Abstract
Background
BMT CTN 1101 is a Phase III randomized controlled trial evaluating the comparative effectiveness of double unrelated umbilical cord blood (dUCB) versus HLA-haploidentical related donor bone marrow (haplo-BM) donor cell sources for blood or bone marrow transplantation (BMT) in patients with hematologic malignancies. Herein, we present the rationale, design and methods of the first cost–effectiveness analysis to be conducted alongside a BMT trial.
Methods
Consenting patients will provide health insurance information to allow calculation of direct medical costs from reimbursement records, and will provide out-of-pocket costs, time costs and health-related quality of life measures through an online survey. These outcomes will inform a cost–effectiveness analysis comparing dUCB and haplo-BM donor cell sources from patient, payer and societal perspectives.
Conclusion
Novel approaches may significantly change the cost, outcomes or availability of BMT. The results of this analysis will be the first to provide a comprehensive evaluation of the comparative effectiveness of these approaches from multiple perspectives.
Keywords: blood and bone marrow transplant, cost–effectiveness, Phase III, randomized controlled trial
Reduced intensity conditioning (RIC) prior to blood or bone marrow transplantation (BMT) has allowed many older and less clinically fit patients with high-risk or advanced hematologic malignancies to receive a potentially curative treatment with allogeneic transplantation [1–5]. Currently, standard practice involves transplantation from a human leukocyte antigen (HLA)-matched sibling or a suitably HLA-matched unrelated donor; however, more than a third of patients do not have a match. Unfortunately, because an unrelated donor search can take up to 4 months [6], many patients succumb to their disease while awaiting identification of a suitably matched donor [7].
New sources for donor cells for RIC BMT, such as bone marrow from HLA-haploidentical family members (haplo-BM), or unrelated double umbilical cord blood (dUCB), may permit timelier BMT for adult patients lacking suitably related or unrelated donors [8–12]. However, the comparative effectiveness of RIC transplantation from haplo-BM relative to dUCB remains to be determined [8]. Accordingly, the National Heart Blood and Lung Institute [13] recently funded a Phase III randomized trial through the BMT Clinical Trials Network (BMT CTN 1101). The primary hypothesis is that progression-free survival will be similar in patients receiving dUCB and haplo-BM transplantation.
Because BMT is costly, advances that extend this option to a larger number of patients with leukemia and lymphoma have important economic consequences. In addition, the acquisition cost of the dUCB graft is significantly higher than haplo-BM, and potentially prohibitive for some patients [12,14,15]. There is also uncertainty about the relative rates of hematopoietic recovery and adverse events, both of which could have clinically meaningful impacts on resource use and quality of life. These factors provide a compelling rationale to conduct a cost–effectiveness analysis (CEA) to evaluate the economic value of dUCB transplantation given its higher cost versus haplo-BM transplantation.
Below, we describe the design of a CEA being conducted alongside BMT CTN 1101 to address these questions. Our study will be the first to provide a high-quality, comprehensive evaluation of the comparative effectiveness of different BMT transplantation graft sources from patient, payer and societal perspectives. Importantly, we will measure a variety of outcomes that can influence decision-making: health insurer costs, patient out-of-pocket costs, lost work productivity and health-related quality of life.
Methods
Study design
BMT CTN 1101 is a Phase III, randomized, multicenter trial designed to evaluate the comparative effectiveness of two graft sources for adult patients requiring alternative donor BMT: first, dUCB; and second, haplo-BM. The trial is being conducted at 37 centers across the USA, has target accrual of 410 patients over 4 years, and involves 3-year follow-up after transplantation [13]. The primary hypothesis of the parent trial is that 2-year progression-free survival is similar after related haplo-BM donor transplantation and dUCB transplantation. Secondary end points include evaluation of treatment-related mortality, relapse and economic impacts.
The economic analysis will be conducted as a cost–utility analysis from the societal perspective. The summary result of this analysis is an estimate of the cost–effectiveness of dUCB versus haplo-BM, stated as the incremental cost per quality-adjusted life year (QALY) gained. Details of this calculation are provided below.
The hypotheses of this ancillary cost–effectiveness analysis are: total direct medical care costs will be significantly greater for patients who receive dUCB compared with those who receive haplo-BM donor cells; out-of-pocket and indirect costs (lost earnings due to illness and its treatment) will be significantly greater for the families of patients transplanted with dUCB compared with those transplanted using haplo-BM donor cells; and dUCB transplantation is not cost-effective relative to Haplo-BM transplantation (from societal and payer perspectives).
The patient and caregiver will be asked to provide information on out-of-pocket costs, work productivity and health-related quality of life at baseline (pretransplant) and during follow-up.
This ancillary cost–effectiveness study was approved by the Fred Hutchinson Cancer Research Center institutional review board and by all participating centers.
Participants
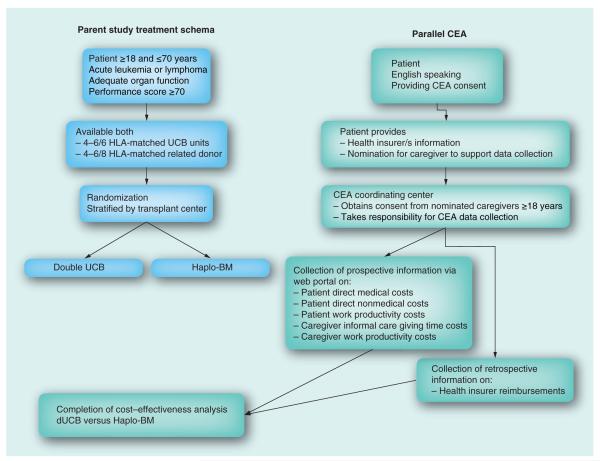
The BMT CTN 1101 study has a target recruitment of 410 patients. This recruitment target is feasible given that the participating centers performed approximately 1800 allogeneic mismatched related or unrelated blood transplantations for patients aged 18–70 years with a hematologic malignancy in 2011, and a similar number in 2012. Trial eligibility criteria include the following: between the ages of 18 and 70 years; diagnosis of a hematologic malignancy; two partially HLA-matched UCB units (mismatched at up to two of six loci including HLA-A, -B, -DRB1) and a partially HLA-mismatched related donor (mismatched at up to four of eight loci including HLA-A, -B, -C, -DRB1), and at least 6 months since a previous autologous transplant. All English-speaking patients will be eligible for the CEA. Patients will be asked to nominate up to two adult, English-speaking caregivers for participation (Figure 1).
Figure 1. BMT CTN 1101 and parallel cost–effectiveness analysis study schema.
CEA: Cost–effectiveness analysis; dUCB: Double unrelated umbilical cord blood; Haplo-BM: Haploidentical related donor bone marrow; UCB: Umbilical cord blood.
Measures
For the economic evaluation, we will collect the following from all study participants and their family members: direct medical care costs through health insurance reimbursements; patient and family out of pocket costs; patient time spent in treatment; family member time spent in caregiving, and health-related quality of life [16]. The time spent in treatment and care-giving will be used to evaluate differences in work productivity costs for patients and caregivers in a separate analysis [16].
Direct costs for medical care
We will use insurance reimbursement records to estimate all direct medical care costs (treatment, procedure, medication, laboratory and facility) from the payer perspective. Healthcare claims records from insurers will be requested for the period beginning 12 months prior to the date of BMT to 2 years following the date of transplantation or death (available from trial records). Insurance records will be obtained for all consenting patients regardless of outcome (i.e., for patients who remain in remission, who relapse and who die). In cases where a patient belongs to an Health Maintenance Organization (HMO) at the time of transplant (i.e., an organization with no external claims and payments), we will ask the HMO to provide reimbursement records to the transplant center where the patient was treated and for internal costing records for medical care consumed in the HMO during the period of observation. Because some patients will have multiple insurance plans and may change plans, we will ask patients periodically to update their insurance information. Insurance records will be requested towards the end of the study follow-up period.
Patient & family out-of-pocket costs
BMT-related out-of-pocket expenditure information will be collected directly from patients and caregivers nominated by the patient. Caregivers are included in the study to provide information during periods when patients are unable to participate due to illness, and to assist with recall and reconciliation of potential expenses [17].
We will capture out of pocket expenses for medical care (e.g., copays, deductibles and uncovered medical bills) and costs for related nonmedical costs (e.g., transportation, travel time and distance, accommodation, child care and telecommunication) incurred by patients and caregivers using an online adaptation of the cost diary method used by Goossens and colleagues [18].
Productivity costs: work loss related to illness & treatment
Patient and caregivers’ time spent away from work for BMT-related care will be estimated using the Work Productivity and Activity Impairment Questionnaire (WPAI) [19]. The WPAI measures work time missed as well as work and activity impairment due to a specific health issue. The value of this time will be based on Bureau of Labor Statistics information matched by age and sex.
Health-related quality of life & QALYs
Health-related quality of life will be derived from the EQ-5D (in the base case) and SF-36 surveys (in secondary analyses) administered prior to transplantation, at 12 months and at 24 months as part of the BMT CTN 1101 trial. [20,21] Both of these surveys can be used to determine utility values, which are measures of preference for health states ranging from 0 (dead) to 1 (ideal health) [22]. We will combine the utilities with survival data to calculate average QALYs [23] for each treatment arm.
Procedures for collecting economic information
Due to the intensity of BMT and the significant impacts on patients and their families, it can be difficult to measure out-of-pocket costs, work productivity and health-related quality of life. Accordingly, we attempt to minimize the burden of surveying, using a secure online portal that can be accessed from any internet-connected computer or smartphone. The website includes questions on common out-of-pocket costs, work productivity (WPAI) and informal caregiving time (using the Dumont method) [24]. There are free text sections for recording uncommon or unique costs. Per their preference, participants are reminded using e-mail, telephone or mailed post cards, to complete information at baseline (pretransplant) and each month for the first 6 months following transplantation and then every 3 months for the remaining 18 months of the study.
Statistical analysis
Estimating costs & outcomes for each treatment arm
We will compute within-trial and projected (lifetime) results, the latter using simulation modeling. Lifetime overall survival (OS) beyond the observation period will be extrapolated using different parametric survival functions (Weibull, Gompertz, exponential, log-normal and generalized gamma distributions) [25] with the base case survival function selected using the Akaike Information Criterion (AIC) [26]. Cox–Snell residuals will be plotted as a confirmatory test to identify the function with the best fit to the observed data [27]. The mean number of life-years for patients in each group will be estimated as the area under the OS curve [28]. QALYs will be estimated from OS by weighting with utility values obtained from the analysis of utility data. Utility estimates beyond the trial observation period will be based on monthly trends in utility as observed for those who survive the year following transplantation. Projected utility weights for the last 6 months of life will be based on utilities for the last 6 months of life for persons who die during the year following transplant. In cases where insufficient numbers of persons have died within 6 months of their survey, we will use patient’s pretransplant utility scores as an estimate for quality of life in the last 6 months of life.
We will partition costs beyond the study observation period into continuing care and death periods. Continuing care costs will be based on monthly trends in costs observed for those who have survived the year following transplant. Death costs, defined as costs of care during the last 6 months of life for persons who have died, will be based on costs of care observed for those who die during the year following transplant. Costs will be modeled based on projected survival.
Calculation of the incremental
cost–effectiveness ratio
The incremental cost–effectiveness ratio (ICER) of haplo-BM versus dUCB is calculated using the following formula:
Where Chaplo-BM and CdUCB refer to average total costs of each alternative, and Ehaplo-BM and EdUCB refer to average total effectiveness for each alternative. The resulting ICER represents the investment required to obtain one additional unit of effect, and can be used to quantify the value provided by alternative A versus alternative B. We will conduct cost–utility analysis; that is, with effectiveness outcomes measured as QALYs. The base case analysis is conducted from a societal perspective, evaluates costs and effects over a lifetime horizon, and discounts future costs and benefits equally (base case discount rate of 3%), as is recommended [29,30].
All analyses will be conducted using the intention-to-treat (ITT) approach. Where substantial crossover occurs, additional per protocol analyses will be completed. Our analyses will address the following hypotheses:
Hypothesis 1: total direct medical care costs will be significantly greater for patients who receive dUCB compared with those who receive haplo-BM donor cells.
Insurance reimbursement records from each participant providing health insurance information will be reviewed and aggregated into two time horizons for the cost analysis; pretransplant (conditioning and attainment of donor cells); and after-transplant care (the time following infusion of donor cells). For the base case analysis, the mean difference in disaggregate costs and total cost between patients (i.e., the incremental cost) who receive dUCB and haplo-BM will be analyzed using the Kaplan–Meier sample average estimation (KMSA) technique [31,32]. Both censoring and skewedness are addressed by the KMSA. Using cost histories from the patients in each study arm, the KMSA technique determines the mean cost (M) over the time period of interest as:
Where Si denotes the probability of the event occurring in the ith month and is the average cost among patients who are alive at the beginning of the ith month and is the estimated survival probability obtained from the Kaplan-Meier curve. Specifically, is the estimated probability of being alive at the beginning of the ith month. [33]. The design of the treatment trial is consistent with independent censoring and the time intervals incorporated into cost data collection provide appropriately narrow time intervals for the KMSA technique. To complete the analysis of direct medical care costs, a regression-based KMSA model developed by Lin will be used to account for baseline patient characteristics that could influence costs, clustering within study centers, and to evaluate the uncertainty provided by the use of these different analytic techniques as an analytic sensitivity analysis [23,34,35].
Hypothesis 2: out-of-pocket and indirect costs (lost earnings due to illness and its treatment) will be significantly greater for the families of patients transplanted with dUCB compared with those transplanted using haplo-BM donor cells.
Direct medical costs paid by patients will be based on records provided by patients and caregivers. Direct nonmedical costs will be disaggregated into transportation, accommodation, telecommunication and other costs. These costs will then be combined to calculate the total out-of-pocket costs incurred. Indirect (productivity) costs for patients and their nominated caregivers will be presented separately as will cost related to informal caregiving. Time spent by family caregivers to provide support to patients will be valued initially using the opportunity cost method, with subsequent valuation using the proxy-good method in sensitivity analyses [36].
A similar analytic approach will be used to calculate out-of-pocket and indirect costs.
Hypothesis 3 (null): dUCB transplantation is not cost effective relative to haplo-BM transplantation from societal or payer perspectives.
Total costs will be analyzed from both a societal and payer perspective [22,30]. The payer perspective considers only reimbursable direct medical care costs. The societal perspective includes direct medical care costs paid by health insurers, direct medical care and nonmedical costs paid by patients, and the value of patient and caregiver time spent during treatment and related care.
If Haplo-BM is less costly and more effective (greater QALYs) than dUCB, haplo-BM is said to dominate dUCB and no numerical estimate of incremental cost–effectiveness is required. Instead, the estimated reduction in cost and improvement in quality-adjusted survival, and the associated uncertainty in these estimates, will be reported. If haplo-BM is less costly and has equal or noninferior effectiveness to dUCB (as determined by the difference in QALYs), the estimated reduction in cost, the equivalence or non-inferiority in QALYs, and the associated uncertainty in these estimates, will be reported. If haplo-BM is more costly and more effective than dUCB, an incremental cost–effectiveness ratio will be calculated and reported with the associated uncertainty characterized (table 1).
Table 1.
Power calculations.
| Scenario | Hypotheses | Sample size (%) | Power for costs |
Power for QAL Ys |
Power for ICE Rs† |
|---|---|---|---|---|---|
| Hypothesis 1: CDMP | Ho: CDMPdUCB = CDMPHaplo-BM | 410 (100) | 0.993 | ||
| Ha: CDMPdUCB ≠ CDMPHaplo-BM | 308 (75) | 0.969 | |||
| 205 (50) | 0.876 | ||||
|
| |||||
| Hypothesis 2: COOPI | Ho: COOPIdUCB = COOPIHaplo-BM | 410 (100) | 0.999 | ||
| Ha: COOPIdUCB ≠ COOPIHaplo-BM | 308 (75) | 0.999 | |||
| 205 (50) | 0.999 | ||||
|
| |||||
| Hypothesis 3: | |||||
| cost–effectiveness analysis | Costs: | ||||
| Haplo-BM dominates dUCB | Ho: CdUCB = CHaplo-BM | ||||
| Ha: CdUCB ≠ CHaplo-BM | 410 (100) | 0.993 | 0.996 | ||
| QALYs: | 308 (75) | 0.969 | 0.981 | ||
| Ho: EdUCB = EHaplo-BM | 205 (50) | 0.876 | 0.906 | ||
| Ha: EdUCB ≠ EHaplo-BM | |||||
|
| |||||
| Haplo-BM more costly and effective than dUCB |
Ha: NB 0 | 410 (100) | 0.989/0.982 | ||
| Ha: NB / 0 | 308 (75) | 0.958/0.94 | |||
| 205 (50) | 0.852/0.818 | ||||
Parameters used in the power calculations include: difference in mean direct medical care costs (payer) between dUCB and haplo-BM (ΔC = US$142,500, SD: $300,000) – three-quarters of the difference and the same variability in costs seen in our preliminary analysis of 42 Seattle Care Alliance transplant patients; difference in mean out-of-pocket and indirect costs for families between dUCB and Haplo-BM (ΔC = $2.6 K, SD: $1.1 K) – half the difference and the same variability in costs seen in a pilot study exploring the out-of-pocket costs associated with hematopoietic cell transplantation [14]; for superiority testing, MCID in QALYs (EMCID = 0.147) based on MCID estimates of utility values provided by the EQ-5D and SF-6D [43], SD two times the MCID in QALYs (SD: EMCID = 0.294); an equivalence margin equal to plus and minus half the MCID in QALYs (EMCID/2 = 0.074) with a SD two-times this value (SD: EMCID/2 = 0.147); a similar negative noninferiority margin (-EMCID/2 = −0.074) and variance (SD: EMCID/2 = 0.147); and, for ICER power calculations, a correlation between cost and effect of 0.25.
Estimates of the power for ICERs are derived from the statistical test of whether NMB is significantly different from zero with NMB calculated as the WTP threshold times the difference in QALYs minus the difference in cost ([WTP.ΔE] – ΔC) [23].
ICER at willingness to pay thresholds of US$50,000/100,000 per QALY.
CDMP: Costs direct medical payer; COOPI: Costs out-of-pocket and indirect; dUCB: Double unrelated umbilical cord blood; Haplo-BM: Haploidentical related donor bone marrow; ICER: Incremental cost–effectiveness ratio; MCID: Minimally clinically important difference; NMB: Net monetary benefit; SD: Standard deviation; QALY: Quality-adjusted life year; WTP: Willingness to pay.
Analyses of uncertainty
In economic evaluations, uncertainty analyses seek to identify factors that are most influential to the outcome (ICER) and to characterize uncertainty around the ICER. We will conduct oneway sensitivity analyses to characterize the uncertainty around the base case results related to each parameter. We will also conduct probabilistic sensitivity analysis to evaluate the expected range of outcomes given the joint uncertainty of all model parameters. To do so, we will define distributions for all of the input parameters, draw 10,000 parameter sets from the distributions, propagate them through the algorithm and obtain the resulting 10,000 cost, QALY and ICER outcomes for analysis. We will plot the probabilistic sensitivity analysis results as cost–effectiveness acceptability curves showing the probability that dUCB and haplo-BM transplantation are cost effective at different willingness-to-pay thresholds (e.g., US$50,000–200,000 per QALY) [37].
Base year cost conversion & discounting
All costs will be adjusted to current US$ at the time of completion of the study. Direct medical costs will be adjusted using the medical consumer price index [38], and nonmedical costs will be adjusted using the US Consumer Price Index for All Urban Consumers [39]. Costs and outcomes incurred beyond 12 months from randomization will be discounted at a rate of 3% annually with sensitivity analyses completed with discount rates of 1 and 5% [30].
Sample size calculations
The targeted sample size for BMT CTN 1101 is 410, with 205 per treatment arm. Our targeted enrollment in the ancillary CEA study is 300 patients and 600 caregivers (two per patient). table 1 presents the estimated power to evaluate each specific aim and the CEA with different sample size assumptions (proportions of the parent study sample). Our calculations allow for 5% censoring due to loss to follow-up and administrative censoring, and we also anticipate that 5% of randomized patients will not receive their assigned transplant.
Discussion
RIC BMT is allowing older and less clinically fit patients to receive potentially curative treatment of their high risk or advanced hematological malignancies with allogeneic transplantation [1–5]. Haplo-BM and dUCB transplantation are new, and potentially valuable, sources of donor cells for RIC BMT for patients who lack available HLA-matched related or unrelated donors [8–12], and thus may expand both the number of eligible patients and survival by shortening waiting times to transplant. However, the relative risks and benefits of these procedures are not well established. The BMT CTN 1101 trial has been designed to determine the relative efficacy of these two donor types for adult patients requiring alternative donor BMT. The ancillary economic analysis will extend that evidence by evaluating the impact of these procedures on direct and indirect (productivity) cost, out-of-pocket burden to patients and families, and health-related quality of life. All of these issues are critical but understudied factors in patient, physician and payer decision-making. For example, many patients travel long distances to transplant centers, and they and their families incur significant financial costs associated with transportation, lodging and loss of income during the patient’s extended recovery period [14]. Additionally, while the long-term impact of BMT on patients’ employment and productivity has been reported [40,41], the short-term impact of BMT on work and income loss has not yet been studied. Since patients typically take an extended leave of absence from work during the transplant and recovery period, advances in BMT that reduce the time to recovery could have important economic benefits to family wage earners.
The design of the CEA has a number of strengths. First, conducting an economic evaluation alongside BMT CTN 1101 has high internal validity and timeliness by utilizing the existing trial structure to collect necessary data. A second important strength is that we collect resource use information directly from health insurers, which enhances the accuracy of our reimbursement estimates and allows comprehensive capture of resource use. Additionally, this study has the strength of including an evaluation of patient and caregiver out-of-pocket costs and lost work productivity during transplantation, which will provide new insights about financial burden of dUCB and haplo-BM transplantation.
Our study also has several important limitations. Most importantly, our ancillary CEA study results may be limited if few BMT CTN 1101 patients choose to enroll. However, past experience shows that patients are likely to participate in the economic component of clinical trials [42]. We may also be limited by our ability to collect insurance records from some private insurers. In this case, we will consider imputation strategies based on clinical, socioeconomic and insurance characteristics of similar trial enrollees. Additionally, patients and caregivers may have difficulty accessing the web portal to complete out-of-pocket cost and productivity surveys. If patients and caregivers are unable or unwilling to use the portal but wish to participate in the study, we will also offer support via telephone for data collection.
Conclusion
We have described the rationale and design of a cost–effectiveness analysis being conducted alongside a multi-center, Phase III, randomized comparative trial of dUCB versus haplo-BM transplantation after reduced intensity conditioning in patients with hematologic malignancies. The cost–effectiveness study will be collecting detailed health insurer, out-of-pocket and caregiver cost information to characterize the economic consequences of using these alternative sources of donor cells. This, combined with our analysis of the quality-adjusted survival outcomes, will provide a comprehensive view of the comparative effectiveness of these alternatives. If one donor cell source is less costly and at least equally effective, the results of the study will have important implications for patient, clinician and health insurer decision-making, as well as the design of future BMT clinical trials.
Future perspective
Retrospective studies have demonstrated that new procedures for blood or bone marrow transplantation from alternative donors such as unrelated umbilical cord blood or related, HLA-haploidentical donors can result in comparable outcomes to those after allogeneic transplantation from HLA-matched related or unrelated donors, currently the standard donor sources. Thus, for the approximately 40% of patients with life-threatening hematological malignancies who do not have suitably matched donors, use of cord blood or HLA-haploidentical bone marrow in allogeneic stem cell transplantation will make allogeneic stem cell transplantation a treatment option. It is well known that blood or marrow transplantation is a high-cost treatment intervention. Umbilical cord blood and adult bone marrow grafts differ with respect to cost of acquisition, time to hematologic recovery, incidence of graft-versus-host disease and spectrum of infections, all of which can significantly impact cost and treatment outcome. Because these two alternative treatment approaches have not been compared directly, many key uncertainties about their comparative effectiveness remain unanswered. The results of this cost–effectiveness analysis alongside the randomized, Phase III clinical trial of cord blood versus haploidentical bone marrow (BMT CTN 1101; NCT01597778) will provide important patient-oriented and economic outcomes for patients and clinicians, as well as help health insurers make informed coverage policy decisions regarding these options.
Executive summary.
Expanding access to allogeneic stem cell transplantation
 Reduced intensity conditioning (RIC) prior to blood or bone marrow transplantation (BMT) has allowed many older and less clinically fit patients with high-risk or advanced hematologic malignancies to receive potentially curative treatment with allogeneic stem cell transplantation.
Reduced intensity conditioning (RIC) prior to blood or bone marrow transplantation (BMT) has allowed many older and less clinically fit patients with high-risk or advanced hematologic malignancies to receive potentially curative treatment with allogeneic stem cell transplantation. New sources for donor cells for RIC BMT, such as bone marrow from HLA-haploidentical family members (haplo-BM), or unrelated double umbilical cord blood (dUCB), may permit timelier BMT for adult patients lacking suitably related or unrelated donors.
New sources for donor cells for RIC BMT, such as bone marrow from HLA-haploidentical family members (haplo-BM), or unrelated double umbilical cord blood (dUCB), may permit timelier BMT for adult patients lacking suitably related or unrelated donors.
Limited evidence about comparative effectiveness of donor cell sources
 There is uncertainty about the comparative effectiveness of haplo-BM and dUCB donor cell sources for RIC BMT, including: the relative rates of hematopoietic recovery and adverse events, economic impact and health-related quality of life impacts.
There is uncertainty about the comparative effectiveness of haplo-BM and dUCB donor cell sources for RIC BMT, including: the relative rates of hematopoietic recovery and adverse events, economic impact and health-related quality of life impacts.
Randomized controlled trial rationale
 Important uncertainties about comparative effectiveness have motivated the design of BMT CTN 1101, a Phase III randomized controlled trial to evaluate progression-free survival in patients with high-risk and advanced hematologic malignancies receiving unrelated umbilical cord blood (dUCB) versus HLA-haploidentical related donor bone marrow (haplo-BM) transplantation.
Important uncertainties about comparative effectiveness have motivated the design of BMT CTN 1101, a Phase III randomized controlled trial to evaluate progression-free survival in patients with high-risk and advanced hematologic malignancies receiving unrelated umbilical cord blood (dUCB) versus HLA-haploidentical related donor bone marrow (haplo-BM) transplantation. An ancillary cost–effectiveness analysis is being conducted alongside BMT CTN 1101 to provide supplemental information about the relative value of these alternative donor cell sources.
An ancillary cost–effectiveness analysis is being conducted alongside BMT CTN 1101 to provide supplemental information about the relative value of these alternative donor cell sources.
Ancillary cost–effectiveness analysis design
 Patients enrolled in the BMT CTN 1101 trial will be offered participation in the ancillary cost–effectiveness analysis.
Patients enrolled in the BMT CTN 1101 trial will be offered participation in the ancillary cost–effectiveness analysis. Consenting patients will provide health insurance information to allow calculation of direct medical costs from reimbursement records.
Consenting patients will provide health insurance information to allow calculation of direct medical costs from reimbursement records. Out-of-pocket costs, time costs and health-related quality of life will be collected through a novel web-based survey instrument.
Out-of-pocket costs, time costs and health-related quality of life will be collected through a novel web-based survey instrument. Efficacy, cost and health-related quality of life outcomes will inform a cost–utility analysis comparing dUCB and haplo-BM graft sources for RIC BMT from patient, payer and societal perspectives.
Efficacy, cost and health-related quality of life outcomes will inform a cost–utility analysis comparing dUCB and haplo-BM graft sources for RIC BMT from patient, payer and societal perspectives.
Conclusion
 This cost–effectiveness study will collect detailed health insurer, out-of-pocket and caregiver cost information to characterize the economic consequences of haplo-BM and dUCB sources of donor cells for RIC BMT.
This cost–effectiveness study will collect detailed health insurer, out-of-pocket and caregiver cost information to characterize the economic consequences of haplo-BM and dUCB sources of donor cells for RIC BMT. The incremental cost–effectiveness ratio will be calculated using cost outcomes and quality-adjusted survival outcomes, and will provide a comprehensive measure of the relative value of these alternative donor cell sources.
The incremental cost–effectiveness ratio will be calculated using cost outcomes and quality-adjusted survival outcomes, and will provide a comprehensive measure of the relative value of these alternative donor cell sources. If one donor cell source is less costly and at least equally effective, the results of the study will have important implications for patient, clinician and health insurer decision-making, as well as the design of future BMT clinical trials.
If one donor cell source is less costly and at least equally effective, the results of the study will have important implications for patient, clinician and health insurer decision-making, as well as the design of future BMT clinical trials.
Acknowledgements
The authors thank L Koepl for technical assistance and editing.
Support for this study was provided by grant U10HL069294 and R01HL116291-01 from the National Heart, Lung and Blood Institute and the National Cancer Institute. JA Roth is supported by grant T32 AG027677 from the National Institute on Aging.
No writing assistance was utilized in the production of this manuscript.
Footnotes
Financial & competing interests disclosure The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
Papers of special note have been highlighted as:
of interest
▪ ▪ of considerable interest
- 1.Giralt S, Logan B, Rizzo D, et al. Reduced-intensity conditioning for unrelated donor progenitor cell transplantation: long-term follow-up of the first 285 reported to the national marrow donor program. Biol. Blood Marrow Transplant. 2007;13(7):844–852. doi: 10.1016/j.bbmt.2007.03.011. ▪ ▪ Provides basic background about reduced-intensity conditioning for unrelated donor transplantation.
- 2.Platzbecker U, Thiede C, Fussel M, et al. Reduced intensity conditioning allows for up-front allogeneic hematopoietic stem cell transplantation after cytoreductive induction therapy in newly-diagnosed high-risk acute myeloid leukemia. Leukemia. 2006;20(4):707–714. doi: 10.1038/sj.leu.2404143. [DOI] [PubMed] [Google Scholar]
- 3.Popat U, Heslop HE, Durett A, et al. Outcome of reduced-intensity allogeneic hematopoietic stem cell transplantation (RISCT) using antilymphocyte antibodies in patients with high-risk acute myeloid leukemia (AML) Bone Marrow Transplant. 2006;37(6):547–552. doi: 10.1038/sj.bmt.1705229. [DOI] [PubMed] [Google Scholar]
- 4.Grigg AP, Gibson J, Bardy PG, et al. A prospective multicenter trial of peripheral blood stem cell sibling allografts for acute myeloid leukemia in first complete remission using fludarabine-cyclophosphamide reduced intensity conditioning. Biol. Blood Marrow Transplant. 2007;13(5):560–567. doi: 10.1016/j.bbmt.2006.12.449. [DOI] [PubMed] [Google Scholar]
- 5.Oran B, Giralt S, Saliba R, et al. Allogeneic hematopoietic stem cell transplantation for the treatment of high-risk acute myelogenous leukemia and myelodysplastic syndrome using reduced-intensity conditioning with fludarabine and melphalan. Biol. Blood Marrow Transplant. 2007;13(4):454–462. doi: 10.1016/j.bbmt.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Confer D, Robinett P. The US National Marrow Donor Program role in unrelated donor hematopoietic cell transplantation. Bone Marrow Transplant. 2008;42(Suppl. 1):S3–S5. doi: 10.1038/bmt.2008.102. [DOI] [PubMed] [Google Scholar]
- 7.Grewal SS, Barker JN, Davies SM, Wagner JE. Unrelated donor hematopoietic cell transplantation: marrow or umbilical cord blood? Blood. 2003;101(11):4233–4244. doi: 10.1182/blood-2002-08-2510. [DOI] [PubMed] [Google Scholar]
- 8.Brunstein CG, Fuchs EJ, Carter SL, et al. Alternative donor transplantation after reduced intensity conditioning: results of parallel Phase 2 trials using partially HLA-mismatched related bone marrow or unrelated double umbilical cord blood grafts. Blood. 2011;118(2):282–288. doi: 10.1182/blood-2011-03-344853. ▪ ▪ Provides the Phase II studies that motivate the BMT CTN 1101 study.
- 9.Brown J, Boussiotis V. Umbilical cord blood transplantation: basic biology and clinical challenges to immune reconstitution. Clin. Immunol. 2012;127(3):286–297. doi: 10.1016/j.clim.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Komanduri K, John L, De Lima M. Delayed immune reconstitution after cord blood transplantation is characterized by impaired thymopoiesis and late memory T-cell skewing. Blood. 2007;110:4543–4551. doi: 10.1182/blood-2007-05-092130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Safdar A, Rodriguez G, De Lima M. Infections in 100 cord blood transplantations: spectrum of early and late posttransplant infections in adult and pediatric patients 1996–2005. Medicine (Baltimore) 2007;86:324–333. doi: 10.1097/MD.0b013e31815c52b0. [DOI] [PubMed] [Google Scholar]
- 12.Stanevsky A, Goldstein G, Nagler A. Umbilical cord blood transplantation: Pros, cons and beyond. Blood Rev. 2009;23(5):199–204. doi: 10.1016/j.blre.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 13.Blood and Marrow Transplant Clinical Trials Network. doi: 10.1016/j.bbmt.2016.07.003. https://web.emmes.com/study/bmt2/protocol/1101_protocol/1101_protocol.html. ▪ ▪ Provides the BMT CTN 1101 trial protocol and related materials.
- 14.Majhail NS, Rizzo D, Hahn T, et al. Patient and caregiver out-of-pocket costs of allogeneic hematopoietic cell transplantation: a pilot study. Bone Marrow Transplant. 2013;48(6):865–871. doi: 10.1038/bmt.2012.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ballen KK, Koreth J, Chen YB, Dey BR, Spitzer TR. Selection of optimal alternative graft source: mismatched unrelated donor, umbilical cord blood, or haploidentical transplant. Blood. 2012;119(9):1972–1980. doi: 10.1182/blood-2011-11-354563. [DOI] [PubMed] [Google Scholar]
- 16.Gold M, Siegel J, Russell L, Weinstein M, editors. Cost–Effectiveness in Health and Medicine. Oxford University Press; Oxford, UK: 1996. [Google Scholar]
- 17.Williams LA. Whatever it takes: informal caregiving dynamics in blood and marrow transplantation. Oncol. Nurs. Forum. 2007;34(2):379–387. doi: 10.1188/07.ONF.379-387. [DOI] [PubMed] [Google Scholar]
- 18.Goossens ME, Rutten-Van Molken MP, Vlaeyen JW, Van Der Linden SM. The cost diary: a method to measure direct and indirect costs in cost–effectiveness research. J. Clin. Epidemiol. 2000;53(7):688–695. doi: 10.1016/s0895-4356(99)00177-8. [DOI] [PubMed] [Google Scholar]
- 19.Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics. 1993;4(5):353–365. doi: 10.2165/00019053-199304050-00006. [DOI] [PubMed] [Google Scholar]
- 20.Brazier J, Roberts J. The estimation of a preference-based measure of health from the SF-12. Med. Care. 2004;42(9):851–859. doi: 10.1097/01.mlr.0000135827.18610.0d. [DOI] [PubMed] [Google Scholar]
- 21.Brazier J, Roberts J, Deverill M. The estimation of a preference-based measure of health from the SF-36. J. Health Econ. 2002;21(2):271–292. doi: 10.1016/s0167-6296(01)00130-8. [DOI] [PubMed] [Google Scholar]
- 22.Drummond M, Sculpher M, Torrance G, O’brien B, Stoddart G. Methods for the economic evaluation of health care programmes (3rd) Oxford University Press; Oxford, UK: 2005. [Google Scholar]
- 23.Glick H, Doshi J, Sonnad S, Polsky D. Economic evaluation in clincial trials. Oxford University Press; Oxford, UK: 2007. [Google Scholar]
- 24.Dumont S, Jacobs P, Turcotte V, Anderson D, Harel F. Measurement challenges of informal caregiving: a novel measurement method applied to a cohort of palliative care patients. Social Sci. Med. (1982) 2010;71(10):1890–1895. doi: 10.1016/j.socscimed.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 25.Lee ET, Go OT. Survival analysis in public health research. Annu. Rev. Public Health. 1997;18:105–134. doi: 10.1146/annurev.publhealth.18.1.105. [DOI] [PubMed] [Google Scholar]
- 26.Akaike H. A new look at the statistical model identification. IEEE Trans. Automat. Contr. 1974;19:716–723. [Google Scholar]
- 27.Cleves M, Gould W, Gutierrez R, Marchenko Y, editors. An Introduction to Survival Analysis Using STATA. Stata Press; TX, USA: 2008. [Google Scholar]
- 28.Willan A, Briggs A. Statistical Analysis of Cost–Effectiveness Data. John Wiley & Sons; Chichester, UK: 2006. [Google Scholar]
- 29.Gold MR, Siegal JE, Russel LB, Weinstein MC. Cost–Effectiveness in Health and Medicine. Oxford University Press; NY, USA: 1996. [Google Scholar]
- 30.Ramsey S, Wilke R, Briggs A, et al. Good research practice for cost–effectiveness analysis alongside clinical trials: the ISPOR RCT-CEA task force report. Value Health. 2005;8:521–533. doi: 10.1111/j.1524-4733.2005.00045.x. ▪ ▪ Provides standard practices for conducting cost–effectiveness analyses alongside clinical trials.
- 31.Etzioni R, Urban N, Baker M. Estimating the costs attributable to a disease, with application to ovarian cancer. J. Clin. Epidemiol. 1996;49(1):95–103. doi: 10.1016/0895-4356(96)89259-6. [DOI] [PubMed] [Google Scholar]
- 32.Etzioni RD, Feuer EJ, Sullivan SD, Lin DY, Hu CC, Ramsey SD. On the use of survival analysis techniques to estimate medical care costs. J. Health Econ. 1999;18(3):365–380. doi: 10.1016/s0167-6296(98)00056-3. [DOI] [PubMed] [Google Scholar]
- 33.Lin DY, Feuer EJ, Etzioni R, Wax Y. Estimating medical costs from incomplete follow-up data. Biometrics. 1997;53(2):419–434. [PubMed] [Google Scholar]
- 34.Willan A, Lin D, Manca A. Regression methods for cost–effectiveness analysis with censored data. Stat. Med. 2005;24:131–145. doi: 10.1002/sim.1794. [DOI] [PubMed] [Google Scholar]
- 35.Lin D. Linear regression analysis of censored medical costs. Biostatistics. 2000;1(1):35–47. doi: 10.1093/biostatistics/1.1.35. [DOI] [PubMed] [Google Scholar]
- 36.Koopmanschap M, Van Exel J, Van Den Berg B, Brouwer W. An overview of methods and applications to value informal care in economic evaluations of healthcare. Pharmacoeconomics. 2008;26:269–280. doi: 10.2165/00019053-200826040-00001. [DOI] [PubMed] [Google Scholar]
- 37.Shiroiwa T, Sung Y, Fukuda T, Lang H, Bae S, Tsutani K. Internation survey on willingness-to-pay (WTP) for one additional QALY gained: what is the threshold of cost–effectiveness? Health Econ. 2010;19:422–437. doi: 10.1002/hec.1481. [DOI] [PubMed] [Google Scholar]
- 38.Arzykulov Z, Chichua N, Smagulova K, Khamidullina G, Mukanova S, Suriya K. Effectiveness of chemotherapy with oxaliplatin, capecitabine and bevacizumab (xelox plus bev) in second line therapy of metastatic colorectal cancer (Mcrc) Ann. Oncol. 2010;21:32–32. [Google Scholar]
- 39.Guan Z, Xu J, Luo R, et al. Bevacizumab plus chemotherapy in chinese patients with metastatic colorectal cancer: efficacy and tolerability results from the artist study. Ann. Oncol. 2010;21:22–22. [Google Scholar]
- 40.Chow K, Coyle N. Providing palliative care to family caregivers throughout the bone marrow transplantation trajectory research and practice: partners in care. J. Hosp. Pall. Nurs. 2011;13(1):7–13. [Google Scholar]
- 41.Kirchhoff AC, Leisenring W, Syrjala KL. Prospective predictors of return to work in the 5 years after hematopoietic cell transplantation. J. Cancer Survivor. 2010;4(1):33–44. doi: 10.1007/s11764-009-0105-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramsey SD, Sullivan SD, Kaplan RM, Wood DE, Chiang YP, Wagner JL. Economic analysis of lung volume reduction surgery as part of the National Emphysema Treatment TrialNETT Research Group. Ann. Thorac. Surg. 2001;71(3):995–1002. doi: 10.1016/s0003-4975(00)02283-9. [DOI] [PubMed] [Google Scholar]
- 43.Walters S, Brazier J. Comparison of the minimally important difference for two health state utility measures: EQ-5D and SF-6D. Qual. Life Res. 2005;14:1523–1532. doi: 10.1007/s11136-004-7713-0. [DOI] [PubMed] [Google Scholar]