Abstract
A family of long wavelength protein kinase fluorescent reporters is described in which the probing wavelength is pre-programmed using readily available fluorophores. These agents can assess protein kinase activity within the optical window of tissue, as exemplified by monitoring endogenous cAMP-dependent protein kinase activity (1) in erythrocyte lysates and (2) in intact erythrocytes using a light-activatable reporter.
Keywords: Biosensors, Peptides, Florescent Probes, Dyes/Pigments, Signal Transduction
In vivo optical imaging must contend with the limitations and opportunities imposed by the optical window of tissue (600 – 1000 nm).[1] Tissue transparency at wavelengths less than 600 nm is limited by the presence of hemoglobin and melanin whereas water is the chief culprit above 1000 nm. Fortunately, a wide array of fluorophores are available that are visualized in the red and near IR[2] and several of these have been covalently attached to antibodies for diagnostic purposes.[3–4] However, with the prominent exception of proteases,[5] there are few long wavelength probes for enzymes, which is especially problematic with respect to intracellular erythrocyte biochemistry.[6] Unlike other mammalian cells, the high hemoglobin content of erythrocytes optically obscures subcellular monitoring at wavelengths less than 600 nm. To address the need for observing biochemical pathways in these cells, and with an eye on potential applications for tissue-based studies, we describe the design of red and far-red probes of protein kinase activity.
Protein kinases catalyze phosphoryl transfer from ATP to hydroxyl residues in proteins and peptides. Although a variety of fluorescent sensors of protein kinases have been described,[7]–[8] strategies have not yet been developed that can tune sensors to specific wavelengths within the optical window of tissue. One appealing approach is to take advantage of the commercial availability of far-red and near-IR fluorophores. Enzyme-catalyzed unquenching of fluorescence, via separation of a fluorophore from a nearby fluorescent quencher, has been successfully applied to proteases.[5] Although we previously used such a strategy for protein kinases, “unquenching” required the presence of a third party, namely stoichiometric amounts of a protein that sequesters the phosphorylated-product.[9] We describe a much simpler and more robust alternative in which the newly introduced phosphate serves as a molecular trigger that drives the release of the fluorescent quencher. This provides access into the biologically useful far-red/near IR wavelength realm as exemplified by visualization of kinase activity in the optically challenging intracellular domain of erythrocytes.
This strategy is outlined in Scheme 1. Our initial efforts focused on the cAMP-dependent protein kinase (PKA) due to its central role in erythrocyte behaviour and the life cycle of the malarial parasite Plasmodium Falciparum.[10] PKA efficiently phosphorylates a diverse array of serine-containing positively charged sequences, and we employed two of these sequences in this study: Aoc-GRTGRRFSY-amide[11] and KRRRLASLAA-amide[12]. Fluorophores were appended to the N-termini of both peptides. The amino-octanoic acid (Aoc) moiety was used as a spacer in one of these to reduce any potential unfavorable steric clashes between the large fluorophores and the kinase active site. As noted below, this proved to be an unnecessary precaution as all the peptides in this study serve as PKA substrates. A total of 14 fluorophores were examined that encompass a nearly 250 nm wavelength range throughout the red/far-red: λex (494 – 727 nm) λem (530 – 752 nm). For comparative purposes, the absorbances of five fluorophore-Aoc-GRTGRRFSY-amide peptides are shown relative to that of hemoglobin (Figure 1). We assessed the ability of a library of 48 negatively charged dyes to quench the fluorescence of the fluorophore-substituted peptides (Table S5). Upon subsequent addition of PKA and ATP, fluorescent recoveries, from modest to dramatic, were observed (Tables 1 and S6–S8). We’ve previously shown that a phosphorylated residue in a PKA phospho-peptide product interacts with nearby arginine residues.[13] As a working hypothesis, we propose that this intramolecular electrostatic interaction displaces the quencher dye from the peptide, resulting in the observed increase in fluorescence.
Scheme 1.

General strategy for the protein kinase-catalyzed unquenching of fluorescent kinase substrates. A positively charged fluorescent kinase substrate is fluorescently quenched upon exposure to a negatively charged quencher dye (Q). Kinase-catalyzed phosphorylation releases Q due to favourable intramolecular electrostatic interactions between the newly introduced phosphate and positively charged substrate residues. Note: the non-fluorescent Q:kinase substrate complex in this study does not exist in a 1:1 stoichiometry.
Figure 1.
Relative wavelength-dependent absorbances of erythrocyte lysate (red) and fluorophore-Aoc-GRTGRRFSY-amide peptides where fluorophore = 5Fam (green), TAMRA (violet), Atto620 (cyan), Atto633 (blue), and Red681 (black).
Table 1.
PKA-catalyzed fluorescence increase (Fl-fold) of fluorophore-substituted peptides (2.5 μM) in the presence in buffer (25 mM Tris-HCl pH 7.5, 1 mM MgCl2, [1] = variable, see Table S6) and in 10% erythrocyte lysates (PBS buffer, 5 mM MgCl2, Halt protease and phosphatase inhibitor cocktail, 1 mM leupeptin, pH 7.4, 150 μM 1) with 1 mM ATP and 10 nM PKA. Structures of the fluorophores are provided in Table S1.
| Fluorophore-peptide | λem, λex(nm) | Cond. | Fl-fold↑w |
|---|---|---|---|
| 5Fam-Aoc-GRTGRRFSY | 492, 518 | Buffer Lysate |
2.5 ± 0.1 1.3 ± 0.1 |
| TAMRA-Aoc-GRTGRRFSY | 550, 580 | Buffer Lysate |
104 ± 24 14.6 ± 0.6 |
| Atto620-Aoc-GRTGRRFSY | 619, 643 | Buffer Lysate |
16.6 ± 0.8 12.8 ± 0.8 |
| Atto633-Aoc-GRTGRRFSY | 635, 655 | Buffer Lysate |
30.2 ± 9.1 3.9 ± 0.2 |
| Red681-Aoc-GRTGRRFSY | 670, 706 | Buffer Lysate |
11.2 ± 0.6 3.4 ± 0.1 |
A few representative reporters are shown in Table 1. These agents respond to phosphorylation at wavelengths that include the red, far-red, and into the near-IR. Some of the most responsive fluorescent changes are observed when Acid Blue 80 (1) is paired with either Fluorophore-Aoc-GRTGRRFSY-amide or Fluorophore-KRRRLASLAA-amide (Table S6). Although the fold changes observed with Evans Blue (2) as the quencher are somewhat less dramatic, this dye appears to have a greater affinity for its peptidic counterparts than 1. Since a Job plot analysis (Figure S10) revealed that the stoichiometry of the quencher:fluorophore-peptide pairs is not 1:1 we are unable to assign Kd values. Instead, we employed EQ50 as an assessment of affinity; i.e. the [Q] that generates a fluorescence intensity halfway between [Q] = 0 and saturating Q. For example, in the case of TAMRA-Aoc-GRTGRRFSY-amide, the EQ50 values are 2.14 ± 0.05 μM for 1 and 0.15 ± 0.05 μM for 2 (Figures S11–S13).
As validation of the presumed ability of these reporters to detect inhibitory activity, we employed fluorophore-Aoc-GRTGRRFSY-amide/2 to examine the potency of two well-known inhibitors of PKA, H89 and KT5720. For example, both inhibitors block the phosphorylation of the Atto633-Aoc-GRTGRRFSY-amide substrate and, under the experimental conditions (1 mM ATP), display IC50 values of 0.43 ± 0.15 and 0.61 ± 0.25 μM, respectively (Figure S14).
PKA plays key roles in erythrocyte senescence, localized vasodilation, and deformation.[14] Given the high hemoglobin concentration (~5 mM) in erythrocytes,[15] clean observation of endogenous PKA activity should be more pronounced at wavelengths beyond 600 nm. We initially evaluated the ability of the fluorophore-Aoc-GRTGRRFSY-amide peptides to monitor endogenous PKA activity in 10% erythrocyte lysates. 5Fam-Aoc-GRTGRRFSY-amide displays very modest fluorescent enhancements upon phosphorylation in the presence of quenchers 1 (1.3-fold, Table 1) or 2 (1.04-fold, Table S10). By contrast, the fluorescent enhancements are significantly more robust with TAMRA-Aoc-GRTGRRFSY-amide [1 (14.6-fold); 2 (7.3-fold)] or Atto633-Aoc-GRTGRRFSY-amide [1 (3.9-fold); 2 (6.3-fold)]. Although the TAMRA-labelled peptide responds vigorously to phosphorylation in terms of absolute fluorescent fold change, the Atto633 species displays a superior signal to noise ratio (64:1 versus 12:1 for the TAMRA-peptide) of the progress curve (Figure 2). Indeed, whereas 90% of the fluorescent signal is lost for the FAM-peptide and 98% for the TAMRA-peptide, only 10% of the signal is compromised in the case of the Atto633-peptide (Figure S17). Furthermore, the FAM and TAMRA fluorescent signals are completely obliterated in 100% erythrocyte lysates, whereas 50% of the original Atto633 signal is still present (Figure 2, insert). We also examined the effect of dye quenchers 1 and 2 on erythrocyte viability and discovered that cellular integrity is markedly reduced upon exposure to 1 (30 μM), but remains unperturbed in the presence of 2 (30 μM) (Figure S18). Consequently, all subsequent studies were performed using the Atto633-Aoc-GRTGRRFSY-amide (1 μM)/2 (2 μM) sensor pair.
Figure 2.
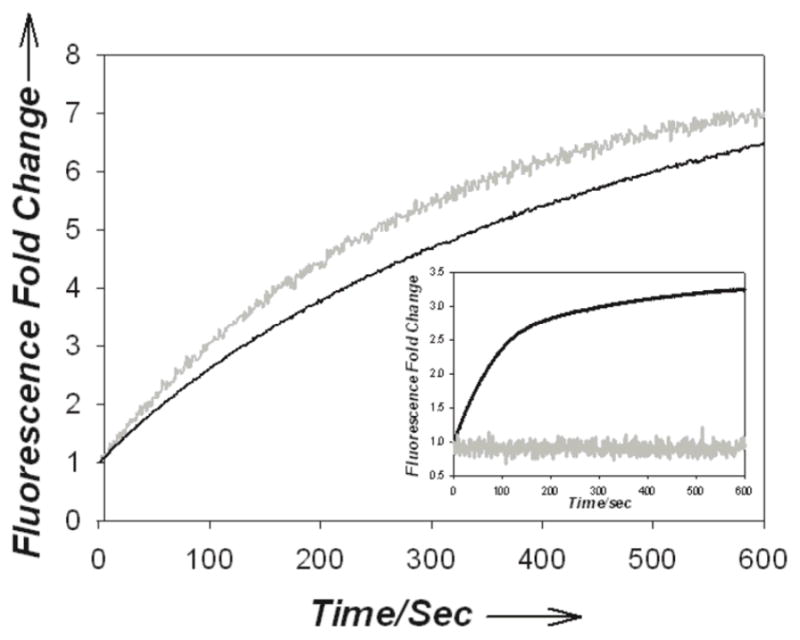
Reaction progress curve of the PKA-catalyzed phosphorylation of fluorophore-Aoc-GRTGRRFSY-amide (1 μM) with quencher 2 (2 μM) in 10% erythrocyte lysates where fluorophore = Atto633 (black), TAMRA (grey). Insert: 100% lysate conditions.
Since erythrocytes possess a number of protein kinases,[6] we examined whether the observed fluorescent response displayed by Atto633-Aoc-GRTGRRFSY-amide/2 is due to endogenous PKA activity. The moderately selective PKA inhibitor H89 blocks the observed fluorescence change and displays an IC50 of 1.14 ± 0.17 μM in lysates (10%) (Figure S15 and Table S11). The highly selective PKA inhibitor KT5720 likewise prevents the phosphorylation-induced fluorescent response (IC50 1.59 ± 0.36 μM). Finally, a PKA-specific antibody was employed to clear PKA from lysates and then phosphoryl-transferase activity was assessed. The decrease in phosphoryl-transferase (80%) activity of the pre-cleared lysate directly corresponds with the amount of PKA removed from the lysate (Figure S16). These results confirm that the fluorescent response in lysates is driven by PKA activity.
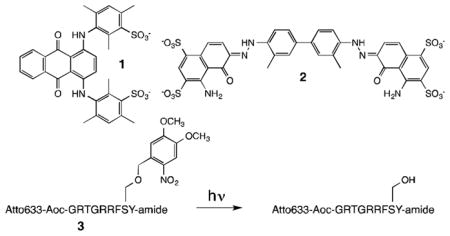
Assessing intracellular enzymatic activity in cells is challenging in a number of ways, including controlling the start point (t = 0) of sensor phosphorylation. Light-activatable analogues provide a means to achieve this end.[16] The serine hydroxyl moiety in Atto633-Aoc-GRTGRRFSY-amide was modified with a light sensitive 4,5-dimethoxy-2-nitrobenzyl (DNMB) functional group (3). As expected, both in buffer and in erythrocyte lysates, the modified serine residue in 3 is not phosphorylated in the absence of photolysis. By contrast, upon illumination, the anticipated fluorescent increase is observed (Figure S19. In addition, the fluorescent response of the photo-chemically activated sensor is blocked in the presence of a PKA inhibitor.
Peptide 3 (1 μM) and quencher 2 (2 μM) were simultaneously introduced into erythrocytes under hypotonic conditions. Loading of the fluorescent peptide alone was confirmed by flow cytometry and confocal microscopy (Figures S20 – S21). Peptide 3/quencher 2-loaded erythrocytes display a weakly fluorescent interior and a moderately fluorescent membrane (Figure 3). We suspect that during sensor loading, a portion of the positively charged peptide binds to and remains associated with the exterior of the cell membrane. The surface of erythrocytes is negatively charged due to sialic acid-adorned proteins.[17] Indeed, this property has been used to decorate red blood cells with a variety of positively charged nanoparticles.[18] The fluorescent ring that outlines the cell presumably reflects the partial or complete displacement of the negatively charged quencher from the surface-bound fluorophore-labelled peptide.
Figure 3.
Confocal images of 2/3-loaded erythrocytes (a) before photolysis and (b) 300 s after photolysis of the encircled cell. Photolysis was performed with 50 mW, 405 nm laser (2% power level) in tornado mode using a dwell time of 20 μs/pixel and 25 frames. Imaging was performed with a 100X objective using 635 nm at 4% laser power. Bar = 5 μm. (c) Fluorescence-fold change as a function of time where 405 nm-mediated photolysis is applied at the third time point (filled circles), in the presence of KT5720 (triangles), and depleted ATP (open circles). See Figure S22 for visual snapshots of the data plotted in c.
PKA activity in intact erythrocytes was assessed using confocal microscopy. Since UV (~360 nm) illumination is known to have an immediate untoward impact on hemoglobin biochemistry,[19] we employed a 405 nm laser to convert the DNMB-peptide 3 to its phosphorylatable counterpart. Upon photolysis, a rapid fluorescent response is observed in the interior of erythrocytes (Figures 3 and S22–S25). By contrast, membrane fluorescence remains unchanged as a function of time, which is expected if the fluorophore-peptide is simply appended on the exterior surface. In addition, the PKA-selective cell-permeable inhibitor KT5720 blocks the increase in Atto633 fluorescence otherwise observed in the erythrocyte interior. Finally, under conditions in which the intracellular content of erythrocytes is depleted of ATP no fluorescent response is observed. These results confirm that intracellular PKA phosphorylates Atto633-GRTGRRFSY-amide.
Electrostatic-driven recognition has served as a basis for the design of other sensors, such as those for heparin.20 As one might expect, the number of opposing charges determines the apparent affinity of the quencher for the fluorophore-peptide.9b We have found that the electrostatic affinity of Aoc-GRTGRRFSY-amide/2 is sufficient to construct a series of red and far-red sensors of protein kinase activity. The desired wavelength of visualization is easily “dialed-in” by appending the appropriate commercially available fluorophore. These reporters possess the requisite photophysical properties to take advantage of the most transparent optical region in tissue as exemplified by visualization of protein kinase activity in erythrocytes.
Experimental Section
See the online Supporting Information for Materials and Methods, Tables, additional Figures, and Schemes
Supplementary Material
Acknowledgments
We thank the National Institutes of Health for financial support (RO1 CA79954), Professor Christian Doerig (Monash Universitt) for bringing to our attention the role of PKA in erythrocyte function, and Prof. Karl Drexhage (ATTO-TEC GmbH; Universität Siegen)
Footnotes
Supporting information for this article is available on the WWW under http://www.angewandte.org or from the author.
References
- 1.Tromberg BJ, Shah N, Lanning R, Cerussi A, Espinoza J, Pham T, Svaasand L, Butler J. Neoplasia. 2000;2:26–40. doi: 10.1038/sj.neo.7900082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lavis LD, Raines RT. ACS Chem Biol. 2008;3:142–155. doi: 10.1021/cb700248m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luo S, Zhang E, Su Y, Cheng T, Shi C. Biomaterials. 2011;32:7127– 7138. doi: 10.1016/j.biomaterials.2011.06.024. [DOI] [PubMed] [Google Scholar]
- 4.Nishimura Y, Yata K, Nomoto T, Ogiwara T, Watanabe K, Shintou T, Tsuboyama A, Okano M, Umemoto N, Zhang Z, Kawabata M, Zhang B, Kuroyanagi J, Shimada Y, Miyazaki T, Imamura T, Tomimoto H, Tanaka T. ACS Chem Neurosci. 2013;4:1183– 1193. doi: 10.1021/cn400010t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edgington LE, Verdoes M, Bogyo M. Cur Opin Chem Biol. 2011;15:798– 805. doi: 10.1016/j.cbpa.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D’Alessandro A, Righetti PG, Zolla L. J Proteome Res. 2010;9:144– 163. doi: 10.1021/pr900831f. [DOI] [PubMed] [Google Scholar]
- 7.(a) Rothman DM, Shults MD, Imperiali B. Trends Cell Biol. 2005;15:502–510. doi: 10.1016/j.tcb.2005.07.003. [DOI] [PubMed] [Google Scholar]; (b) Morris MC. Biochim Biophys Acta. 2013;1834:1387–1395. doi: 10.1016/j.bbapap.2013.01.025. [DOI] [PubMed] [Google Scholar]; (c) Li Y, Xie W, Fang G. Anal Bioanal Chem. 2008;390:2049–2057. doi: 10.1007/s00216-008-1986-z. [DOI] [PubMed] [Google Scholar]; (d) Wu D, Sylvester JE, Parker LL, Zhou G, Kron SJ. Biopolymers. 2010;94:475–486. doi: 10.1002/bip.21401. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Martic S, Kraatz HB. Chem Sci. 2013;4:42– 59. [Google Scholar]
- 8.Nhu NVT, Morris MC. Prog Mol Biol Transl Sci. 2013;113:217– 274. doi: 10.1016/B978-0-12-386932-6.00006-5. [DOI] [PubMed] [Google Scholar]
- 9.(a) Sharma V, Agnes RS, Lawrence DS. J Am Chem Soc. 2007;129:2742–2743. doi: 10.1021/ja068280r. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Agnes RS, Jernigan F, Shell JR, Sharma V, Lawrence DS. J Am Chem Soc. 2010;132:6075– 6080. doi: 10.1021/ja909652q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.(a) Wurtz N, Chapus C, Desplans J, Parzy D. Parasitology. 2011;138:1–25. doi: 10.1017/S003118201000096X. [DOI] [PubMed] [Google Scholar]; (b) Merckx A, Bouyer G, Thomas SLY, Langsley G, Egee S. Trends Parasitology. 2009;25:139– 144. doi: 10.1016/j.pt.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 11.Glass DB, Cheng HC, Mende-Mueller L, Reed J, Walsh DA. J Biol Chem. 1989;264:8802– 10. [PubMed] [Google Scholar]
- 12.Flotow H, Thomas G. J Biol Chem. 1992;267:3074–8. [PubMed] [Google Scholar]
- 13.Prorok M, Sukumaran DK, Lawrence DS. J Biol Chem. 1989;264:17727– 17733. [PubMed] [Google Scholar]
- 14.(a) Sprague RS, Ellsworth ML, Stephenson AH, Lonigro AJ. Am J Physiol Cell Physiol. 2001;281:C1158–C1164. doi: 10.1152/ajpcell.2001.281.4.C1158. [DOI] [PubMed] [Google Scholar]; (b) Jindal HK, Ai Z, Gascard P, Horton C, Cohen CM. Blood. 1996;88:1479–1487. [PubMed] [Google Scholar]; (c) Sprague RS, Bowles EA, Hanson MS, DuFaux EA, Sridharan M, Adderley S, Ellsworth ML, Stephenson AH. Microcirculation. 2008;15:461– 471. doi: 10.1080/10739680701833804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Francis SE, Sullivan DJ, Goldberg DE. Annu Rev Microbiol. 1997;51:97– 123. doi: 10.1146/annurev.micro.51.1.97. [DOI] [PubMed] [Google Scholar]
- 16.(a) Brieke C, Rohrbach F, Gottschalk A, Mayer G, Heckel A. Angew Chem Int Ed Engl. 2012;51:8446–8476. doi: 10.1002/anie.201202134. [DOI] [PubMed] [Google Scholar]; (b) Klan P, Solomek T, Bochet CG, Blanc A, Givens R, Rubina M, Popik V, Kostikov A, Wirz J. Chem Rev. 2013;113:119–191. doi: 10.1021/cr300177k. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Lee HM, Larson DR, Lawrence DS. ACS Chem Biol. 2009;4:409–427. doi: 10.1021/cb900036s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eylar EH, Madoff MA, Brody OV, Oncley JL. J Biol Chem. 1962;237:1992– 2000. [PubMed] [Google Scholar]
- 18.Mai TD, d’Orlye F, Menager C, Varenne A, Siaugue J-M. Chem Comm. 2013;49:5393– 5395. doi: 10.1039/c3cc41513a. and references cited therein. [DOI] [PubMed] [Google Scholar]
- 19.Pan L, Wang X, Yang S, Wu X, Lee I, Zhang X, Rupp RA, Xu J. PLOS One. 2012;7:e44142. doi: 10.1371/journal.pone.0044142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fu X, Chen L, Li J, Lin M, You H, Wang W. Biosens Bioelectron. 2012;34:227– 31. doi: 10.1016/j.bios.2012.02.008. and references cited therein. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




