases in culture and in vivo. Journal of virology. 2011;85:7594–7602. doi: 10.1128/JVI.00290-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suspene R, Guetard D, Henry M, Sommer P, Wain-Hobson S, Vartanian JP. Extensive editing of both hepatitis B virus DNA strands by APOBEC3 cytidine deaminases in vitro and in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2005a;102:8321–8326. doi: 10.1073/pnas.0408223102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suspene R, Henry M, Guillot S, Wain-Hobson S, Vartanian JP. Recovery of APOBEC3-edited human immunodeficiency virus G->A hypermutants by differential DNA denaturation PCR. The Journal of general virology. 2005b;86:125–129. doi: 10.1099/vir.0.80426-0. [DOI] [PubMed] [Google Scholar]
- Suspene R, Sommer P, Henry M, Ferris S, Guetard D, Pochet S, Chester A, Navaratnam N, Wain-Hobson S, Vartanian JP. APOBEC3G is a single-stranded DNA cytidine deaminase and functions independently of HIV reverse transcriptase. Nucleic acids research. 2004;32:2421–2429. doi: 10.1093/nar/gkh554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda E, Tsuji-Kawahara S, Sakamoto M, Langlois MA, Neuberger MS, Rada C, Miyazawa M. Mouse APOBEC3 restricts friend leukemia virus infection and pathogenesis in vivo. Journal of virology. 2008;82:10998–11008. doi: 10.1128/JVI.01311-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor BJ, Nik-Zainal S, Wu YL, Stebbings LA, Raine K, Campbell PJ, Rada C, Stratton MR, Neuberger MS. DNA deaminases induce break-associated mutation showers with implication of APOBEC3B and 3A in breast cancer kataegis. eLife. 2013;2:e00534. doi: 10.7554/eLife.00534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi NR, Gilliland KL, Zhao W, Liu W, Thiboutot DM. Gene array expression profiling in acne lesions reveals marked upregulation of genes involved in inflammation and matrix remodeling. The Journal of investigative dermatology. 2006;126:1071–1079. doi: 10.1038/sj.jid.5700213. [DOI] [PubMed] [Google Scholar]
- Tsai K, Thikmyanova N, Wojcechowskyj JA, Delecluse HJ, Lieberman PM. EBV tegument protein BNRF1 disrupts DAXX-ATRX to activate viral early gene transcription. PLoS pathogens. 2011;7:el002376. doi: 10.1371/journal.ppat.1002376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vartanian JP, Guetard D, Henry M, Wain-Hobson S. Evidence for editing of human papillomavirus DNA by APOBEC3 in benign and precancerous lesions. Science. 2008;320:230–233. doi: 10.1126/science.1153201. [DOI] [PubMed] [Google Scholar]
- Weck KE, Barkon ML, Yoo LI, Speck SH, Virgin HI. Mature B cells are required for acute splenic infection, but not for establishment of latency, by murine gammaherpesvirus 68. Journal of virology. 1996;70:6775–6780. doi: 10.1128/jvi.70.10.6775-6780.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weck KE, Kim SS, Virgin HI, Speck SH. B cells regulate murine gammaherpesvirus 68 latency. Journal of virology. 1999a;73:4651–4661. doi: 10.1128/jvi.73.6.4651-4661.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weck KE, Kim SS, Virgin HI, Speck SH. Macrophages are the major reservoir of latent murine gammaherpesvirus 68 in peritoneal cells. Journal of virology. 1999b;73:3273–3283. doi: 10.1128/jvi.73.4.3273-3283.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller SK. Herpes simplex virus reorganizes the cellular DNA repair and protein quality control machinery. PLoS pathogens. 2010;6:e1001105. doi: 10.1371/journal.ppat.1001105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q, Chen D, Konig R, Mariani R, Unutmaz D, Landau NR. APOBEC3B and APOBEC3C are potent inhibitors of simian immunodeficiency virus replication. The Journal of biological chemistry. 2004;279:53379–53386. doi: 10.1074/jbc.M408802200. [DOI] [PubMed] [Google Scholar]
- Zerboni L, Sen N, Oliver SJ, Arvin AM. Molecular mechanisms of varicella zoster virus pathogenesis. Nature Review Microbiology. 2014;12:197–210. doi: 10.1038/nrmicro3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Yang B, Pomerantz RJ, Zhang C, Arunachalam SC, Gao L. The cytidine deaminase CEM15 induces hypermutation in newly synthesized HIV-1 DNA. Nature. 2003;424:94–98. doi: 10.1038/nature01707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng YH, Irwin D, Kurosu T, Tokunaga K, Sata T, Peterlin BM. Human APOBEC3F is another host factor that blocks human immunodeficiency virus type 1 replication. Journal of virology. 2004;78:6073–6076. doi: 10.1128/JVI.78.11.6073-6076.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu FX, King SM, Smith EJ, Levy DE, Yuan Y. A Kaposi’s sarcoma-associated herpesviral protein inhibits virus-mediated induction of type I interferon by blocking IRF-7 phosphorylation and nuclear accumulation. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:5573–5578. doi: 10.1073/pnas.082420599. [DOI] [PMC free article] [PubMed] [Google Scholar]