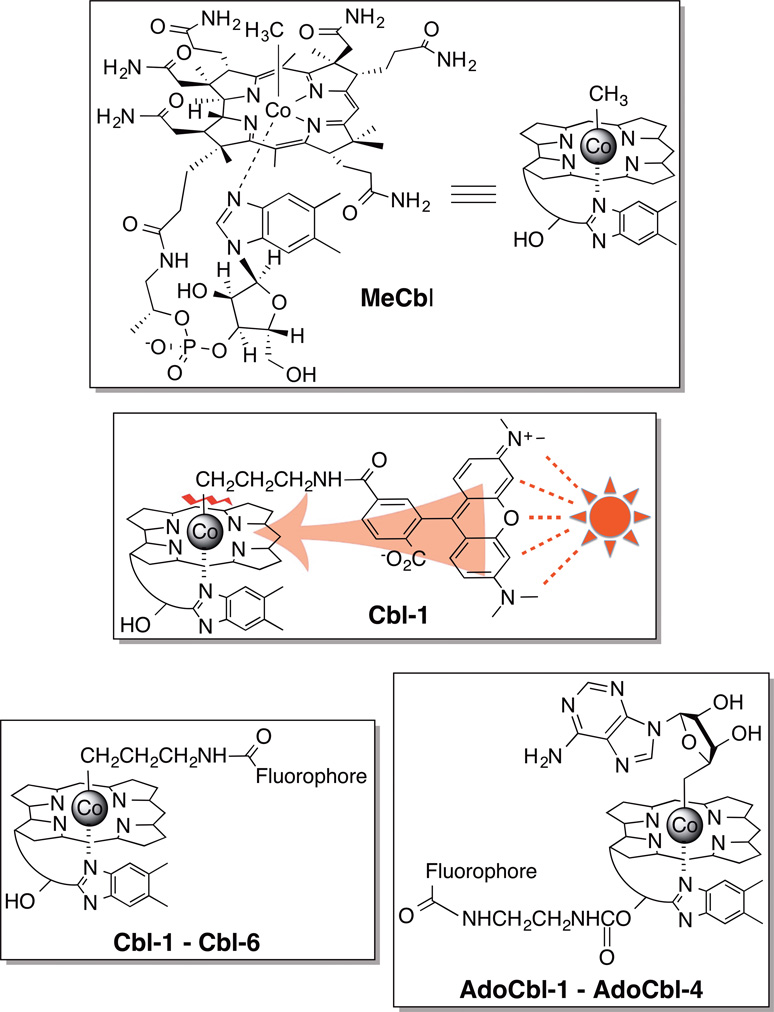
Photo-responsive species are used to manipulate intracellular biochemical pathways, light-sensitive nanoparticles employed to site-selectively deliver cytotoxic agents, and photo-initiated hydrogels applied as biomaterial replacements.[1] However, the application of these molecular entities is dependent upon short wavelengths (<450 nm), which inflict biological damage and are unable to take advantage of the optical window of tissue (600 – 1300 nm).[2] Furthermore, the narrow wavelength-response range limits the ability to design a family of photo-activatable species that can be orthogonally triggered at distinct wavelengths.[3] We recently reported expansion of the photo-responsive toolkit to green light (approximately 560 nm),[4] however these compounds do not absorb light that falls within the optical window of tissue. With these challenges in mind, we report herein wavelength tunable photo-responsive species based on the vitamin B12 cobalamin ring framework. These species are easily encoded, using commercially available fluorophores,[5] to respond to specific wavelengths throughout the visible spectrum and into the near IR.
A fluorescence increase is observed upon photolysis of a 5-carboxytetramethyl-rhodamine (TAMRA)-substituted cobalamin (Cbl-1) due to the ability of cobalamin to quench the fluorescence of attached fluorophores (Figure 1).[4, 6] The latter is a likely consequence of contact quenching between the fluorophore and the corrin ring system.[6b] Contact quenching, unlike fluorescence energy resonance transfer, does not require an overlap between the emission and absorption wavelengths of the fluorophore and quencher, respectively, for energy transfer to transpire. Given the fact that the Co-alkyl bond is weak (<30 kcal/mol),[7] it occurred to us that fluorophores excited at wavelengths beyond that absorbed by cobalamin (>560 nm) could transfer their excited state energy to the corrin ring and thus promote Co-C bond scission (Fig. 1). Indeed, Cbl-1 suffers a photolysis conversion rate (8.0 ± 0.4 × 10−3 s−1) twice that of its non-fluorophore-containing counterparts, MeCbl and Cbl-2 (3.8 ± 0.2 × 10−3 s−1 and 4.2 ± 0.2 × 10−3 s−1, respectively) (Figs. 1, S1 – S4), suggesting that excitation of an appended fluorophore could play a role in promoting photocleavage of the Co-C bond.
Figure 1.
Methylcobalamin (MeCbl) and cobalamin-fluorophore conjugates: Cbl-1 (TAMRA), Cbl-2 (Acetyl), Cbl-3 (SulfoCy5), Cbl-4 (Atto725), Cbl-5 (Dylight 800), Cbl-6 (Alexa700), AdoCbl-1 (TAMRA), AdoCbl-2 (SulfoCy5), AdoCbl-3 (Atto725), AdoCbl-4 (Dylight800).
Several fluorophores were appended to the alkylcobalamin framework, all with excitation wavelengths longer than that absorbed by the corrin ring (Cbl-2 – Cbl-6). A number of features are apparent from the results presented in Table 1. First, illumination at the corrin absorption wavelength (546 nm) completely converts all derivatives to their photolyzed products if sufficient light exposure is applied (25 min under the conditions described in the Supplementary Information). However, at a much shorter illumination time (5 min), Cbl-1 is more completely photolyzed at 546 nm than its counterparts, presumably due to the significant TAMRA absorbance in this region (ε = 90,000 cm−1 M−1). Second, all compounds undergo photolysis at their excitation wavelengths, even at those wavelengths that are far beyond the absorbance of the corrin ring. The cobalamin-SulfoCy5 conjugate Cbl-3 (λex 650 nm, λem 660 nm), when illuminated at 646 ± 10 nm, is photocleaved to hydroxocobalamin (B12a) and SulfoCy5 products as assessed by UV-Vis (Fig. S5) and LC/MS (Table S2, Scheme S7). The Atto725 (Cbl-4, λex 730 nm, λem 750 nm) and Dylight800 (Cbl-5, λex 775 nm, λem 794 nm) conjugates likewise undergo photolysis at wavelengths absorbed by the appended fluorophores, namely 730 ± 10 and 780 ± 10 nm, respectively (Figs. S6 – S7, Tables S3 and S4). Third, all cobalamin-fluorophore conjugates are stable in the dark. Fourth, exposure of these conjugates to wavelengths that they do not absorb has no effect on their structural integrity. The TAMRA derivative, Cbl-1, is stable at 646 nm and longer (Table 1). Cbl-3, with an appended fluorophore that absorbs 646 nm light, is unaffected by 727 and 777 nm illumination.
Table 1.
Percent photolysis of cobalamin-fluorophore conjugate upon illumination at 546 nm (5 min), 646 nm (5 min), 700/727 nm (20 min) and 777 nm (10 min). Percent photolysis was calculated using the maximal observed fluorescent increase as 100% photolysis (see Figs S8, S10, S12, S14, S17, and S24).
| Cobalamin Conjugate |
Fluorophore | λab/ex (nm) [a] | Percent Photolysis at Applied Wavelength (nm) | |||
|---|---|---|---|---|---|---|
| 546 | 646 | 700/727[b] | 777 | |||
| Cbl-1 | TAMRA | 546 | 100 ± 17 | [c] | [c] | [c] |
| Cbl-3 | SulfoCy5 | 546, 646* | 13 ± 21 | 100 ± 21 | [c] | [c] |
| Cbl-4 | Atto725 | 546, 646, 727*, 777 | 62 ± 8 | 35 ± 5 | 100 ± 5 | 9 ± 5 |
| Cbl-5 | Dylight800 | 546, 727, 777* | 22 ± 12 | [c] | 20 ± 12 | 100 ± 12 |
| Cbl-6 | Alexa700 | 546, 646, 700* | 56 ± 3 | 48 ± 5 | 100 ± 3 | [c] |
| Cbl-Bod | Bodipy650 | 546, 646* | 48 ±12 | 100 ± 12 | [c] | [c] |
Absorbance/excitation of the cobalamin-fluorophore conjugate where * is the major fluorophore excitation band.
All compounds were photolyzed at 727 nm, except Cbl-6, which was photolyzed at 700 nm (3 min).
<5% photolyzed under the experimental conditions.
The results in Table 1 suggest that it should be possible to selectively photolyze specific compounds from a mixture of cobalamin-substituted derivatives by employing the appropriate illumination sequence and/or choice of wavelengths. Due to the slight photolysis observed for Cbl-4 at 777 nm, the latter was replaced with the Alexa700-appended conjugate Cbl-6, which lacks an absorption band at 777 nm. Only Cbl-5 photolysis occurs when illuminating a mixture of Cbl-5, Cbl-6, Cbl-3, Cbl-1 at 777 nm [Fig. 2(a)]. Subsequent exposure of the mixture to 700 nm elicits the conversion of Cbl-6 to its photoproducts without affecting Cbl-3 or Cbl-1 [Fig. 2(b)]. A final sequential illumination at 646 and 546 nm [Figs. 2(c) and 2(d)] furnishes the stepwise photolysis of Cbl-3 and Cbl-1, respectively. As expected, 546 nm illumination of a mixture of these Cbl derivatives failed to furnish selective photolysis, which is predictable based on the data in Table 1. By contrast, since Cbl-3 and Cbl-5 are photochemically distinct at 646 nm and 777 nm (Table 1, Fig. S19), they can be individually photo-manipulated without the need to resort to a specific illumination sequence (Fig. S20). The more than 200 nm bandwidth of the cobalamin-fluorophore conjugates in Table 1 furnishes the spectral range upon which specific compounds in a mixture can be orthogonally communicated with and acted upon. Indeed, since the dissociation energy of the Co-C bond is estimated to be less than 30 kcal/mol,[7] it may be feasible to sensitize bond cleavage up to and beyond 1000 nm.
Figure 2.
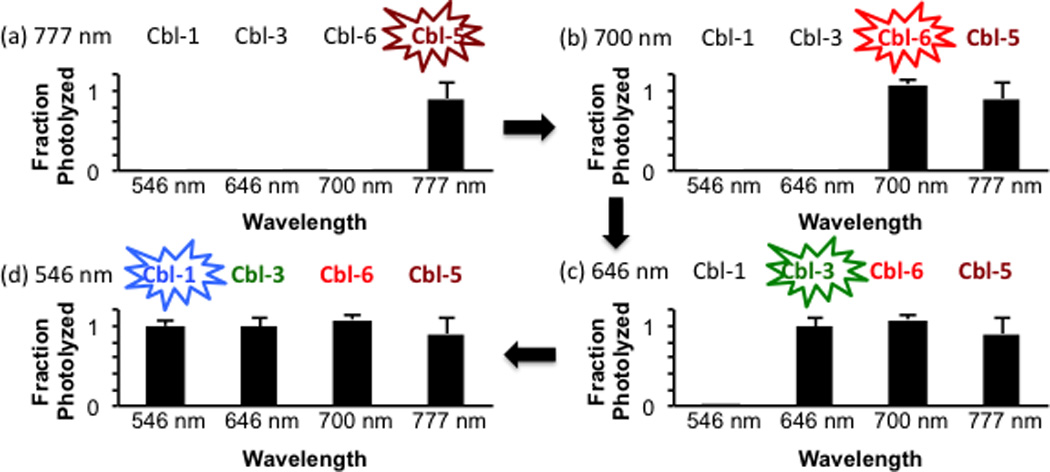
Selective photolysis of individual cobalamin-conjugates from a mixture using serial illumination [(a) 777 nm → (b) 700 nm → (c) 646 nm → (d) 546 nm] sequentially photolyzes Cbl-5, Cbl-6, Cbl-3, and Cbl-1, respectively.
Fluorophores appended to the ribose 5′-OH of alkylcobalamins also promote photocleavage of the Co-alkyl linkage (Figure 1, AdoCbl-1 – AdoCbl-4). Our initial studies employed coenzyme B12 (AdoCbl) derivatives since Schwartz and Frey previously identified the adenosine products of photolysis.[8] LC/MS confirmed that 546 nm illumination of AdoCbl (no fluorophore) and AdoCbl-1 (TAMRA) generates the expected products (Tables S6 – S7). Furthermore, both AdoCbl and AdoCbl-1 are resistant to photolysis at wavelengths beyond 600 nm. In a fashion analogous to the behavior displayed by the Cbl-3 – Cbl-6 series, the fluorophore-substituted AdoCbl-2 (SulfoCy5), AdoCbl-3 (Atto725), and AdoCbl-4 (Dylinght800) derivatives produce the anticipated adenosine products at the wavelengths absorbed by their appended fluorophores (Tables S8 – S10). In short, photolytic release of compounds attached to the Co of cobalamin is tunable, in a predictable fashion, using commercially available fluorophores appended to either the ribose ring or the metal ligand.
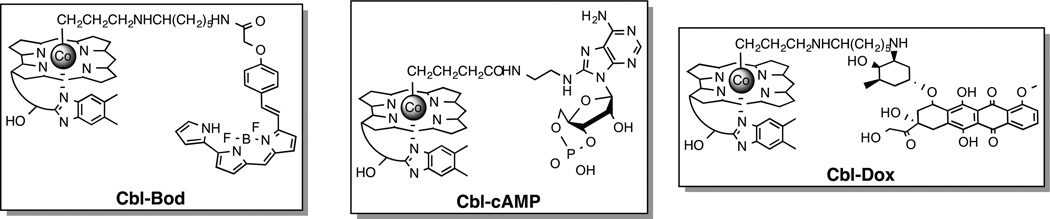
Light-responsive small molecules are commonly prepared by covalently modifying a functional group essential for biological activity with a photocleavable moiety,[1] whereas genetically expressed light-responsive proteins are typically designed by engineering a link between light-induced conformational changes and biochemical activities.[9] Cobalamins offer a potentially attractive alternative since conjugates of the latter appear to be retained by endosomes.[10] There has been a herculean effort to create delivery strategies that avoid these organelles since endosomally-embedded bioactive species are unable to interact with their intracellular targets. This liability represents an opportunity for cobalamin conjugates: cell permeable bioactive species can potentially be endosomally sequestered, which should preclude their action until released by light. With this in mind, the Bodipy650 derivative Cbl-Bod was prepared (Fig. 3, Schemes S2 – S4). Bodipy650 is cytotoxic by virtue of a mitochondria-targeting based mechanism.[11] However, the corresponding Cbl-Bod conjugate, unlike Bodipy650, is endosomal as judged by an endosomal marker (Pearson’s coefficient 0.89; Fig. 4). Furthermore, it remains endosomally sequestered even after 5 h in the dark. Illumination of cells containing Cbl-Bod at 650 nm furnishes a fluorescent increase (230 ± 6%, Figs. S26 – S27) similar to that observed in the spectrofluorometer (220 ± 30%), signaling liberation of the Bodipy650 from the quenching effect of the cobalamin. Under these conditions, Bodipy650 is simultaneously transferred from endosomes to mitochondria, as assessed by the mitochondrial marker, Mitotracker Green (Pearson’s coefficient: 0.81). This supports the notion that endosomal capture of cobalamin derivatives can be exploited as a means to sequester cell-permeable bioactive agents from their intracellular targets. As an aside, it should be feasible to employ non-endosomal strategies for sequestration and release of light sensitive cobalamin derivatives as well.[12]
Figure 3.
The Bodipy650-, cAMP-, and doxorubicin-cobalamin conjugates Cbl-Bod, Cbl-cAMP, and Cbl-Dox, respectively.
Figure 4.
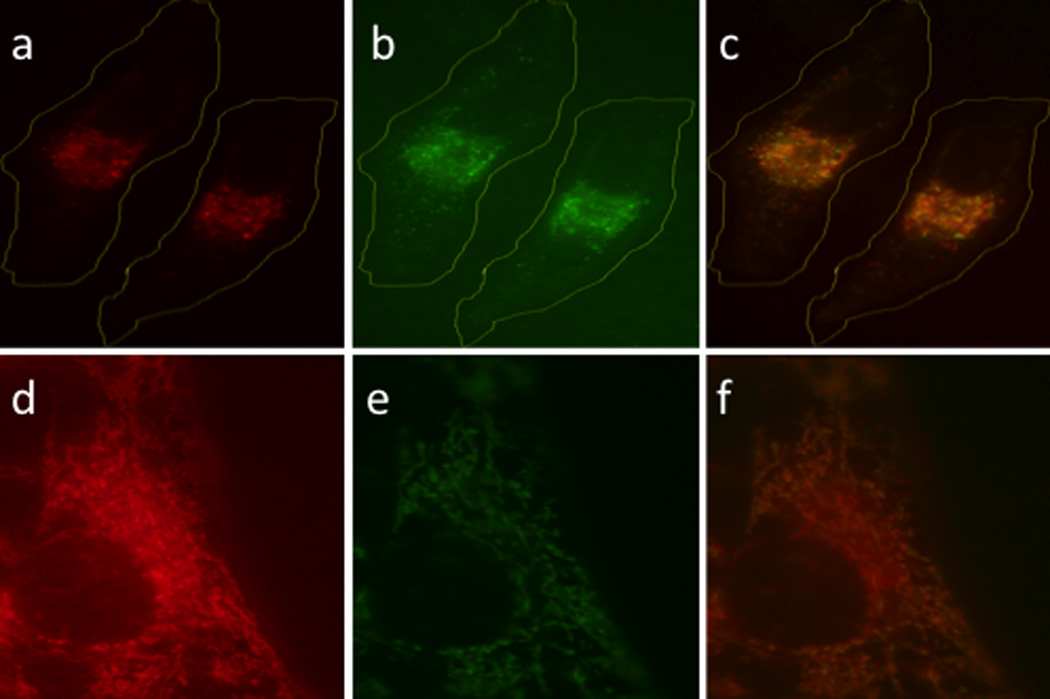
Red light induced translocation of Bodipy 650 in HeLa cells. (a) Cbl-Bod before photolysis (b) Rhodamine B-dextran endosomal marker (c) overlay of (a) and (b). (d) Cbl-Bod after photolysis (e) MitoTracker Green mitochondria marker (f) overlay of (d) and (e).
We examined the use of light to control the activity of a cAMP-dependent protein kinase (PKA) signaling pathway. A cobalamin-appended analog of cAMP, Cbl-cAMP, was prepared (Fig. 3, Scheme S10). cAMP-induced activation of PKA is known to promote dramatic morphological changes in cells, including loss of stress fibers and cell-rounding.[13] In the absence of illumination, Cbl-cAMP has no effect on the morphology of REF52 cells. Upon illumination in the presence of Cbl-cAMP, REF52 cells undergo the expected loss of their stress fiber content as well as cell shrinking and rounding (Fig. 5). In addition, an 8-substituted acetylated-cAMP analog (Ac-cAMP, Scheme S9) was prepared and used as a control. Both Ac-cAMP and a commercially available cell-permeable cAMP analog [8-(4-chloro-phenylthio)-2’-O-methyladenosine-3’,5’-cyclic monophosphate; CPT-cAMP] are active in the absence of light, producing morphological effects on REF52 cells analogous to those displayed by Cbl-cAMP upon illumination (Fig. S28). These results demonstrate that the cobalamin in Cbl-cAMP precludes cytosolic penetration by an appended cAMP derivative (cf. Ac-cAMP is cell permeable) and that illumination releases cAMP from its cobalamin constraint.
Figure 5.
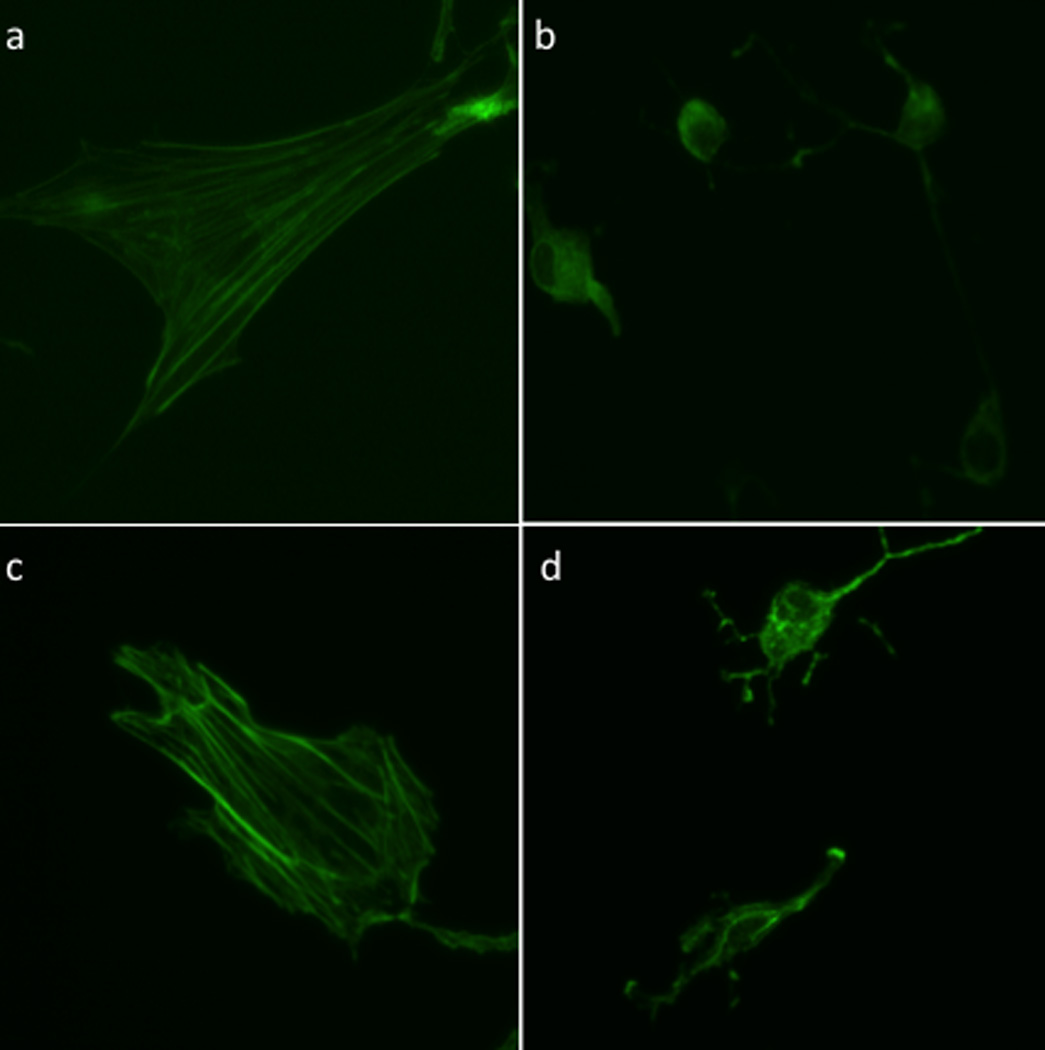
Light-dependent Cbl-cAMP-mediated rearrangement of actin cytoskeleton in rat embryonic fibroblasts (REF-52). Ref-52s were exposed to Cbl-cAMP, illuminated at 530 nm, and stained with Dylight 488-phalloidin to visualize the actin stress fibers. (a) No treatment, media alone (b) CPT-cAMP as a positive control (c) Cbl-cAMP, no illumination (d) Cbl-cAMP with illumination. Images are representative of 10 – 20 cells.
There is significant interest in the use of light to site-selectively deliver therapeutic agents in vivo. For example, in the arena of chemotherapy, strategies that limit cytotoxicity to targeted regions offer the promise of reduced side effects and the application of otherwise too-toxic-to-use agents. Doxorubicin highlights this need since this valuable anticancer agent displays severe cardiotoxicity.[14] We examined the cytotoxicity of the cobalamin-doxorubicin conjugate Cbl-Dox (Fig. 3, Scheme S11) in HeLa cells, illumination times (Fig. 6). Light-only treatment, or Cbl-Dox exposure in the absence of photolysis, has no effect on cell viability. In addition, Cbl-Dox exposure in the absence of illumination is completely innocuous. By contrast, Cbl-Dox exposure with increasing illumination time furnishes a light dose-dependent increase in cell death that ultimately recapitulates that produced by doxorubicin alone.
Figure 6.
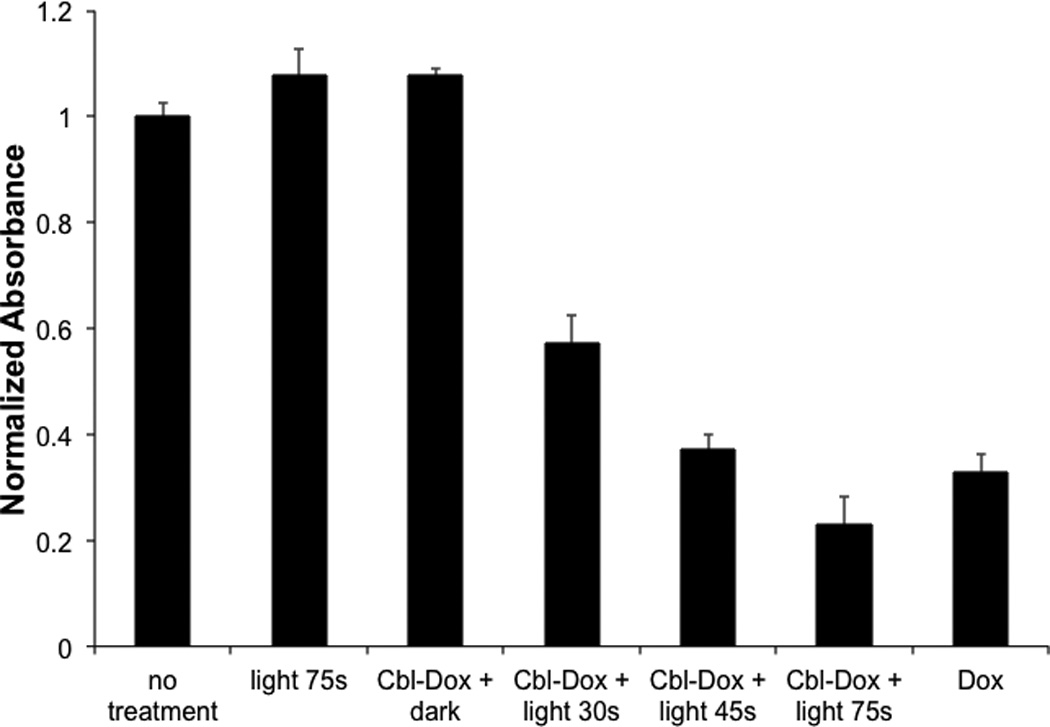
Light-induced release of doxorubicin from Cbl-Dox induces apoptosis of HeLa cells. Cells were loaded with 10 µM Cbl-Dox and illuminated for 30, 45, and 75 s at 530 nm. Cell viability was assessed, after 24 h, via an MTS assay (absorbance measured at 520 nm and normalized to the untreated control). The data shown are the average of three experiments ± SEM.
The chemistry of photo-responsive agents has been, for the most part, limited to long UV/short visible wavelengths (300 – 450 nm).[1,3,4] This narrow region of the electromagnetic spectrum (a) lacks the desired tissue-penetrating depth displayed by 650 – 1000 nm wavelengths, (b) inflicts bio-molecular damage which can have undesirable short and long term in vivo consequences, and (c) can support only a limited number of orthogonal wavelength-responsive agents. Although the corrin ring of B12 is unable to efficiently absorb light beyond 550 nm, we found that commercially available fluorophores can be used as antennas to capture long wavelength light, which promotes scission of the Co-C bond at wavelengths up to 800 nm. Indeed, since the dissociation energy of this bond is estimated to be less than 30 kcal/mol,[7] it may be feasible to sensitize bond cleavage up to and beyond 1000 nm. In summary, the ability to control the molecular properties of bioactive species with long visible and near-IR light has implications in drug delivery, material fabrication, nanotechnology, and the spatiotemporal control of cellular behavior.
Experimental Section
See the online Supporting Information for Materials and Methods, Tables, additional Figures, and Schemes
Supplementary Material
Acknowledgments
We thank the National Institutes of Health for financial support (RO1 CA79954).
Footnotes
Supporting information for this article is available on the WWW under http://www.angewandte.org or from the author.
References
- 1.(a) Brieke C, Rohrbach F, Gottschalk A, Mayer G, Heckel A. Angew Chem Int Ed Engl. 2012;51:8446–8476. doi: 10.1002/anie.201202134. [DOI] [PubMed] [Google Scholar]; (b) Klan P, Solomek T, Bochet CG, Blanc A, Givens R, Rubina M, Popik V, Kostikov A, Wirz J. Chem Rev. 2013;113:119–191. doi: 10.1021/cr300177k. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Lee HM, Larson DR, Lawrence DS. ACS Chem Biol. 2009;4:409–427. doi: 10.1021/cb900036s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tromberg BJ, Shah N, Lanning R, Cerussi A, Espinoza J, Pham T, Svaasand L, Butler J. Neoplasia. 2000;2:26–40. doi: 10.1038/sj.neo.7900082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.(a) Goguen BN, Aemissegger A, Imperiali B. J Am Chem Soc. 2011;133:11038–11041. doi: 10.1021/ja2028074. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Hagen V, Dekowski B, Nache V, Schmidt R, Geissler D, Lorenz D, Eichhorst J, Keller S, Kaneko H, Benndorf K, Wiesner B. Angew Chem Int Ed Engl. 2005;44:7887–7891. doi: 10.1002/anie.200502411. [DOI] [PubMed] [Google Scholar]; (c) Kantevari S, Matsuzaki M, Kanemoto Y, Kasai H, Ellis-Davies GC. Nat Methods. 2010;7:123–125. doi: 10.1038/nmeth.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Menge C, Heckel A. Org Lett. 2011;13:4620–4623. doi: 10.1021/ol201842x. [DOI] [PubMed] [Google Scholar]; (e) Priestman MA, Sun L, Lawrence DS. ACS Chem Biol. 2011;6:377–384. doi: 10.1021/cb100398e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Priestman MA, Shell TA, Sun L, Lee HM, Lawrence DS. Angew Chem Int Ed Engl. 2012;51:7684–7687. doi: 10.1002/anie.201202820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lavis LD, Raines RT. ACS Chem Biol. 2008;3:142–155. doi: 10.1021/cb700248m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(a) Jacobsen DW. Methods Enzymol. 1980;67:12–19. doi: 10.1016/s0076-6879(80)67005-0. [DOI] [PubMed] [Google Scholar]; (b) Lee M, Grissom CB. Org Lett. 2009;11:2499–2502. doi: 10.1021/ol900401z. [DOI] [PubMed] [Google Scholar]; (c) Rosendahl MS, Omann GM, Leonard NJ. Proc Natl Acad Sci U S A. 1982;79:3480–3484. doi: 10.1073/pnas.79.11.3480. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Smeltzer CC, Cannon MJ, Pinson PR, Munger JD, Jr, West FG, Grissom CB. Org Lett. 2001;3:799–801. doi: 10.1021/ol006825v. [DOI] [PubMed] [Google Scholar]
- 7.(a) Halpern J, Kim SH, Leung TW. J Am Chem Soc. 1984;106:8317–8319. [Google Scholar]; (b) Kozlowski PM, Kumar M, Piecuch P, Li W, Bauman NP, Hansen JA, Lodowski P, Jaworska M. J Chem Theo Comp. 2012;8:1870–1894. doi: 10.1021/ct300170y. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz PA, Frey PA. Biochemistry. 2007;46:7284–7292. doi: 10.1021/bi700077v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moglich A, Moffat K. Photochem Photobiol Sci. 2010;9:1286–1300. doi: 10.1039/c0pp00167h. [DOI] [PubMed] [Google Scholar]
- 10.(a) Bagnato JD, Eilers AL, Horton RA, Grissom CB. J Org Chem. 2004;69:8987–8996. doi: 10.1021/jo049953w. [DOI] [PubMed] [Google Scholar]; (b) Gupta Y, Kohli DV, Jain SK. Crit Rev Ther Drug Carrier Syst. 2008;25:347–379. doi: 10.1615/critrevtherdrugcarriersyst.v25.i4.20. [DOI] [PubMed] [Google Scholar]
- 11.(a) Awuah SG, You Y. RSC Advances. 2012;2:11169–11183. [Google Scholar]; (b) Kamkaew A, Lim SH, Lee HB, Kiew LV, Chung LY, Burgess K. Chem Soc Rev. 2013;42:77–88. doi: 10.1039/c2cs35216h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nguyen LT, Oien NP, Allbritton NL, Lawrence DS. Angew Chem Int Ed Engl. 2013;52:9936–9939. doi: 10.1002/anie.201305510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oishi A, Makita N, Sato J, Iiri T. J Biol Chem. 2012;287:38705–38715. doi: 10.1074/jbc.M112.401547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.(a) Patil RR, Guhagarkar SA, Devarajan PV. Crit Rev Ther Drug Carrier Syst. 2008;25:1–61. doi: 10.1615/critrevtherdrugcarriersyst.v25.i1.10. [DOI] [PubMed] [Google Scholar]; (b) Volkova M, Russell R., 3rd Curr Cardiol Rev. 2011;7:214–220. doi: 10.2174/157340311799960645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.(a) Bochet CG. Angew Chem Int Ed Engl. 2001;40:2071–2073. doi: 10.1002/1521-3773(20010601)40:11<2071::AID-ANIE2071>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]; (b) Kotzur N, Briand B, Beyermann M, Hagen V. J Am Chem Soc. 2009;131:16927–16931. doi: 10.1021/ja907287n. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.