Abstract
SecA is an essential ATPase in bacterial Sec-dependent protein translocation pathway, and equilibrates between monomers and dimers in solution. The question of whether SecA functions as monomers or dimers in membranes during the protein translocation is controversial. We previously constructed a tail-to-head SecAA tandem dimer, and showed it is fully functional by complementation in vivo and protein translocation in vitro, indicating that SecA can function at least as a dimer in the membrane without dissociating into monomers. In this study, we further constructed genetically a tail-to-head SecAAA trimer, which is functional in complementing a temperature-sensitive secA mutant. The purified SecAAA trimer per protomer is fully active as SecAA tandem dimers in ATPase activity, in protein translocation in vitro and in ion channel activities in the oocytes. With these functional tail-to-head trimer SecAAA and tandem SecAA, we examined their surface topology in the presence of liposomes using AFM. As expected, the soluble SecAAA without lipids are larger than SecAA. However, the ring/pore structures of SecAAA trimers were, surprisingly, almost identical to the SecA 2-monomers and SecAA dimers, raising the intriguing possibility that the SecA may exist and function as hexamer ring-structures in membranes. Cross-linking with formaldehyde showed that SecA, SecAA and SecAAA could form larger oligomers, including the hexamers. The molecular modeling simulation shows that both tail-to-head and tail-to-tail hexamers in the membranes are possible.
Introduction
In bacteria, many secreted proteins are transported post-translationally across the cytoplasmic membranes by the general secretary pathway (Sec-pathway). SecA, SecYEG and other Sec proteins are key components in this process [1–5]. The prevailing model depicts that SecYEG-SecDF•YajC complexes form the essential translocation core channel; and motor protein SecA undergoes ATP-driven cycles of membrane insertion/de-insertion, pushes the preprotein through the channel in a step-wise fashion [3,6–8]. We have recently shown that in addition to this high-affinity SecA-SecYEG-SecDF•YajC protein-conducting channel, there is an additional SecA-alone low-affinity protein-conducting channel [9] with lower specificity and less efficiency that can be transformed into the high-affinity channels [10]. This additional SecA-alone channel has been supported by the observations that SecA integrates into membranes [4,11–14] and forms ring-like pore structures in the presence of phospholipids bilayers as observed by transmission electron microscopy and atomic force microscopy (AFM). This ring-like pore structures are not formed in solution or in uncharged phosphatidylcholine [15]. Such SecA-liposomes are functional in promoting in vitro protein translocation and eliciting ion channel activity in the oocytes and in the patch-clamp electrophysiological recordings [9]. These results indicate that SecA may also form the core and can play a structural role in the protein translocation process. A model of SecA-alone channel has been presented [14].
SecA is distributed between the inner membrane and cytosol [11,16], and functions in the membrane. The soluble SecA equilibrates between dimer and monomer [17,18]. However, the functional oligomeric state of SecA in the membrane has been the subject of considerable controversy. There were reports that monomer is the functional status of SecA in the membrane [19–21], while others showed that SecA functions as a dimer. We earlier showed that SecA tail-to-head tandem dimer, SecAA, has similar in vivo and in vitro activities as wild-type SecA [22]. Moreover, SecAA and SecA can form ring-like pore structures with similar sizes upon interaction with anionic phospholipids [22], suggesting that SecA can function at least as a dimer without dissociating into monomers. These results also show that SecA can function in a tail-to-head configuration.
In this study, we further constructed a genetically tail-to-head SecAAA trimer and show that the trimers are fully functional in vivo and in vitro per protomer as SecA monomers and SecAA dimers. Interestingly, SecAAA upon interaction with lipids also forms a ring structure similar to SecA and SecAA in sizes and shapes as observed by AFM, suggesting the possible existence of functional hexamers. Crosslinking and molecular modeling data further support the possible existence of SecA hexamers.
Materials and Methods
Bacterial strains, plasmids, and media
Escherichia coli DH5α was used for plasmid isolation and for subcloning DNA fragments. pET5asecA* which harbors a mutation of A to G at nucleotide #2098 [23], resulting in SecAM700V that has no effect on SecA activity, was used for all SecA constructions. E. coli BL21.19 [24] harboring pET5asecAA and pET5a/secAAA were used for complementation assays and overproduction of SecA derivatives. To construct pET5a/secAA with linker from tail-to-head an entire secA was PCR amplified from pET5asecA using 5’ primer containing NdeI, and 3’ primer including the linker sequences (BamHI and XhoI) and NdeI. DNA fragment was then digested with NdeI and inserted into the NdeI site of pET5a/secA to yield pET5a/secAA containing tail-to-head linker (TCA GGA TCC ATT CTC GAG CAT) sequences between two secAs.
pET5a/secAAA was constructed from pET5a/secAA by PCR amplification of an entire secA using primers containing BamHI and XhoI at 5’ and 3’, respectively. The PCR generated DNA fragment was digested with BamHI and XhoI and inserted into the same sites of pET5a/secAA. The resulting pET5a/secAAA contains two linkers (Ser-Gly-Ser-Thr-Ser-Ile-His and Pro-Gly-Ser-Ile-Leu-Glu-His). All the plasmid constructions were verified at the DNA Sequencing Core Facility (Department of Biology in Georgia State University). Strains for SecAA and SecAAA overproduction were cultured in TAG medium (10 g/L Tryptone, 5 g/L NaCl, A salts [22] and 0.5% glucose) supplemented with 100 µg/ml amplicillin.
Complementation test
Overnight BL21.19 cultures harboring vector pET5a alone or pET5asecAA and pET5a/secAAA were inoculated into fresh LB/Amp and grow at 30°C. Log-phase growing cells were then adjusted to the same density of 0.5 O.D. 600 nm. 100 µl volumes of serial 10-flod dilutions were applied onto the plates, incubated at 42 °C and plates containing 30 to 300 colonies were used to obtain colony forming units. Duplicate control plates were incubated at 30 °C as described previously [22].
Expression and purification of SecA tandem trimer and dimer
All protein purification steps and centrifugations were performed at 4 °C. SecAA and SecAAA overexpressed cells were cultured at 30 °C until O.D. 600 nm of 1.2 and induced with 0.5 mM IPTG. Proteins were overexpressed at 30 °C for 1.5 hours. Cells were harvested by centrifugation in a Beckman JA-10 rotor at 5,000 rpm for 15 min and resuspended in 3 ml/g wet cells of TKMD buffer (10 mM Tris-HCl, pH 7.6, 50 mM KCl, 10 mM MgOAc and 1 mM DTT) with 20% (NH4)2SO4 to prevent SecAAA degradation. The protease inhibitor cocktail (Roche) and PMSF was added before cell suspensions were passed through a French Press cell (SLM-Aminco) at 15,000 psi. Cell lysates were centrifuged at 90,000 rpm for 30 minutes using a Beckman TLA-100.3 rotor to separate membrane fractions from supernatant. SecAA and SecAAA were more stable in the membrane fractions which were used for purification as follows. Membrane fractions were dissolved in DTKM buffer containing 1.25% n-octyl-D-glucoside (OG) for 2 hours at 4 °C followed by centrifugation at 90,000 rpm for 30 min. to remove insoluble materials. Solubilized proteins were precipitated by 35% (NH4)2SO4 and dissolved in 0.5 ml DTKM. After centrifugation to remove the debris, the supernatant was applied onto a Sephacryl-300 (50/100) column using DTKM buffer containing 1M NaCl, 1mM DTT, 0.5 mM EDTA; the high salt was used to dissociate SecA monomers during purification [18]. Fractions containing SecAA or SecAAA were combined and precipitated by 60% (NH4)2SO4. Protein pellets were dissolved in 200 µl 100 mM NH4HCO3 and loaded onto a Superose 6 HR gel-filtration column (Amersham Bioscience Corp) and eluted in 100 mM (NH4)HCO3 buffer containing 1 mM DTT at a flow rate of 0.4 ml/min. Fractions contained SecAA and SecAAA were combined, aliquoted and stored at −80 °C. Protein concentration was determined by the Bradford assay (Bio-Rad) using BSA as a standard.
ATPase assay
The ATPase activity assays were carried out as reported previously [25]. Liposomes were prepared from E. coli total lipid mixture (Avanti, Polar Lipids, Inc) by sonication in an ice-cold water bath.
In vitro protein translocation assay
Protein translocations were carried out as previously described [26], using SecA-depleted BA13 membrane vesicles [1]. Protein synthesis was performed with an S30 extract of SecA-depleted BL15.5 as previously described [1]. The messenger was total RNA from strain HJM114 containing plasmids pOmp9 and pCI857 [27]. The membrane vesicles containing the 35S-labelled OmpA protein after proteinase K treatment were isolated, analyzed in 12% SDS-PAGE, autoradiographed and quantified by densitometer [1,22]. The input amount of 35S-labelled proOmpA used in the translocation assay was used as 100%. The results were averages from four separated experiments.
Xenopus oocyte preparation and channel activity recording
The preparation of oocytes from Xenopus laevis, injection of the E. coli membranes and precursors, and the recording of ion channel activities were performed as described previously [9,28]. 50 nl of solution containing 60 ng of BA-13 membranes depleted of SecA, and 17.5 ng proOmpA with indicated amounts of purified SecAA and SecAAA were injected into oocytes. The channel activities were determined after the injected oocytes were incubated for 3 hrs at 23 °C and recorded for 1 min as described [9,28].
Cross-linking assay
Cross-linkings with purified SecA, SecAA and SecAAA in vitro with 1-Ethyl-3-(3-dimethyl-aminopropyl) carbodiimide (EDC) was performed as described [19,23] or with formaldehyde (Sigma Chemical Co) [29] in vitro at indicated concentrations. Cross-linked products were incubated at 60°C for 10 mins [29], analyzed by 7% SDS-PAGE and followed by Coomassie Blue staining.
Atomic Force Microscopy
AFM images were obtained with a non-contact mode CP-Autoprobe (Park Scientific, Sunnyvale, CA) as previously described [15].
Results and Discussion
SecA trimers are as active as SecA dimers in vivo and in vitro
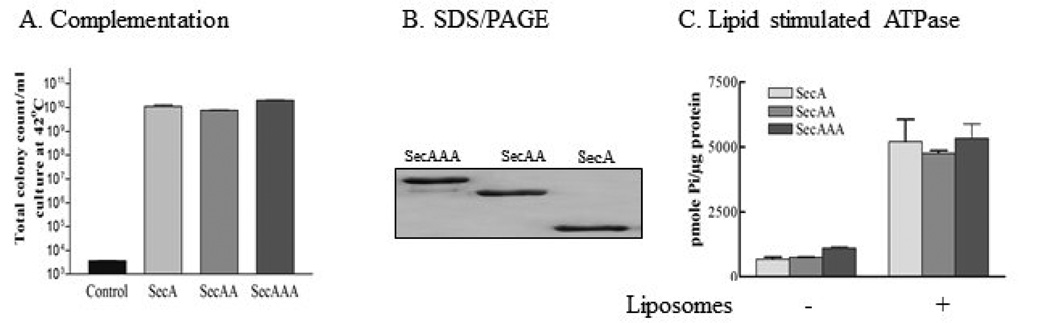
Our previous studies show that the tail-to-head tandem SecAA dimer without linkers behaved identically to wild-type SecA in complementation, protein translocation activity and morphology [22]. It has been shown that SecA tends to form higher oligomers as evident by chemical crosslinking [17]. To test the possibility that SecA can function as higher oligomers in the membrane, we constructed tail-to-head triple SecA with linkers. As a control, we also construct SecAA dimer with a short linker. First the in vivo complementation activity by colony formation units of SecA trimers was tested using E. coli BL21.19 harboring various plasmids including pET5a/secAAA or pET5a/secAA. The dominant presences of SecAA and SecAAA at 42 °C in the cells were confirmed with direct boiling of unbroken cells (data not shown). The results show that SecAAA trimers are fully functional in complementation of SecA in vivo as SecAA dimers and SecA monomers (Fig. 1A).
Fig. 1. SecAAA trimer is functional in vivo.
(A) Complementation assay was carried out with BL21.19 cells harboring vector pET5a alone, pET5a/secA, pET5a/secAA and pET5a/secAAA. The dominant presences of SecAA and SecAAA at 42 °C in the cells were confirmed with direct boiling in the presence of SDS with whole cells. (B) Purified SecAAA and SecAA detected by SDS-PAGE/Coomassie Blue gels, showing >95% purity. (C) SecA dimers and trimers possess similar intrinsic ATPase activity in vitro as wild-type SecA, and both can be stimulated by liposomes to the same extent as wild-type SecA; 2 µg of each purified SecA, SecAA and SecAAA was incubated with 10 µg of liposomes at 30 °C for 30 minutes.
To examine the in vitro activity of the SecA, we purified SecAAA and SecAA from membrane fractions of E. coli BL21.19 harboring pET5a/secAAA or secAA overproducing dimeric or trimeric SecA as described in the Materials and Methods. In most studies, SecA were purified from soluble fractions since SecA exists in soluble and membrane forms equally in E. coli [11]. However, the cytosol fractions of SecAAA oligomers are not stable, subjecting to protease digestion, thus we isolated the SecA oligomers from membranes in the presence of 20% (NH4)2SO4 and protease inhibitors during cell breakage. We also used high salt (1 M NaCl) at final steps of purification by gel filtration to dissociate SecA monomers. The purity of the purified proteins was shown in Fig.1B. The SecAA purified from membranes were as active as from soluble form which protease susceptibility, unlike SecAAA, is not an issue (SI Fig 1, and data not shown). The purified SecAAA and SecAA possesses low level intrinsic ATPase, similar to soluble monomeric SecA [27], and could be stimulated by liposomes to the same extent as the wild-type monomeric SecA per SecA protomer amounts (Fig. 1C).
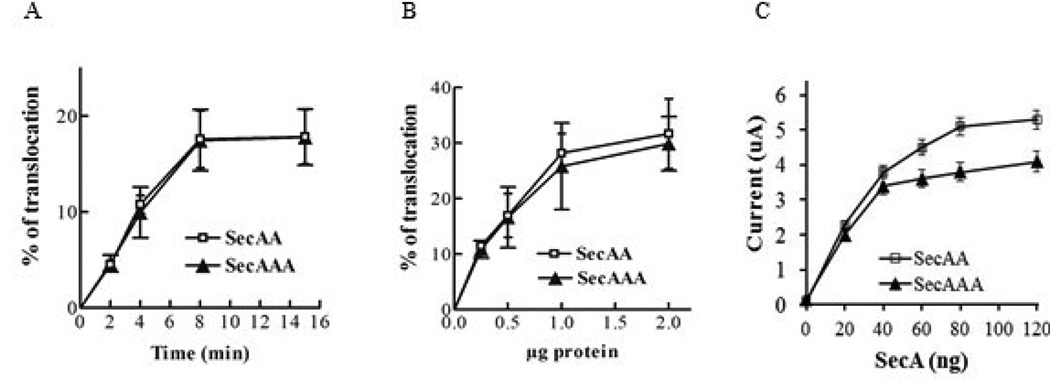
As SecA functions in the membrane, we next determined the protein translocation in vitro and channel activities in oocytes of these purified dimeric SecAA and trimeric SecAAA using SecA-depleted membranes [9,28]. We previous showed that purified soluble SecAA is fully active as monomeric SecA in proOmpA translocation [22]. We found SecAAA isolated from membranes were as active as SecAA in protein translocation with proOmpA in time course (Fig. 2A) and in amount of protein per SecA protomer. (Fig. 2B). We previously also showed that SecA can promote ion channel activity with membranes and precursors injected into oocytes [28], and soluble SecAA as well as monomeric SecA were active in eliciting channel activity in liposomes, reflecting the formation of protein-conducting channel [9]. Therefore we determined whether the purified SecAAA possesses the similar ion channel activity as with dimeric SecAA. The results showed that both SecAA and SecAAA oligomers can elicit almost the same ion channel activity per SecA protomer in the membrane (Fig 2C). Taken these data together, we conclude that the purified trimeric SecA is almost fully functional as in dimeric SecA and monomeric SecA in vivo and in vitro.
Fig. 2. Trimeric SecA has similar in vitro translocation and channel activities as dimeric SecA.
(A & B). In vitro protein translocation efficiency of [35S] Met-labelled proOmpA in the presence of indicated amount of SecAA dimers or SecAAA trimers purified from membranes. (C). Ion channel activity of membrane SecAA and SecAAA in oocytes. n=15
SecAAA forms ring-like pore structures similar to SecAA as observed in AFM
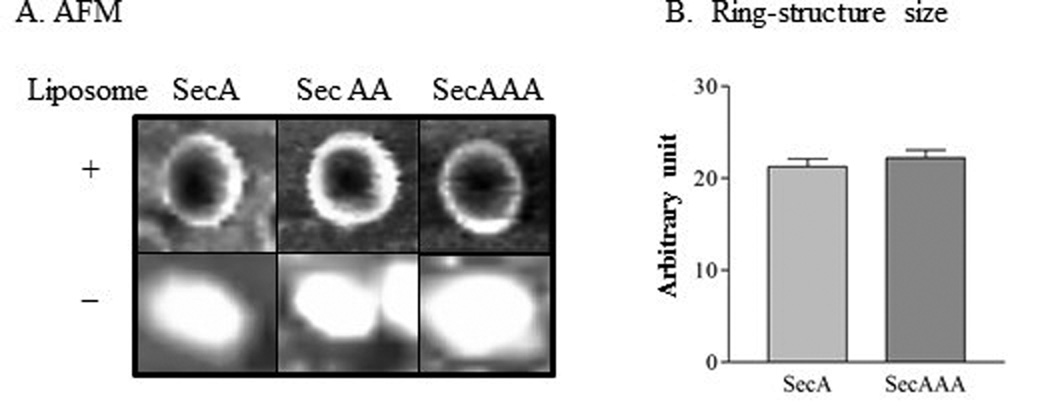
Our early data show that SecA and SecAA can form ring-like pore structures with similar size and topology in the presence of anionic phospholipids bilayers [22]. We tested whether SecAAA can also form ring-like structures. In the absence of liposomes, the SecAAA was larger than SecAA as expected (Fig.3A). In the presence of liposomes, SecAAA indeed underwent conformational changes and formed ring-structures with an indented pore as monomeric SecA and dimeric SecAA [15,22] (Fig. 3A). Surprisingly, similar ring-structures as dimeric SecAA in morphology as observed under AFM (Fig.3A; SI Fig.2) and in size (Fig.3B) as previously reported for SecA [15], indicating the similar ring structures in sizes and in topology could all be formed by a trimeric SecAAA, dimeric SecAA and monomeric SecA. To reconcile the paradoxical observations that trimeric SecAAA and dimeric SecAA are similar in activity per protomer and yet similar in sizes of pore-ring structures in the liposomes even though SecAAA is bigger than SecAA in solution (Fig. 3 without liposomes), we conceive a model of hexamer (3×SecAA or 2×SecAAA) in the membrane. We first utilized the simulation and built a molecular model, based on the approximated ring diameter of about 80–100Å, the pore size of about 30–40 Å and the dimension of the side view of a single monomer, a hexamer model was assembled using VMD program [30], then was optimized by Molecular Mechanics (MM) and Molecular Dynamics (MD) using CHARMM program [31–34]. The simulation of a tail-to-head model of SecA with the ring diameter of 92 Å and the pore size of 31 Å with a 90 Å depth spanning the lipid bilayer were determined (SI. Fig.3). Interestingly, a tail-to-tail SecA hexamer can also be obtained with a similar dimension. Thus, molecular modelings reveal that SecA hexamers could be fitted with the diameter of about 9 nm and depth of 9 nm with a central pore cavity of about 3 nm in both tail-to-head or tail-to-tail for monomeric SecA, SecAA dimer and SecAAA trimer that is close to sizes as estimated approximately by SecA ring-like structure observed by AFM and transmission electron microscope [15]. It is possible that the hexamer structures if indeed exist, the protomer SecA may be arranged in tail-to-head or tail-to-tail combination.
Fig. 3. AFM images of SecA, SecAA and SecAAA in the presence or absence of phospholipids.
(A). The samples for AFM image were prepared with or without liposomes. (B). The average AFM imaged pore-structures were measured. n=100.
Oligomeric states of SecA: In vitro cross-linking of SecA
It is known that SecA can be crosslinked as dimers, or even higher order oligomers in vitro [17,22,35–37]. Parts of the controversy of whether Sec functions as dimers were the contradictory observations that depending on the cross-linked conditions, some cross-linked SecA dimers were active [36, 38] where the other were not [21]. Thus we determined the conditions for the cross-linkings. Using zero-length cross-linker formaldehyde, SecA was capable of forming higher-order oligomers than dimers, such as hexamers (Fig. 4A). These formaldehyde cross-linked oligomers were not stable upon boiling as expected [29]. Interestingly, under the conditions used, cross-linking products were observed under high salt which shifts the equilibrium toward monomer [18]. Such cross-linked high oligomers were obtained using formaldehyde but not EDC as the cross-linker (Fig. 4A). In addition, the formation of SecA oligomers was also reported to be favoring at higher concentration of SecA [18]. Our higher order cross-linked products, however, could be observed at a low protein concentration as 0.25 mg/ml which is much lower than the estimated physiological concentration of 0.8 mg/ml (Fig. 4B). The cross-linking conditions with formaldehyde were used to further examine the oligomeric states of monomeric SecA, dimeric SecAA and trimeric SecAAA. We found that monomeric SecA could be cross-linked as dimers as expected, but it also appeared with same molecular size as SecAAA trimers (Fig. 4A and 4B), suggesting that SecA could exist as dimers as well as trimers. Interestingly, these three protomers could all be cross-linked to higher oligomers, including hexamers, as a function of incubation time and even at lower concentration of SecAA and SecAAA (Fig. 4B).
Fig. 4. Cross-linking analyses.
(A) Purified SecA at different concentrations was subjected to 0.2% formaldehyde and EDC cross-linking and salt strengths as indicated. (B) Purified SecA, SecAA and SecAAA were subjected to 0.2% formaldehyde cross-linking at 23 °C for the indicated time. The cross-linked profiles were analyzed as described in the Materials and Methods.
Taken all in vivo and in vitro data together with molecular modeling, it appears that trimeric SecAAA can function actively as monomeric SecA and dimeric SecAA, and that SecA may exist and function as a hexamer in the membrane.
Supplementary Material
Highlights.
Tail-to-head SecAAA trimers are active as SecA and SecAA dimers in complementation
Purified SecAAA trimers are as fully active as SecAA dimer per protomer in vitro
SecA, SecAA and SecAAA form similar size pore-ring structures in liposomes.
Molecular modeling shows possibility of SecA hexamer channels in the membranes.
SecA, SecAA, and SecAAA can be cross-linked to higher oligomers
Acknowledgement
This work was supported in part by NIH grant GM34766. The Biology Core facility was supported in part by George Research Alliance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cabelli RJ, Chen L, Tai PC, Oliver DB. SecA protein is required for secretory protein translocation into E. coli membrane vesicles. Cell. 1988;55:683–692. doi: 10.1016/0092-8674(88)90227-9. [DOI] [PubMed] [Google Scholar]
- 2.Cunningham K, Lill R, Crooke E, Rice M, Moore K, Wickner W, Oliver D. SecA protein, a peripheral protein of the Escherichia coli plasma membrane, is essential for the functional binding and translocation of proOmpA. Embo J. 1989;8:955–959. doi: 10.1002/j.1460-2075.1989.tb03457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manting EH, Driessen AJ. Escherichia coli translocase: the unravelling of a molecular machine. Mol Microbiol. 2000;37:226–238. doi: 10.1046/j.1365-2958.2000.01980.x. [DOI] [PubMed] [Google Scholar]
- 4.Watanabe M, Blobel G. SecA protein is required for translocation of a model precursor protein into inverted vesicles of Escherichia coli plasma membrane. Proc Natl Acad Sci U S A. 1993;90:9011–9015. doi: 10.1073/pnas.90.19.9011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wickner W, Leonard MR. Escherichia coli preprotein translocase. J Biol Chem. 1996;271:29514–29516. doi: 10.1074/jbc.271.47.29514. [DOI] [PubMed] [Google Scholar]
- 6.Driessen AJ, Nouwen N. Protein translocation across the bacterial cytoplasmic membrane. Annu Rev Biochem. 2008;77:643–667. doi: 10.1146/annurev.biochem.77.061606.160747. [DOI] [PubMed] [Google Scholar]
- 7.Economou A, Wickner W. SecA promotes preprotein translocation by undergoing ATP-driven cycles of membrane insertion and deinsertion. Cell. 1994;78:835–843. doi: 10.1016/s0092-8674(94)90582-7. [DOI] [PubMed] [Google Scholar]
- 8.Papanikou E, Karamanou S, Economou A. Bacterial protein secretion through the translocase nanomachine. Nat Rev Microbiol. 2007;5:839–851. doi: 10.1038/nrmicro1771. [DOI] [PubMed] [Google Scholar]
- 9.Hsieh YH, Zhang H, Lin BR, Cui N, Na B, Yang H, Jiang C, Sui SF, Tai PC. SecA alone can promote protein translocation and ion channel activity: SecYEG increases efficiency and signal peptide specificity. J Biol Chem. 2011;286:44702–44709. doi: 10.1074/jbc.M111.300111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsieh YH, Zhang H, Yang H, Jiang C, Sui S-F, Tai Phang C. Reconstitution of SecA-dependent protein-conducting channels as functionally efficient as in membranes: Transformation of low-affinity SecA-liposome channels to high-affinity SecA-SecYEG-SecDF. YajC. Biochem Biophys Res Commun. 2013;431:388–392. doi: 10.1016/j.bbrc.2013.01.042. [DOI] [PubMed] [Google Scholar]
- 11.Cabelli RJ, Dolan KM, Qian LP, Oliver DB. Characterization of membrane-associated and soluble states of SecA protein from wild-type and SecA51(TS) mutant strains of Escherichia coli. J Biol Chem. 1991;266:24420–24427. [PubMed] [Google Scholar]
- 12.Rajapandi T, Oliver D. Integration of SecA protein into the Escherichia coli inner membrane is regulated by its amino-terminal ATP-binding domain. Mol Microbiol. 1996;20:43–51. doi: 10.1111/j.1365-2958.1996.tb02487.x. [DOI] [PubMed] [Google Scholar]
- 13.Ramamurthy V, Oliver D. Topology of the integral membrane form of Escherichia coli SecA protein reveals multiple periplasmically exposed regions and modulation by ATP binding. J Biol Chem. 1997;272:23239–23246. doi: 10.1074/jbc.272.37.23239. [DOI] [PubMed] [Google Scholar]
- 14.You Z, Liao M, Zhang H, Yang H, Pan X, Houghton JE, Sui SF, Tai PC. Phospholipids induce conformational changes of SecA to form membrane-specific domains: AFM structures and implication on protein-conducting channels. PLoS One. 2013;8:e72560. doi: 10.1371/journal.pone.0072560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang HW, Chen Y, Yang H, Chen X, Duan MX, Tai PC, Sui SF. Ring-like pore structures of SecA: implication for bacterial protein-conducting channels. Proc Natl Acad Sci U S A. 2003;100:4221–4226. doi: 10.1073/pnas.0737415100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akita M, Shinkai A, Matsuyama S, Mizushima S. SecA, an essential component of the secretory machinery of Escherichia coli, exists as homodimer. Biochem Biophys Res Commun. 1991;174:211–216. doi: 10.1016/0006-291x(91)90507-4. [DOI] [PubMed] [Google Scholar]
- 17.Benach J, Chou YT, Fak JJ, Itkin A, Nicolae DD, Smith PC, Wittrock G, Floyd DL, Golsaz CM, Gierasch LM, Hunt JF. Phospholipid-induced monomerization and signal-peptide-induced oligomerization of SecA. J Biol Chem. 2003;278:3628–3638. doi: 10.1074/jbc.M205992200. [DOI] [PubMed] [Google Scholar]
- 18.Woodbury RL, Hardy SJ, Randall LL. Complex behavior in solution of homodimeric SecA. Protein. Sci. 2002;11:875–882. doi: 10.1110/ps.4090102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Or E, Boyd D, Gon S, Beckwith J, Rapoport T. The bacterial ATPase SecA functions as a monomer in protein translocation. J Biol Chem. 2005;280:9097–9105. doi: 10.1074/jbc.M413947200. [DOI] [PubMed] [Google Scholar]
- 20.Or E, Navon A, Rapoport T. Dissociation of the dimeric SecA ATPase during protein translocation across the bacterial membrane. Embo J. 2002;21:4470–4479. doi: 10.1093/emboj/cdf471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Or E, Rapoport T. Cross-linked SecA dimers are not functional in protein translocation. FEBS Lett. 2007;581:2616–2620. doi: 10.1016/j.febslet.2007.04.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang H, Na B, Yang H, Tai PC. Additional in vitro and in vivo evidence for SecA functioning as dimers in the membrane: dissociation into monomers is not essential for protein translocation in Escherichia coli. J Bacteriol. 2008;190:1413–1418. doi: 10.1128/JB.01633-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Das S, Stivison E, Folta-Stogniew E, Oliver D. Reexamination of the role of the amino terminus of SecA in promoting its dimerization and functional state. J Bacteriol. 2008;190:7302–7307. doi: 10.1128/JB.00593-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mitchell C, Oliver D. Two distinct ATP-binding domains are needed to promote protein export by Escherichia coli SecA ATPase. Mol. Microbiol. 1993;10:483–497. doi: 10.1111/j.1365-2958.1993.tb00921.x. [DOI] [PubMed] [Google Scholar]
- 25.Lill R, Dowhan W, Wickner W. The ATPase activity of SecA is regulated by acidic phospholipids, SecY, and the leader and mature domains of precursor proteins. Cell. 1990;60:271–280. doi: 10.1016/0092-8674(90)90742-w. [DOI] [PubMed] [Google Scholar]
- 26.Chen L, Rhoads D, Tai PC. Alkaline phosphatase and OmpA protein can be translocated posttranslationally into membrane vesicles of Escherichia coli. J. Bacteriol. 1985;161:973–980. doi: 10.1128/jb.161.3.973-980.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geller BL, Movva NR, Wickner W. Both ATP and the electrochemical potential are required for optimal assembly of pro-OmpA into Escherichia coli inner membrane vesicles. Proc. Natl. Acad. Sci. USA. 1986;83:4219–4222. doi: 10.1073/pnas.83.12.4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin BR, Gierasch LM, Jiang C, Tai PC. Electrophysiological studies in Xenopus oocytes for the opening of Escherichia coli SecA-dependent protein-conducting channels. J Membr Biol. 2006;214:103–113. doi: 10.1007/s00232-006-0079-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manting EH, van der Does C, Driessen AJ. I n vivo cross-linking of the SecA and SecY subunits of the Escherichia coli preprotein translocase. J. Bacteriol. 1997;179:5699–5704. doi: 10.1128/jb.179.18.5699-5704.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Humphrey W, Dalke A, Schulten K. VMD: visual molecular dynamics. J Mol Graph. 1996;14:33–38. 27–38. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- 31.Brooks BR, Bruccoleri RE, Olafson BD, States DJ, Swaminathan S, Karplus M. CHARMM: a program for macromolecular energy, minimization, and dynamics calculations. J. Comput. Chem. 1983;4:187–217. [Google Scholar]
- 32.Nicklaus MC. Conformational energies calculated by the molecular mechanics program CHARMm. J. Comput. Chem. 1997;18:1056–1060. [Google Scholar]
- 33.Patel S, Brooks CL., 3rd CHARMM fluctuating charge force field for proteins: I parameterization and application to bulk organic liquid simulations. J Comput Chem. 2004;25:1–15. doi: 10.1002/jcc.10355. [DOI] [PubMed] [Google Scholar]
- 34.Patel S, Mackerell AD, Jr, Brooks CL., 3rd CHARMM fluctuating charge force field for proteins: II protein/solvent properties from molecular dynamics simulations using a nonadditive electrostatic model. J Comput Chem. 2004;25:1504–1514. doi: 10.1002/jcc.20077. [DOI] [PubMed] [Google Scholar]
- 35.Driessen AJ. SecA, the peripheral subunit of the Escherichia coli precursor protein translocase, is functional as a dimer. Biochemistry. 1993;32:13190–13197. doi: 10.1021/bi00211a030. [DOI] [PubMed] [Google Scholar]
- 36.Jilaveanu LB, Oliver D. SecA dimer cross-linked at its subunit interface is functional for protein translocation. J Bacteriol. 2006;188:335–338. doi: 10.1128/JB.188.1.335-338.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van der Wolk JP, Boorsma A, Knoche M, Schafer HJ, Driessen AJ. The low-affinity ATP binding site of the Escherichia coli SecA dimer is localized at the subunit interface. Biochemistry. 1997;36:14924–14929. doi: 10.1021/bi971766n. [DOI] [PubMed] [Google Scholar]
- 38.de Keyzer J, van der Sluis EO, Spelbrink RE, Nijstad N, de Kruijff B, Nouwen N, van der Does C, Driessen AJ. Covalently dimerized SecA is functional in protein translocation. J Biol Chem. 2005;280:35255–35260. doi: 10.1074/jbc.M506157200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.