Abstract
The fruit fly (Drosophila melanogaster) has long been a premier model for developmental biologists and geneticists. The utility of Drosophila for toxicology studies has only recently gained broader recognition as a tool to elaborate molecular genetic mechanisms of toxic substances. In this article two practical applications of Drosophila for developmental toxicity assays are described. The first assay takes advantage of newly developed methods to render the fly embryo accessible to small molecules, toxicants and drugs. The second assay engages straightforward exposures to developing larvae and easy to score outcomes of adult development. With the extensive collections of flies that are publicly available and the ease with which to create transgenic flies, these two assays have a unique power for identifying and characterizing molecular mechanisms and cellular pathways specific to the mode of action of a number of toxicants and drugs.
Introduction
The use of alternative small organism models in toxicology has grown tremendously in the last decade. While the fruit fly (Drosophila melanogaster) has been a premier model for developmental biologists and geneticists, its utility for toxicology studies has only recently seen a widespread emergence. Currently, Drosophila are being used for mechanistic studies of a number of priority environmental contaminants and toxicants including mercury (Rand et al., 2009), lead (Hirsch et al., 2003), arsenic (Ortiz et al., 2009), manganese (Bonilla et al., 2012), ethanol (Guarnieri and Heberlein, 2003), nanoparticles (Posgai et al., 2011), pesticides (Gupta et al., 2007) and solvents (Wasserkort and Koller, 1997). In the realm of drug discovery, Drosophila models of human disease, notably neurodegenerative diseases such as Parkinson’s and Alzheimer’s disease (Botella et al., 2009; Sang and Jackson, 2005), are contributing to identification of beneficial compounds. However, the use of the fly model in screening for toxicity in the drug development pipeline remains an area for development. The relevance of the Drosophila model for understanding the human condition under stress of toxicants has largely been accepted as we have come to understand the abundance of highly conserved genes and pathways controlling development, stress response and xenobiotic metabolism across these divergent species (Mackay and Anholt, 2006; Misra et al., 2011; Sykiotis and Bohmann, 2010).
There are numerous advantages of the fruit fly for laboratory studies. Of note are its short life cycle (embryo to adult in approx. 10 days at 25°C), simple genetic architecture (~15,000 genes harbored on four chromosome) and the ease and low cost of maintenance in the lab relative to other animal models. The Drosophila model is endowed with almost a century of genetic and molecular characterization of its development. As a result, the molecular genetic “toolbox” and accompanying databases for gene expression and activity is as comprehensive as any experimental model. The fruit fly is holometabolous, having two motile life stages; larval and adult. Developmental programs of tissue and organ morphogenesis in both the embryonic and the larva-pupae periods are known in cellular and molecular detail. Each of these life stages offers a robust platform for design of assays of development, which we demonstrate with the protocols below.
A goal of this Chapter is to entice the newcomer to the Drosophila model for investigations in toxicology mechanisms. Several considerations of the features and value of this model are discussed in the Commentary section below. The protocols presented here also aim to provide the experienced fly researcher with methods to approach toxicological and pharmacological research questions. We focus on two protocols where toxin exposures are administered at the embryonic and larval stages, respectively. These methodologies are designed with two distinct goals with respect to the endpoints that are evaluated and their utility in resolving toxic mechanisms. In the case of the embryo assay, toxicant activity can be characterized according to abnormal phenotypes that result in developing organ systems (e.g. the nervous system). Alternatively, the larval (eclosion) assay presents a convenient platform for screening effects of various genetic backgrounds on the overall developmental susceptibility or tolerance to a toxicant of interest. We present these protocols in the context of our own studies that examine the developmental neurotoxicity of methylmercury.
Maintaining and handling Drosophila cultures
The ease and economy of establishing cultures for laboratory research is one aspect of Drosophila that makes it a preferred animal model for life science and biomedical researchers. Flies are cultured on a simple cornmeal-molasses-yeast-agar medium in 50 mL vials or scaled up to 250 mL bottles. While flies are happy to grow just about anywhere, it is beneficial to have a dedicated room for fly handling. Having this space will help contain flies, and yeast, from getting into other areas of the lab, not to mention the lunch room! Cultures can be maintained at room temperature, although reproducibility of experiments benefits greatly from maintaining stocks at a stable temperature (either 25°C or 18°C) and a controlled humidity environment (~60%), which requires investment in a dedicated fly incubator chamber. Other investments will be a stereo dissecting microscope with light source and a CO2 anesthetizing station with a block and blowgun. Consumable supplies and reagents are readily available from a number of vendors.
The methods of Drosophila culture and handling are well documented in several published books and online resources. Presenting a protocol for fly culturing is outside the scope of this article and readers are referred to the publications and online resources below. Particularly useful books to get started are:
-
◦
Drosophila Manual (Carolina Biological Supply, #452620)
-
◦
Drosophila Protocols, Chap. 35 (Cold Spring Harbor Laboratory Press, 2000, (Ashburner and Roote, 2000))
-
◦
Drosophila melanogaster: Practical Uses in Cell and Molecular Biology (Methods in Cell Biology Vol. 44, Chap. 2 (Matthews, 1994) (Harder to find, but an excellent book!)
Online resources to reference when getting started are: http://www.ceolas.org/fly/intro.html http://flystocks.bio.indiana.edu/ (under “Fly work” tab).
Basic Protocol 1
Collection and staging of embryos
The female fly lays fertilized eggs, or embryos, which are capable of developing into motile larva in approximately 22 hours. Due to this rapid development, care is taken to collect embryos in a narrow time frame (1–2 hours) such that embryos are at a similar stage over the course of an experiment. Aging of a timed collection can be done to obtain embryos of a desired developmental stage prior to, or subsequent to, treatments. (Note: for the embryo assay, aging the embryos at 18°C is critical prior to the permeabilization steps). Eclosion assays (Protocol 4) involve chronic exposure of the toxin to larvae over a four-day period and are therefore less sensitive to developmental staging during embryo collection. Embryos collected over a 16–18 hour period (overnight) can be used for eclosion assays.
Materials
A breeding population of approximately 500 flies
Embryo collection cage (Flystuff.com #59–101)
Grape-agar plates, 10cm (made from Flystuff.com, #47–102)
Yeast paste
25°C incubator
18°C incubator
1.1 Embryo collection for embryo assays
Anaesthetize and deposit a breeding population of flies (~500) in an embryo collection cage. Prepare a grape-agar plate dressed with a flattened “dab” of yeast paste (hereafter called a “grape plate”). Affix grape plate to the bottom of the cage.
Allow flies to acclimate to the cage by culturing for a day or two at 25°C, changing the grape plate in the morning and evening.
-
To begin collection of staged embryos, place a fresh grape plate on the cage first thing in the morning. Replace the grape plate after one hour, and discard this initial embryo collection
embryos should be frozen before disposal.
Collect embryos for 2 hours and replace with fresh grape plate.
Place lid on collected embryo plate and set plate in 18°C incubator. Incubate embryos for the time sufficient to reach desired stage for toxin treatment (see Support Protocol 1, embryo staging). Proceed to permeabilization protocol (Basic Protocol 2)
Additional 2-hour embryo layings can be collected in succession throughout the day and placed in 18°C for aging.
1.2 Embryo collection for eclosion assays
Place a fresh grape plate on a population cage at the end of the day (~5PM). Incubate cage at 25°C overnight.
Collect plate in the morning (~9AM). Place a lid on the plate and place back in 25°C incubator.
Age the embryos for 24–30 hours to establish a plate full of 1st instar (L1) larvae.
Proceed to eclosion assay (Basic Protocol 4)
Basic Protocol 2
Embryo permeabilization
Delivery of toxins and small molecules to the embryonic tissues relies on treatments to render the eggshell permeable. The protocol below is a modification of a previously described method that utilizes a novel d-limonene based embryo permeabilization solvent (EPS, (Rand et al., 2010)). There are two steps to treating the eggshell for permeabilization. First, the outer chorionic layers are removed by brief immersion in bleach, which exposes the impermeable waxy layer. Subsequent EPS treatment diminishes the waxy layer, making the shell permeable to solutes. This protocol will result in a batch of embryos that will display a range of permeability that results from heterogeneous rates of eggshell hardening among embryos, even when they are closely staged. However, the protocol also employs application of fluorescent dye, which enables determination of permeability on an individual basis, both in live embryos and in post-fixation preparations.
Materials
Bleach(Fisher #SS290-4, final solution diluted to 50% with H2O)
EPS (embryo permeabilization solvent, recipe below)
MBIM (modified basic incubation medium, recipe below)
MBIM with 0.1% Tween 20 (MBIM-T)
PBS (saline phosphate buffer)
50mL glass beaker
Flat-bottomed nitex basket
Notched-bottom nitex development basket (see (Rand et al., 2010))
Six 60mm plastic dishes
CY5 carboxylic acid dye (10mM stock made in DMSO, Lumiprobe #23090)
1.5mL microfuge tube, clear
soft bristled paintbrush
disposable pipets
Kimwipes
Nutator (VWR #82007-202)
A microscope equipped with epiflourescence, including far-red detection.
2.1 Dechorionate the embryos
Prepare 50mL of 50% bleach in a dish. Adjust a faucet to have a gentle flow of room temperature water.
Using tap water and a paintbrush, wash embryos from grape plate into a flat-bottomed nitex basket. Rinse away excess yeast under tap water.
Immerse the basket containing the embryos in the bleach for 2 minutes. Using a disposable pipet squirt the embryos occasionally with the bleach.
Rinse embryos completely, holding under tap water to remove bleach.
Set the basket in dish with tap water and proceed to EPS treatment.
2.2 Permeabilization with EPS
Prepare 1:40 dilution of EPS in MBIM (3mL final volume) in 50mL beaker. (EPS forms a white emulsion when diluted)
Prepare six 60mm dishes with 10mL PBS to be used for rinsing.
Remove excess water from the bottom of the embryo basket with a Kimwipe.
-
Immerse the basket in the diluted EPS.
Note: time of EPS exposure is critical. Useful range is from 30 seconds to 1.5 minutes, correlating with younger to older embryo age.
Remove excess EPS from outside of basket with Kimwipe.
Rinse embryos by successive immersion in PBS in the 60mm dishes (10–15 seconds per dish). Gently squirt embryos with PBS to facilitate rinsing using disposable pipet.
2.3 Dye treatment to identify permeabilized embryos
Prepare 50µM CY5 dye in 1 mL of MBIM-T in a 1.5mL microfuge tube.
Remove excess PBS from bottom side of embryo basket with Kimwipe.
Transfer embryos gently to dye solution using a paintbrush.
Cap tube and invert briskly to bring embryos into suspension.
Place tube on its side on a Nutator rocker table for 15 minutes.
Remove tube and flick side briskly to dislodge any embryos from the sides.
Place tube upright in rack and let embryos settle for ~30 seconds.
Open tube and withdraw dye solution without disrupting settled embryos.
Place 1 mL MBIM-T in tube, cap and invert several times to wash embryos.
Let embryos settle, and repeat MBIM-T wash 2 more times.
Let embryos settle and remove MBIM-T. Proceed with embryo treatment and development.
Basic Protocol 3
Developmental effects of toxin exposure in the embryo
Toxin exposures can be done acutely or chronically. Acute exposures are achieved by including the toxin in the dye treatment step (e.g. 15 minute exposure). Chronic exposures are achieved by addition of toxin to the medium in the development basket set up described below. Dose response should be established empirically in either presentation. Many compounds require DMSO to be soluble in concentrated stock solutions. Final concentrations of DMSO should not exceed 1% in embryo treatments. Development of embryos can be achieved in MBIM medium alone or with a 50:50 mixture of MBIM and M3 medium, the latter being more favorable for longer developmental periods.
Prepare 6 mL development medium (MBIM or MBIM:M3) with toxin at desired concentration in 60mm dish.
Place notched-bottom nitex basket in a medium 60mm dish.
Using a paintbrush, gently transfer embryos to the mesh in the development basket. Arrange embryos in a monolayer.
Place a lid on the dish and continue incubation at 25°C until desired stage. Proceed with endpoint analysis of live embryos or fixation for immunostaining.
Support Protocol 1
Endpoint analysis of developmental toxin exposure: immunostaining
A critical parameter of this protocol is determining the degree of permeability of each embryo, which can vary widely. This is necessary to judge the accessibility of the embryo to the toxin, and hence the exposure that is incurred. Permeability can be determined by observation of the CY5 dye uptake in either live or fixed embryos under far-red excitation with a fluorescence microscope. It is necessary to correlate the level of dye uptake with the phenotype of the embryo resulting from toxin exposure to make conclusions of the toxin effect. It is also prudent to determine empirically the level dye uptake (permeability) that correlates with fully viable embryos, which can vary with time of EPS treatment and age of embryos. Consideration of optimizing these parameters is discussed below in the Commentary and previous publications (Rand, 2014; Rand et al., 2010).
Effects of toxins on embryogenesis can be monitored through a wide array of endpoints. A very simple endpoint is development to late stages (e.g. Stage 17 or hatching to larval stage), which can be scored via direct observation under brightfield microscopy. Stage 17 embryos can be determined by the degree of condensation of ventral nerve cord and other easily identifiable structures (Ashburner et al., 2005). Hatching can be scored easily by counting empty vitelline membranes and unhatched embryos (Rand et al., 2009). Alternatively, autoflourescence of yolk proteins in the blue wavelengths can be resolved in live embryos, which is very effective for revealing development of the midgut and hindgut (Rand et al., 2010). More informative endpoints can be resolved using standard immunological methods to reveal morphological and cellular phenotypes in discrete tissues and organs. These later methods can also take advantage of vital dye reporters (e.g. GFP) that can be monitored in either live embryos or fixed preparations (Rand et al., 2010). A protocol for fixation and immunostaining of toxin treated permeabilized embryos is presented below (adapted from (Patel, 1994). Note: Dechorionation with 50% bleach, the typical first step in embryo immunostaining, is not necessary at this stage as it has already been done prior to permeabilization).
Materials
PBT (phosphate buffer with 1% BSA and 0.1% Triton X100, recipe below)
PBT, NGS, NDS (PBT with 5% normal goat and donkey serum, recipe below)
PEM (PIPES buffer with EGTA and MgSO4, recipe below)
8% PFA (paraformaldehyde prepared in H2O).
Heptane (Fisher #H3501)
Methanol (Fisher #A412)
Microfuge tube (1.7mL, clear polypropylene)
1° and 2° antibodies
Nutator (VWR #82007-202)
Vortex
Glass slides (VWR, Superfrost Plus, #48311-703)
Glass cover slips (18 ×18mm, Fisher #12-541A)
Clear nail polish
Embryo fixation. (All washing and incubation steps are done with rocking on a Nutator in a 1.5mL microfuge tube. Solutions are exchanged by allowing embryos to settle, drawing off supernatant and replacing with fresh solution).
Transfer embryos from notched development basket to 1.5mL microfuge tube containing 1 mL PBT using a paintbrush. Invert tube to re-suspend embryos and wash for 1 minute. Draw off PBT and repeat with one more wash. Remove all PBT.
-
Add 250 µL 2X PEM and 250µL 8%PFA to embryos. Add 500µL heptane. Invert several times to mix, then incubate on Nutator for 25 minutes.
(Note: two phases form with these solutions)
-
Draw off lower (aqueous) layer and discard. Add 750µL of methanol. Vortex the tube for 30 seconds
(Note: set vortex on ¾ speed. This aggressive mixing removes the vitelline membrane from the fixed embryonic tissue).
Allow embryos to settle to bottom of tube (Vitelline membranes will remain at the interface). Draw off all liquid and discard.
-
Wash embryos three times in 1 mL of MeOH. Proceed to immunostaining.
(Note: embryos may be stored in MeOH at −20°C for many months)
Immunostaining embryos (All washing and incubation steps are done with rocking on a Nutator in a 1.5mL microfuge tube. Solutions are exchanged by allowing embryos to settle, drawing off supernatant and replacing with fresh solution).
Wash embryos 3 × 10 minutes in 1mL of PBT.
Block embryos in PBT, NGS/NDS for 30 minutes, remove blocking solution.
Add 1° antibody in 300µL PBT, NGS/NDS (Dilution dependent on antibody type and concentration). Incubate overnight at 4°C.
Wash 3 × 10 minutes in PBT.
Add 2° antibody in 300µL PBT, NGS/NDS (Dilution dependent on antibody type and concentration). Incubate at room temperature for 1 hour.
Wash 3 × 10 minutes in PBT. Draw off all PBT.
Add 100µL of mounting medium and gently re-suspend embryos by tapping the tube. Allow embryos to settle and equilibrate in mounting medium at least 1 hour (Commercial mounting medium can be used, or see recipe below).
Mount embryos on a slide by placing 35µL of suspended embryos on a slide and gently covering with cover slip. Seal edges with nail polish. Observe under fluorescence microscopy. (*Examples of immunostaining of methylmercury treated embryos can be seen below.)
Basic Protocol 4
Developmental effects of toxin exposure in larvae: the Eclosion assay
Effect of toxins on fly development can be assessed by an eclosion assay. Eclosion is the term for the emergence of the adult fly from the pupal case. In the assay, first instar larvae (L1) are reared on medium containing the toxin of interest and eclosion is scored as the number of adults that successfully leave the pupal case. The assay therefore measures effects of toxin exposure over a substantial developmental period (larvae-pupae). However, the ease of scoring and the relatively high throughput of the assay make it very desirable for screening genetic and nutritional influences on a toxic insult. In some cases where eclosion rates are low or absent, development to the late pupal stage (dark pupa) can be used as an effective endpoint to differentiate tolerance to a toxin. Scoring is generally executed 13 days after L1 larvae are seeded on fresh food medium. This assay can be used to evaluate behavior of a single fly strain on various concentrations and compositions of toxin or drug treatment, or to investigate the effects of different genetic backgrounds on a single type of toxic exposure. The steps for larval exposure, development and scoring are outlined below, using an example of treatments with methylmercury from our own studies.
Materials
Jazz- Mix (Fisher Scientific #AS153)
Water (from lab purification system)
Bulk polystyrene fly vials (Genesee Scientific, #AS 520)
Vial plugs (Flystuff.com # 32-116BF)
Cheese cloth (Flystuff.com # 53–100)
Stirring hot plate
Thermometer
Cool water bath
2nd stir plate
2 L glass beaker
100 ml glass beaker
Digital scale (1 kg range)
Stir bar
Standard microwave oven
25 mL serological pipettes
Motorized pipet filler/dispenser
Disposable transfer pipets
Adjustable pipets and tips (10µL and 200µL)
Elongated forceps
Stereomicroscope and light source
L1 stage larvae (see Basic Protocol 1.2)
4.1 Media preparation
Eclosion assays are typically performed in 50mL fly vials containing 10mL of food. Assays are done with three replicates for each sample condition (e.g. each dose of the toxin and/or each genetic background).
Determine volume of food needed for assays and weigh out Jazz-Mix Drosophila fly food accordingly (0.189 g/ml). (Volume calculation = #vials × 10mL. Add 10% to this volume to avoid coming-up short when distributing the food to the vials).
Pre-boil the calculated volume of water in a 2-liter beaker in a microwave oven (approximately 4 minutes).
Put 2-liter beaker on a pre-heated (set on ~350°C) stirring hot plate and add a large stir bar.
Slowly add pre-weighed Jazz-Mix to the beaker and cover.
Allow mixture to reach a boil, reduce heat (~250°C); then, on a low boil, continue with stirring for 10 minutes to ensure the agar in the mix dissolves thoroughly.
Label a series of empty vials accordingly with the concentrations of the toxin that will be in the food in each vial (e.g. see Table 1).
Transfer 2-liter beaker to a cool water bath on a stir plate and continue stirring until food mixture reaches 65°C. Transfer 2-liter beaker back to the pre-heated stirring hot plate set at 85°C to prevent solidification of the mixture.
-
Prepare concentration series of toxin in food: replicates for each concentration are prepared sequentially, starting with lowest concentration. For example, the amounts needed for 2 series of replicates of food with the toxin methylmercury (MeHg) is given below in Table 1. In this example, 2 strains of flies are being used, therefore 2 strains×3 replicates×10 mL Jazz-Mix = 60 mL food needed for each treatment concentration. A stock concentration of 50mM MeHg in DMSO is utilized to prepare each MeHg treatment DMSO is used for a vehicle control.
(*Warning: MeHg is a potent neurotoxin and should only be handled with appropriate protective gloves and clothing).
Pour off 60mL of warm food into a 100 mL beaker. Add 18µL of DMSO and mix thoroughly with a disposable pipet. Pipet 10 ml of mixture into each of six vials labeled “0”. Repeat this process for the next concentration (e.g. 60mL food + 6µL MeHg + 12 µL DMSO, for 5µM MeHg food). When all the food is distributed in the vials, cover with cheese cloth and allow to cool overnight.
The next day, cap vials with plugs. Use food immediately or store in a plastic bag under refrigeration. Food should be used within 3 days to avoid desiccation.
Table 1.
| # of Fly Strains |
[MeHg] final concentration (µM) |
Jazz Food (ml) |
MeHg (µl of 50mM stock in DMSO) |
DMSO (µl) |
|---|---|---|---|---|
| 2 | 0 | 60 | --- | 18 |
| 5 | 60 | 6 | 12 | |
| 10 | 60 | 12 | 6 | |
| 15 | 60 | 18 | --- |
4.2 Transfer of Larvae
Fifty L1 larvae are seeded to each vial. Ten larvae at a time are picked up from the grape plate and transferred to the surface of the food medium in the vial. To assist in counting, five positions (four “dots” and one “line”) are marked with a pen on the outside of the vial to determine where larvae are deposited in the food on the inside.
Position grape plate under microscope with lighting from the side to highlight larvae. Pick up 10 larvae using a probe or elongated forceps that can contact the food surface in the vial.
Holding the vial in one hand (preferably the non-dominate hand) place the thumb on the first “dot” on the vial. Carefully lower the probe containing larvae into the food media in the vial adjacent to the dot and swipe the end of the probe through the food to release the larvae. Rotate the vial and position thumb on next “dot”. Repeat by transferring 10 more L1 larvae and advancing thumb to next “dot”. Once the long vertical line is reached, and the final 10 larvae are transferred (50 in all), the vial is plugged. Write the date and any other relevant information on the vial.
The eclosion assay is performed in triplicate for each fly strain. Each series of a replicate for a given strain (e.g. 0, 5, 10, 15µM MeHg) should be seeded with larvae from the same grape plate to maintain consistency and accuracy in larval staging across the toxin concentrations. The second and third replicate series can be seeded with L1 larvae from the same plate or a grape plate collected on the subsequent day, but having the same timing of laying and aging.
Vials seeded with larvae are placed in a humidified chamber (~60%) at 25°C.
4.4 Scoring development
Scoring development is done 13 days after transferring L1 larvae to vials. All flies that have eclosed are counted, whether dead or alive.
Anesthetize live flies with CO2 and lay out on a CO2 block. Count flies by direct inspection. (Sorting flies into groups of five can assist counting).
Under the microscope, count the number of dead eclosed flies that remain in the food and on the sides of the vial. Count only the flies that have fully exited their pupal casing.
On a score sheet, tally the number of flies that have eclosed. Additional info on toxin efficacy can be gained by scoring live and dead flies separately.
Prepare tables and plots of the percent of eclosion (sum of live and dead flies) versus toxin concentration.
Note: Plots of live flies only can give an additional read-out of the tolerance of the flies to the toxin. As an alternative to scoring eclosed flies, counting the number of flies that make it to the dark pupae stage and beyond (including eclosion) may be useful, as this can give some idea as to what stage of development the toxic insult targets (e.g. pupal versus larval). In addition, some toxic insults may exert an overall developmental delay. In this instance, observing and scoring development past day 13 is necessary to document this effect.
Supporting Protocol 2
4.1 Eclosion assay: testing effects of nutritional additives
Evaluation of the effect of additives on the toxicity of MeHg or other toxicological compounds can be performed. For example, the addition of caffeine at various concentrations to food preparations containing MeHg may be employed to assess tolerance via eclosion. The procedure for the eclosion assay in examining additives is the same as for a single toxin; however, media preparation is adjusted to include the additive. Appropriate control media and media containing the additive are prepared from a single batch of Jazz- mix.
Outlined below is a procedure using supplementation of caffeine to MeHg-containing food preparations.
Additional Materials
Caffeine (AKT Laboratories, #C0221)
In this example, caffeine is added at 2mM final concentration, which is previously shown to illicit effects on MeHg toxicity (Rand et al., 2012).
Prepare caffeine at a 100 mM working concentration in dH2O. Dissolve caffeine by vigorously shaking at 37°C.
Prepare Jazz mix. (Refer to 4.1 Media Preparation).
To 60mL of warm Jazz mix add MeHg (or vehicle control, DMSO) and caffeine (or vehicle control, H2O) to yield concentrations designated in Table 2 below. Stir thoroughly to achieve homogeneity of the toxic compound and additive.
Pipet 10 ml of mixture into each of six vials labeled for the appropriate MeHg and caffeine concentrations.
Cover vials with cheese cloth. Cool overnight. Plug vials the following day and proceed to larval eclosion assay. (*An example of outcomes from this assay can be seen in Figure 4 below).
Table 2.
| # of Fly Strains |
[MeHg] (µM) |
Jazz Food (ml) |
MeHg (µl) | DMSO (µl) |
Caffeine (ml) | H2O (ml) |
|---|---|---|---|---|---|---|
| 2 | 0 | 60 | --- | 18 | --- | 1.2 |
| 0 + Caffeine | 60 | --- | 18 | 1.2 | --- | |
| 5 | 60 | 6 | 12 | --- | 1.2 | |
| 5 + Caffeine | 60 | 6 | 12 | 1.2 | --- | |
| 10 | 60 | 12 | 6 | --- | 1.2 | |
| 10 + Caffeine | 60 | 12 | 6 | 1.2 | --- | |
| 15 | 60 | 18 | --- | --- | 1.2 | |
| 15 + Caffeine | 60 | 18 | --- | 1.2 | --- |
Figure 4. Transfer of Larvae for eclosion assay. (See description in section 4.2).
4.2 Eclosion assay: testing transgenic flies
Assaying traits associated with genetic variations is a hallmark of the utility of Drosophila. The ease with which to obtain transgenic or mutant flies with altered expression or activity of a single gene of interest make this model uniquely powerful for investigating mechanisms of toxicology. The example below describes use of the conventional GAL4>UAS transgene expression system is to investigate the activity of the endogenous CYP6g1 gene, the Drosophila homolog of human CYP3A4, in augmenting the toxicity of MeHg. This gene is highly expressed in the fat body (FB), which mediates many functions analogous to the vertebrate liver. Results of this experiemetnal set up are previously reported in (Rand et al., 2012). Steps below refer to basic fly handling required for creating transgene expressing L1 larvae.
Materials
Gal4 and UAS fly stocks
Small embryo cage (Flystuff.com #59–100)
Grape-agar plates, 6cm (made from Flystuff.com, #47–102)
Yeast paste
Prepare bottle cultures of the fat body GAL4 “driver” (FBGAL4) stock at 25°C. Two bottle cultures prepared in parallel will ensure the collection of plenty of virgin female flies.
Prepare bottle cultures of desired responder lines, e.g., UAS-CYP6g1 and UAS-CYP6g1RNAi and a control wild type line. One bottle culture of each of these should be sufficient as fewer males than females are required in subsequent crosses.
Collect virgin GAL4 driver females and UAS responder males. Virgin collection is achieved by collecting newly eclosed females prior to their mating and follows well-documented protocols (see (Ashburner and Roote, 2000)). Males can be collected from a healthy population of adults at any time.
Establish mating of GAL4 and UAS lines. Combine approximately 150 virgin females (FBGAL4) and 100 males (UASCYPg61, UASCYP6g1RNAi, or wt control) in a small collection cage with a grape plate and yeast. Allow mating to proceed for at least two days with changes of grape plates twice a day.
Collect embryos as in Basic Protocol 1.2. Proceed to Eclosion assay with progeny L1 larvae of the F1 generation.
Reagents and Solutions
Basic Protocol1
EPS (embryo permeabilization solvent)
9 mL d-limonene (Ultra high purity grade, Florida Chemical Company)
500 µL Cocamide DEA (Ninol 11CM, Stepan Chemical, Northfield Il)
500 µL Ethoxylated alcohol (Bio-soft 1–7, Stepan Chemical, Northfield Il)
Solutions of surfactants (Ninol 11CM and Bio-soft 1–7) are warmed in 37°C bath. Put 9mL of d-limonene into a glass scintillation vial with a small stir bar. Pipet the Ninol 11CM and Bio-soft1–7 to the d-limonene (5% final concentration). Mix and remove stir bar. (EPS stock solution is good for approximately 2 months. Warm EPS at 37°C and mix prior to use).
MBIM (modified basic incubation medium, from (Strecker et al., 1994))
| 2.2g | MgCl2•6H2O |
| 2.97g | MgSO4•7H2O |
| 0.42g | NaH2PO4 |
| 12.1g | Glutamic acid |
| 6.05g | Glycine |
| 0.66g | Malic Acid |
| 0.027g | Sodium acetate |
| 2.2g | Glucose |
| 0.99g | CaCl2•2H2O |
pH 6.8, adjust with drops of 10N NaOH and then sterile filter. Store frozen in 50mL aliquots.
MBIM-T (MBIM with 0.1% Tween 20)
50mL MBIM
50µL Tween-20
M3 medium (Shields and Sang M3 insect culture medium, Sigma S8398)
Prepared according to manufacturer’s instructions
Fixation and staining solution recipes
PBT (PBS, 1% BSA, 0.1% TX100, 200mL)
190 mL of standard PBS solution, pH 7.2
2 g bovine serum albumin (Sigma A9647)
200 µL triton X-100 (Fisher BP151-100)
bring final volume to 200 mL with H2O.
PBT, NGS/NDS
1.5 mL PBT
75 µL normal goat serum (Sigma G9023)
75 µL normal donkey serum (Sigma D9663)
2× PEM (200mM PIPES, 4mM EGTA, 2mM MgSO4, pH 6.9, 100 mL)
6.93 g PIPES (Sigma P3768)
0.19 g EGTA (Sigma E8145)
0.046 g MgSO4 (00627 Fluka)
8% PFA (paraformaldehyde)
4 g paraformaldehyde (Acros #41678)
50 mL H2O
Prepare PFA in a fume hood. Add PFA to water stirring in beaker. Add a couple of drops of 10N NaOH to raise pH and allow PFA to go into solution. Warming the solution will assist in solubilizing the PFA. Prepare 500 µL aliquots of clear 8% PFA solution, freeze at −20°C. Thaw aliquot completely in 37°C water bath immediately before use.
Mounting medium (20mM TRIS, 0.5% n-propyl gallate, 50% glycerol, pH 8.5, 20mL)
267µL of 1.5M TRIS, pH8.8
9.7mL H2O
0.5mL 20% n-propyl gallate (Sigma P3130, stock: 200mg/mL in DMSO)
10 mL glycerol (Fisher G33-500)
Mix TRIS and water first, adjust pH to 8.5. Add n-propyl gallate, mix. Stir in glycerol until thoroughly mixed.
Commentary
Background information
Growth and Development during the life cycle of the fly
For the experimental biologist, the multiple life stages of the fly is an asset in that fundamental mechanisms of cellular growth, differentiation, patterning and specification as well as organogenesis are played out in two scenarios: when the embryo forms a larva and when the larva transitions to adult via pupal metamorphosis. While these life stage transitions necessarily invoke unique cellular events and call on specific molecular pathways, in many contexts, e.g. neurogenesis and neuron/glial differentiation and specification, the same molecular signaling pathways are invoked at the embryonic and at the larval-pupal development stages. Thus, in one model platform there is the opportunity to probe the effects of toxicants on a given molecular pathway in two or more contexts. The protocols above outline methods of applying toxicant or drug to the embryo and to the larvae for assays that monitor effects across these critical developmental transitions.
Drosophila are holometabolous insects, meaning they undergo complete metamorphophosis and go through four life stages: embryo, larvae, pupae and adult. Embryogenesis is complete in 22–24 hours at standard incubation temperatures of 25°C, culminating in hatching of the first instar larva (L1). Larval growth spans approximately four days and is punctuated by two molts between the first-second instars and second-third instars, occurring at approximately 24 hours and 48 hours after larval hatch, respectively. The animal grows more than 30 times its body weight from newly hatched larvae to the wandering third instar stage (L3) (Ashburner et al., 2005). The pupal stage lasts approximately four days and is characterized by identifiable “white” and “dark” stages occurring as metamorphosis progresses and formation and tanning of the adult cuticle happens. Eclosion is the term describing the emersion of the adult fly from the pupal case.
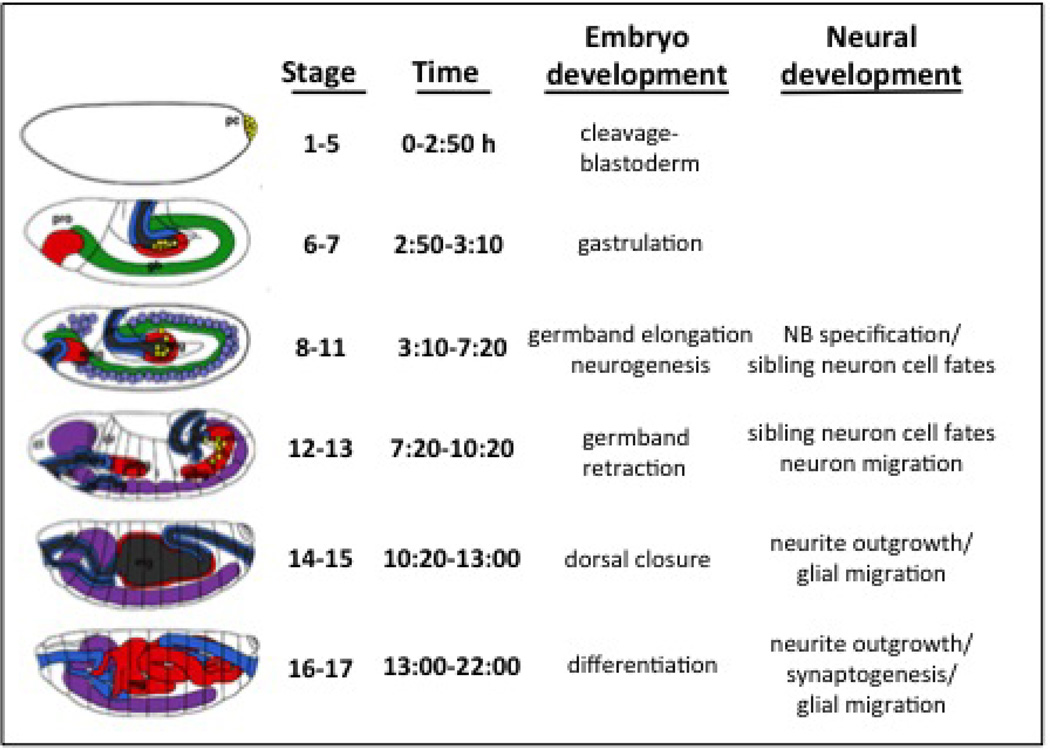
Embryogenesis and metamorphosis in this organism have been studied extensively. There are 17 stages of embryogenesis and 15 stages of metamorphosis, these being identifiable with morphological features that can be discerned with light microscopy (Ashburner et al., 2005). Fundamental stages of embryogenesis are illustrated in Figure 1. Evaluation of endpoints in embryo treatments requires a familiarity with features of embryo development (see (Ashburner et al., 2005), (Hartenstein, 1993)). In contrast, the eclosion assays call upon simple skills of scoring adults or distinguishing identifiable white and dark pupae features.
Figure 1. Stages of embryo development.
Anterior is left and dorsal is up. Neuroblasts (light purple, at stage 8–11). Ventral nerve cord (VNC) and brain neurons (purple, at stage 12–17). Endoderm/midgut (red). Mesoderm (green). Foregut/Hindgut (Blue). (See text for discussion. Adapted from (Hartenstein, 1993))
The GAL4>UAS system: a molecular geneticists toolbox
The value of using Drosophila in toxicology studies is set against a backdrop of the fly model being perhaps the most developed molecular genetic laboratory model available. In short, current tools enable functional analyses of essentially every gene in the genome at any point in development and within any tissue. The methods most pertinent for developmental toxicology assays fall along two lines: 1) constructs and reagents allowing for visualization of cellular, tissue and/or organ morphology and 2) constructs and reagents that either induce or report changes in gene expression. The GAL4>UAS system is the “bread-and-butter’ method used by Drosophila researchers and has been described in detail in several publications (Brand et al., 1994; Duffy, 2002). This system utilizes artificial constructs of DNA introduced to the fly chromosome, that encode the yeast-derived transcriptional unit of the GAL4 transcription factor and its cognate DNA target known as the upstream activating sequence (UAS). Neither of these genetic elements alone have an effect on the fly. However, promoter-specific expression of GAL4 can elicit tissue specific expression of a gene of interest that is preceded by the UAS. This method has been exploited to perform tissue specific reporter gene expression (e.g., GFP or lacZ) as well as over-expression of endogenous or heterologous (human) genes. Knockdown of gene expression can also be achieved with expression of inhibitory RNA hairpin constructs in a targeted manner. For the toxicologist, this technology permits an easy read out of teratogenic effects of chemicals in the embryo, in either live or fixed tissue. Importantly, this technology can be used to probe the function of genes, e.g. phase I, II or III metabolism genes, in modifying effects of applied toxins.
Critical parameters and troubleshooting
Embryo staging
The stages of Drosophila embryogenesis have been described in great detail (Ashburner et al., 2005; Hartenstein, 1993) (Figure 1). Treatments at the onset of critical developmental events (e.g. neurogenesis) can be targeted by carefully aging a brood of embryos laid in a narrow time frame. For practical purposes a 2-hour collection of embryos is typically required to obtain sufficient numbers of embryos for downstream treatments and handling. Where needed, closer developmental staging can be achieved using a narrower collection window (e.g. 30min to 1hour), which may require a larger population at the outset. Below is a schematic illustrating features of the embryo at distinct developmental stages based on development at 25°C. It is important to note that development of the embryo at 18°C occurs at half the rate of development at 25°C.
Embryo permeabilization
Permeabilization steps outlined above will typically yield a batch of embryos that display a wide range of permeability, as seen by various levels of CY5 dye uptake. This is normal and is a result of non-uniform rates of eggshell hardening that likely occur between individual embryos (Li and Li, 2006). It is therefore critical to use the level of CY5 uptake as a guide in identifying embryos that will have encountered toxicant exposure. This can be achieved in two ways: 1) sorting of embryos based on CY5 intensity immediately after the dyeing step or 2) relying on residual CY5 fluorescent signal in endpoint analysis (e.g. post fixation and immunostaining). The latter process is less time consuming and avoids excess manipulation of the embryos. However, it does require sensitive imaging capabilities in the far-red wavelengths since the CY5 signal is seen to diminish with fixation (approximately 10-fold). A typical pattern seen with CY5 (and several other dyes) is a concentration of the dye to the yolk contents in the lumen of the gut at later embryonic stages. This is a good first indication that normal development has occurred in the case of control treated embryos. In addition, over-permeabilization can result in failed embryo development, which is typically seen to correlate with an over-abundance of CY5 signal. The pattern of CY5 localization in conjunction with toxin treatment can also serve as an indicator of gross teratogenicity. Displacement of the entire yolk to the anterior compartment of the embryo has been seen in severe MeHg exposures in embryos (data not shown).
Anticipated results
Examples of the outcomes from treatments in Protocols 2 and 3 can be seen in Figure 2 below. An example of how these protocols are effective for assay toxicants is seen with the effects of methylmercury in Figure 3. Transfer of L1 larvae to food vials from the grape plate is pictured in Figure 4. Typical outcomes of the eclosion assay (Protocol 4) are seen in Figure 5 below. Results of these treatments and assays are described in the accompanying figure legends.
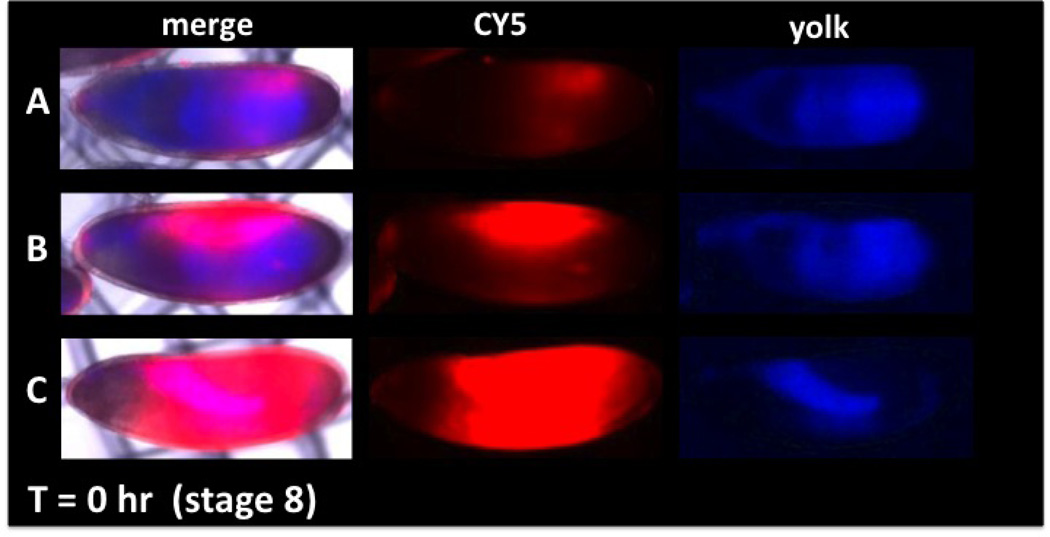
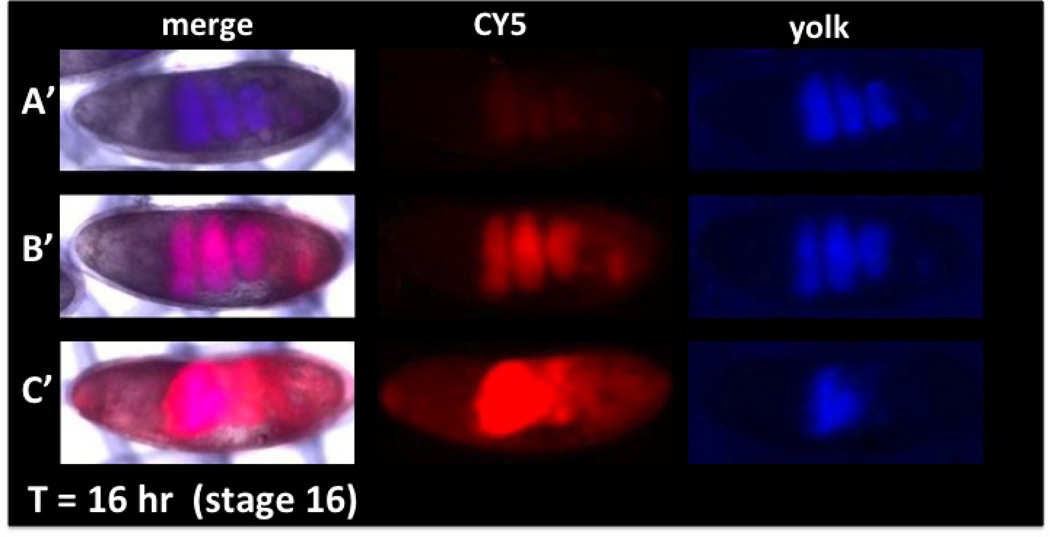
Figure 2. Permeability and viability determination with CY5 dye uptake and distribution in embryos.
(Anterior is left and dorsal is up). The degree of permeability can be assessed by CY5 uptake (red) and compared to embryo age determined by distribution of yolk (blue). A range of permeability is seen in embryos of the same stage (A, B, C, CY5). Viability can be determined by incubating embryos for a period of time (Basic Protocol 3) and observing the distribution of yolk to the involutions of the forming midgut (A’, B’, C’ yolk). Over-permeabilization (C, C’) results in decreased viability seen by the failure of midgut formation (C’, merge and yolk) and an incomplete sequestration of dye in the gut (C’ CY5). Correlation of CY5 dye uptake and viability (assessed by yolk blue autofluorescence) allows identification of optimally permeabilized viable embryos (see B and B’). Methods: A two-hour collection of embryos was aged for two hours at 18°C to obtain embryos at ~stage 8. EPS permeabilization and CY5 dye treatments were done as in Protocol 2. Embryos were placed in a notched basket with MBIM culture medium (Protocol 3) and visualized with brightfield, blue and far-red epifluorescence (A, B, C). Embryos were then aged at room temperature for 16 hours and imaged again under the same image capture settings (A’, B’, C’).
Figure 3. Post-fixation determination of toxin effects in permeabilized embryos.
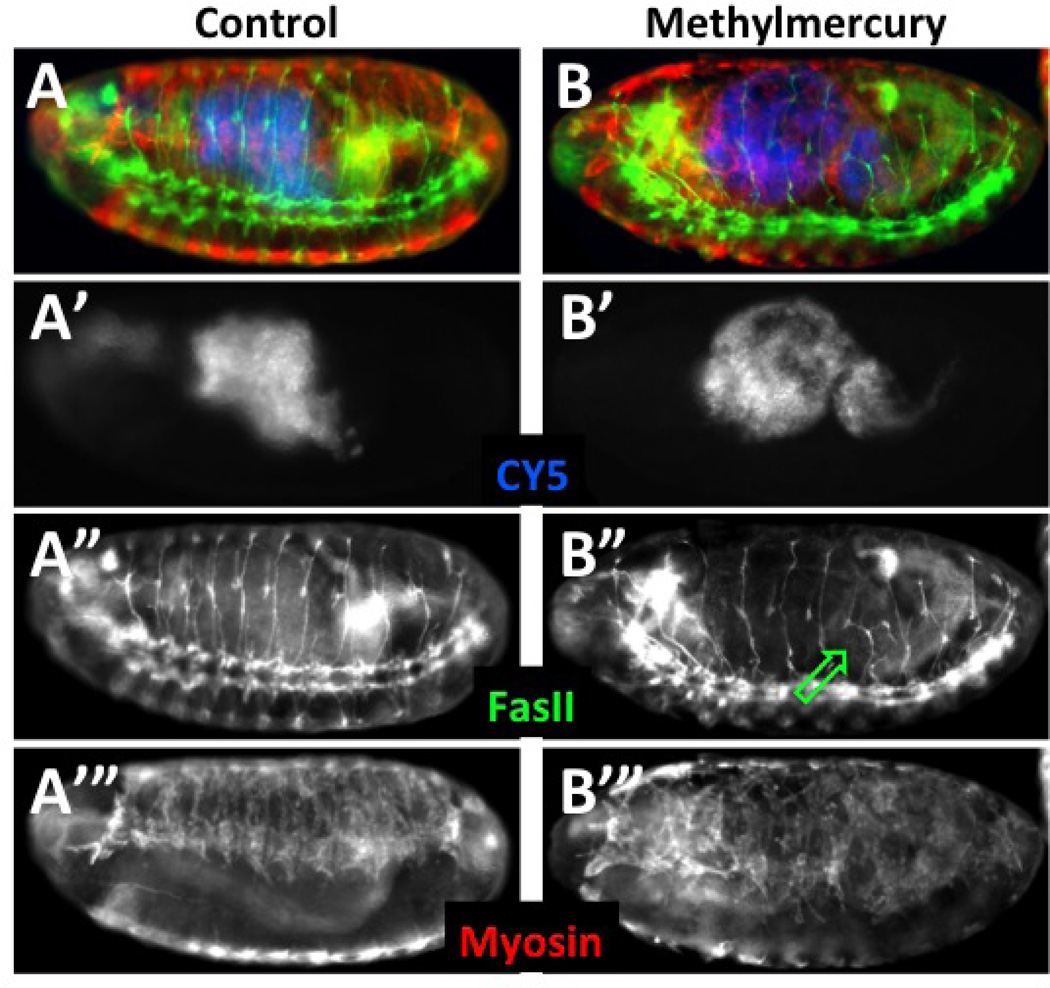
Embryos were collected and aged at 18°C to obtain a brood of ~stage 8 embryos. At which time they were permeabilized according to Protocol 2 and treated with CY5 dye. Embryos were then developed in notched baskets with either MBIM medium alone or with the addition of 50µM methylmercury and development continued at room temperature for eight hours. Embryos were then fixed and stained as in Protocols 3.1 and 3.2. Embryos that were permeabilized at the outset are identified by direct detection of CY5, which localizes to the lumen of the gut (A’ and B’, pseudo-colored blue in A and B). Segmental and intersegmental motor neurons are stained with anti-FasII antibody (A” and B”, green in A and B) and somatic muscle revealed with anti myosin heavy chain antibody (A’” and B’”, red in A and B). Embryos are equally permeablized as seen by the levels of CY5 dye (A’, B’). Methylmercury is seen to disrupt normal motor neuron projections in the posterior abdominal segments (B”, green arrow). Patterning of developing muscles is similarly seen to be affected strongly by methylmercury (B’” compared to A’”).
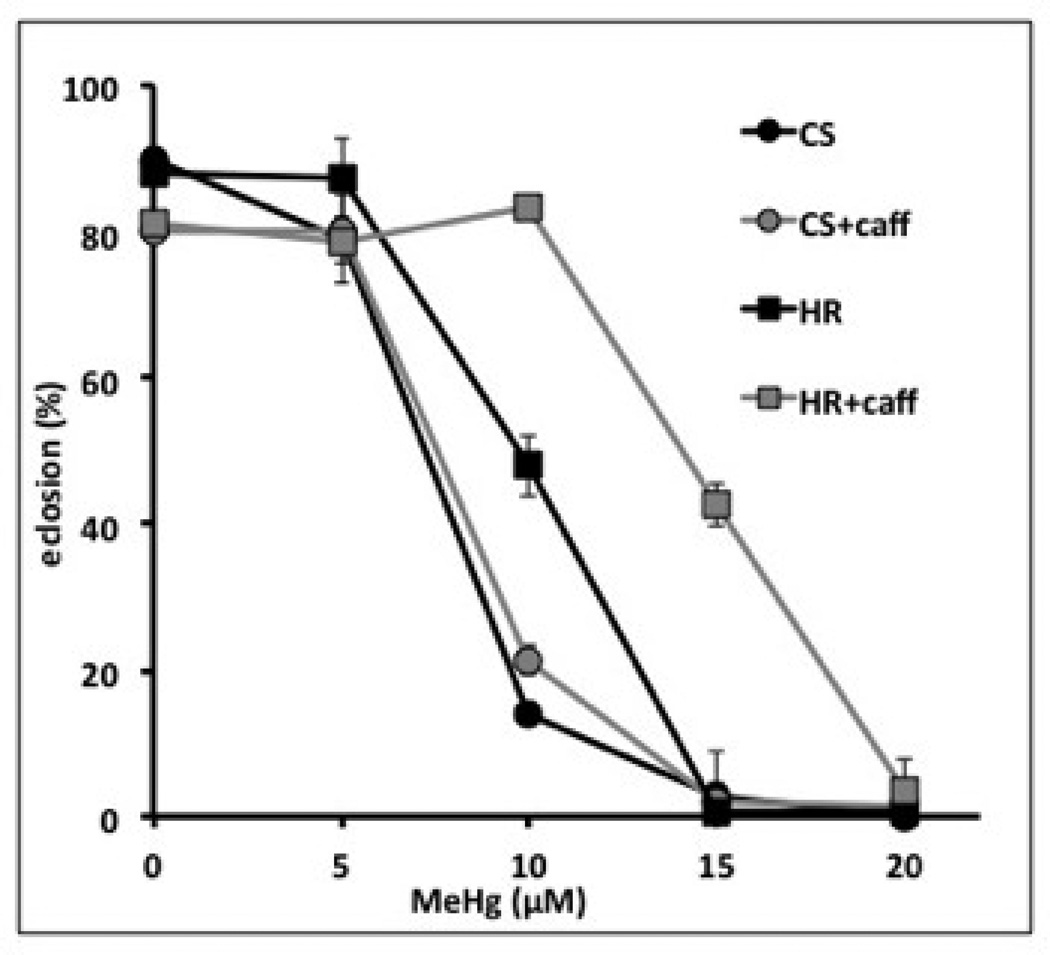
Figure 5. Developmental toxicity of methylmercury and beneficial effects of caffeine in two fly strains.
Eclosion assays were performed as in Protocol 4 using the Canton S (CS) and Hikone R (HR) strains of flies. These strains have previously been reported to differ in their tolerance to methylmercury using this assay (Rand et al., 2012). A reduced rate of eclosion is seen with increasing methylmercury (MeHg) in the food medium. The HR strain exhibits more tolerance to MeHg, as seen by higher rates of eclosion on 10µM and 15µM MeHg compared to CS. Addition of 2mM caffeine to the food increases tolerance in both strains seen by increased rates of eclosion on 10µM (CS and HR) and 15µM MeHg (HR) relative to MeHg treatment alone.
Time Considerations
Basic protocol 1: 3 days
Basic protocol 2: 1 hour
Basic protocol 3: approx. 24 hours
Basic protocol 3.1: 1 hour
Basic protocol 3.2: 20 hours
Basic protocol 4.1: 1.5 hour
Basic protocol 4.2: 1–2 hours
Basic protocol 4.3: 1–1.5 hours
Acknowledgments
This work was supported by NIH/NIEHS R03ES021581 (awarded to M.D.R.) and the University of Rochester Environmental Health Center (NIH/NIEHS P30 ES001247).
Literature Cited
- Ashburner M, Golic K, Hawley R. Drosophila: A Laboratory Handbook. 2nd ed. New York: Cold Spring Harbor Laboratory Press; 2005. [Google Scholar]
- Ashburner M, Roote J. Laboratory Culture of Drosophila. In: Sullivan W, Ashburner M, Hawley R, editors. Drosophila Protocols. New York: Cold Spring Harbor Laboratory Press; 2000. pp. 585–599. [Google Scholar]
- Bonilla E, Contreras R, Medina-Leendertz S, Mora M, Villalobos V, Bravo Y. Minocycline increases the life span and motor activity and decreases lipid peroxidation in manganese treated Drosophila melanogaster. Toxicology. 2012;294:50–53. doi: 10.1016/j.tox.2012.01.016. [DOI] [PubMed] [Google Scholar]
- Botella JA, Bayersdorfer F, Gmeiner F, Schneuwly S. Modelling Parkinson's disease in Drosophila. Neuromolecular medicine. 2009;11:268–280. doi: 10.1007/s12017-009-8098-6. [DOI] [PubMed] [Google Scholar]
- Brand AH, Manoukian AS, Perrimon N. Ectopic expression in Drosophila. Methods in cell biology. 1994;44:635–654. doi: 10.1016/s0091-679x(08)60936-x. [DOI] [PubMed] [Google Scholar]
- Duffy JB. GAL4 system in Drosophila: a fly geneticist's Swiss army knife. Genesis. 2002;34:1–15. doi: 10.1002/gene.10150. [DOI] [PubMed] [Google Scholar]
- Guarnieri DJ, Heberlein U. Drosophila melanogaster, a genetic model system for alcohol research. Int Rev Neurobiol. 2003;54:199–228. doi: 10.1016/s0074-7742(03)54006-5. [DOI] [PubMed] [Google Scholar]
- Gupta SC, Siddique HR, Mathur N, Mishra RK, Mitra K, Saxena DK, Chowdhuri DK. Adverse effect of organophosphate compounds, dichlorvos and chlorpyrifos in the reproductive tissues of transgenic Drosophila melanogaster: 70kDa heat shock protein as a marker of cellular damage. Toxicology. 2007;238:1–14. doi: 10.1016/j.tox.2007.05.017. [DOI] [PubMed] [Google Scholar]
- Hartenstein V. Atlas of Drosophila Development. New York: Cold Spring Harbor Press; 1993. [Google Scholar]
- Hirsch HV, Mercer J, Sambaziotis H, Huber M, Stark DT, Torno-Morley T, Hollocher K, Ghiradella H, Ruden DM. Behavioral effects of chronic exposure to low levels of lead in Drosophila melanogaster. Neurotoxicology. 2003;24:435–442. doi: 10.1016/S0161-813X(03)00021-4. [DOI] [PubMed] [Google Scholar]
- Li JS, Li J. Major chorion proteins and their crosslinking during chorion hardening in Aedes aegypti mosquitoes. Insect biochemistry and molecular biology. 2006;36:954–964. doi: 10.1016/j.ibmb.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay TF, Anholt RR. Of flies and man: Drosophila as a model for human complex traits. Annu Rev Genomics Hum Genet. 2006;7:339–367. doi: 10.1146/annurev.genom.7.080505.115758. [DOI] [PubMed] [Google Scholar]
- Matthews K. Care and Feeding of Drosophila melanogaster. In: Goldstein LSB, Fryberg EA, editors. Drosophila melanogaster: Practical uses in cell and molecular biology. Vol. 44. New York: Academic Press; 1994. pp. 13–32. [DOI] [PubMed] [Google Scholar]
- Misra JR, Horner MA, Lam G, Thummel CS. Transcriptional regulation of xenobiotic detoxification in Drosophila. Genes & development. 2011;25:1796–1806. doi: 10.1101/gad.17280911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz JG, Opoka R, Kane D, Cartwright IL. Investigating arsenic susceptibility from a genetic perspective in Drosophila reveals a key role for glutathione synthetase. Toxicol Sci. 2009;107:416–426. doi: 10.1093/toxsci/kfn192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel N. Imaging Neuronal Subset and other Cell Types in Whole Mount Drosophila Embryos and Larvae using Antibody Probes. In: LSB G, EA F, editors. Drosophila melanogaster: Practical uses in cell and molecular biology. Vol. 44. New York: Acedemic Press; 1994. pp. 445–487. [DOI] [PubMed] [Google Scholar]
- Posgai R, Cipolla-McCulloch CB, Murphy KR, Hussain SM, Rowe JJ, Nielsen MG. Differential toxicity of silver and titanium dioxide nanoparticles on Drosophila melanogaster development, reproductive effort, and viability: size, coatings and antioxidants matter. Chemosphere. 2011;85:34–42. doi: 10.1016/j.chemosphere.2011.06.040. [DOI] [PubMed] [Google Scholar]
- Rand MD. A method of permeabilization of Drosophila embryos for assays of small molecule activity. Journal of Visualized Experiments. 2014 doi: 10.3791/51634. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand MD, Dao JC, Clason TA. Methylmercury disruption of embryonic neural development in Drosophila. Neurotoxicology. 2009;30:794–802. doi: 10.1016/j.neuro.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand MD, Kearney AL, Dao J, Clason T. Permeabilization of Drosophila embryos for introduction of small molecules. Insect biochemistry and molecular biology. 2010;40:792–804. doi: 10.1016/j.ibmb.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand MD, Lowe JA, Mahapatra CT. Drosophila CYP6g1 and its human homolog CYP3A4 confer tolerance to methylmercury during development. Toxicology. 2012;300:75–82. doi: 10.1016/j.tox.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang TK, Jackson GR. Drosophila models of neurodegenerative disease. NeuroRx. 2005;2:438–446. doi: 10.1602/neurorx.2.3.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strecker TR, McGhee S, Shih S, Ham D. Permeabilization, staining and culture of living Drosophila embryos. Biotech Histochem. 1994;69:25–30. doi: 10.3109/10520299409106257. [DOI] [PubMed] [Google Scholar]
- Sykiotis GP, Bohmann D. Stress-activated cap'n'collar transcription factors in aging and human disease. Science signaling. 2010;3:re3. doi: 10.1126/scisignal.3112re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserkort R, Koller T. Screening toxic effects of volatile organic compounds using Drosophila melanogaster. Journal of applied toxicology: JAT. 1997;17:119–125. doi: 10.1002/(sici)1099-1263(199703)17:2<119::aid-jat415>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]