Summary
The cooperation of tumor infiltrating lymphocytes and tertiary lymphoid tissue in early stage colorectal carcinoma further corroborates the strong immune influences on tumor progression and patient outcome. Immune factors in the tumor microenvironment may warrant inclusion in pathology reports and staging systems for prognostication and prediction of therapeutic response.
In this issue of Clinical Cancer Research, DiCaro and colleagues from the Department of Immunology and Inflammation in Rozzano, Italy explore the relationship between tumor infiltrating lymphocytes (TIL), tertiary lymphoid tissue (TLT), and patient outcome in colorectal carcinoma [1].
The most common classification system for the staging and prognostication of cancer is the American Joint Committee on Cancer/Union for International Cancer Control (AJCC/UICC) TNM classification. The current system stratifies for patient survival, though patients within single TNM stages may demonstrate a range of clinical outcomes, indicating the potential for further refinement. In the current system, histologic features such as the depth of tumor invasion are used to predict disease progression, which is a ‘tumor-autonomous assumption’ [2]. Conspicuously, the potential role of the local tumor microenvironment in promoting or curtailing tumor progression is not represented [3]. Furthermore, while the TNM stage of a patient is often used to drive treatment decisions, the current system does not incorporate factors that may predict response to therapy.
The presence of TIL, specifically those with cytotoxic, memory, or Th1 phenotypes within a tumor are recognized to be of positive prognostic significance in a broad range of solid tumor types, including colorectal carcinoma, melanoma, and non-small cell lung carcinoma (NSCLC) [4]. In Stage I–III colorectal carcinoma, the spatial organization (centrally within tumor parenchyma and at the peripheral invasive edge where the tumor interfaces with stroma) of specific TIL subsets have been shown to have greater prognostic significance than TNM stage [5]. These findings have led investigators to advocate for the inclusion of an ‘Immunoscore’ indicating the presence of specific immune infiltrates as a component of new tumor classification schemes. Such a TNM-I system is currently being validated by an international consortium [2].
In addition to substantiating the relationship of TIL and better prognosis in early stage colorectal carcinoma, Di Caro and colleagues studied the impact of TLT on patient outcome. The relationship between the presence of tertiary lymphoid tissue (TLT) and improved patient outcome was first described in NSCLC [6]. TLT is an organized structure, consisting of B-cell dense follicles, supported by interfollicular dendritic cells, and surrounded by T-cell zones. The presence of high endothelial venules (HEV) in these structures has also been noted, and are thought to facilitate recruitment and subsequent T-cell activation and differentiation for a sustained anti-tumor adaptive immune response [6]. Di Caro and colleagues performed a standardized quantitative analysis of area occupied by TLT at the invasive margin compared to the total digitized tumor surface. They identified TLT in 79% of 351 different specimens analyzed, and demonstrated that like dispersed TIL, having a high density of TLT was associated with better prognosis in patients with Stage II disease.
Some of the most interesting findings from Di Caro and colleagues’ study emerged when they looked at the relationship between TIL and TLT. They found that the density of TLT at the invasive margin correlated with the density of dispersed TIL only in patients who did not experience relapse, suggesting that a clinical benefit requires synchronization of these two components. The authors went on to provide additional evidence that TLT mediates the recruitment of lymphocytes to the local tumor microenvironment in a murine model of inflammation-mediated colorectal carcinomagenesis (AOM/DSS). They adoptively transferred GFP labeled splenocytes and demonstrated that after 24 hours, the GFP labeled cells localized to TLT in the AOM/DSS mice while very few were seen in the lymphoid tissue of control mice. In addition, they demonstrated increased HEV density within the TLT of AOM/DSS mice compared to lymphoid tissue in control mice, further supporting the notion that these structures facilitate lymphocyte recruitment. The supporting role of TLT in the local immune response has been inferred [6,7], and this report now provides functional evidence for the role of these tertiary lymphoid structures in providing access for TILs into the tumor microenvironment. While the presence of TLT implies the local generation of a specific anti-tumor immune response, it is still unclear what proportion of the response is actually generated at this site versus another location, such as a draining lymph node. It also remains to be determined whether the B-cell component of TLT indicates a humoral contribution to the immune response.
A better understanding of the tumor-host interaction also has a number of implications for cancer immunotherapy. A growing body of evidence suggests an association between an inflamed tumor environment and response to immunotherapies, including cancer vaccines and checkpoint blockade. This makes sense mechanistically, as many of these agents, e.g., anti-PD-1, likely protect or potentiate an ongoing immune response, as opposed to generating a de novo anti-tumor immune response [8]. Patients whose tumors contain TLT may be better positioned to respond to this novel class of agents, since their tumors can support trafficking of TIL. Many features of the immune contexture in tumors are being investigated for their relationship to therapeutic response [9], and the findings of Di Caro, et al, support the inclusion of TLT as one of these features.
These findings also call attention to tumors with a non-inflamed phenotype. As Di Caro and colleagues have shown, patients with early stage disease who relapsed were more likely to have low TIL and TLT densities. These tumors may avoid the immune system through immune exclusion or ignorance and may require additional intervention to generate an inflamed phenotype [10]. Tumors lacking TLT likely have less local capacity for T cell trafficking, broad tumor antigen exposure, and rapid response to potential tumoral antigen shift, resulting in less efficient and effective anti-tumor immunity. It is also not evident whether current immunotherapeutic regimens demonstrate significant activity in tumors lacking activated TIL. Thus in this group of patients, therapies may need to be sequenced. For example, traditional cytotoxic agents, targeted inhibitors, or cancer vaccines may be used first to induce the presence of activated TIL and even TLT [10,11]. Once the tumor has established a gateway for T-cell influx and subsequent priming, an immunotherapeutic regimen such as checkpoint blockade may be administered to protect the resultant immune response.
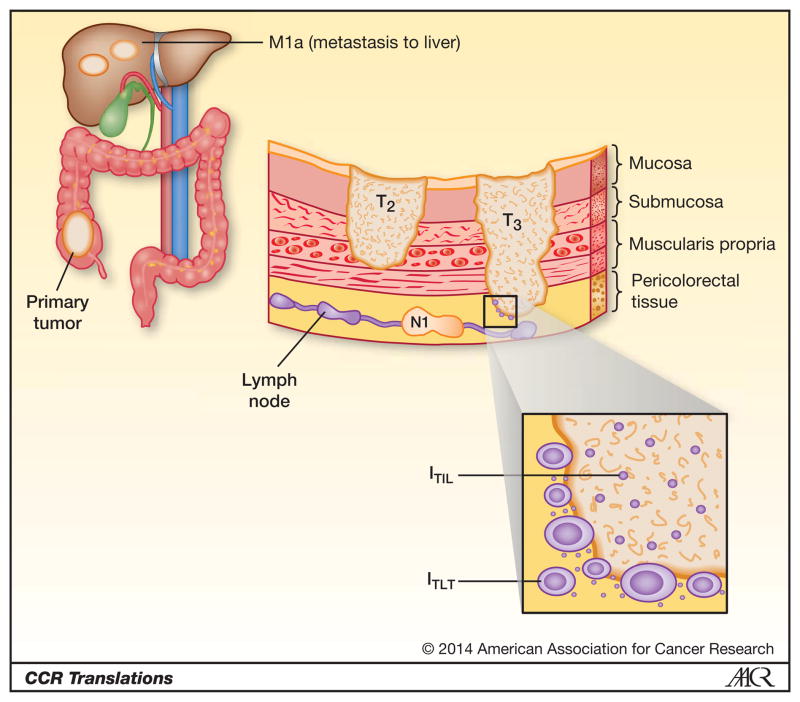
In the era of personalized medicine, it is likely that a parameter representing the immune microenvironment will be included in future surgical pathology reports and staging systems, Figure 1. Many of the original findings leading to the current Immunoscore were generated in studies of colorectal carcinoma. The functional significance of various immune cell subsets in different cancer types will require additional investigation, as early studies indicate that some immune cell subsets, such as Tregs, have ambivalent prognostic importance in different tumor types [4]. Additionally, the current Immunoscore focuses on the host’s adaptive immune response to tumor, and does not include features of potential adaptive immune resistance by tumor, such as PD-L1 expression, which could protect tumors from immune destruction [8]. Other important practical considerations include how best to characterize the local immune milieu within tumors, i.e., with routine hematoxylin and eosin review of TIL grade [8, 12], by immunohistochemical analysis of immune cell subsets and presence of TLT [2,4,5], or molecularly using gene expression signatures [4,9]. Thresholds will have to be determined as well as standardized methods for quantification that translate across institutions. Once these issues are addressed, however, the AJCC/UICC staging system, at least for specific tumor types, may indeed transition to TNM-I.
Figure 1.
The current staging system is based on characteristics of the tumor and includes the depth of tumor invasion (T), presence of tumor in lymph nodes (N) and evidence of metastases (M). The in situ component of the host immune response provides additional prognostic and therapeutic information. Shown here is tertiary lymphoid tissue (TLT) at the invasive tumor margin of colorectal carcinoma and in the peritumoral stroma, as well as tumor infiltrating lymphocytes (TIL) in both the core of the tumor and at the invasive margin. An immune-descriptive parameter representing features such as TIL and TLT will likely be included in future staging systems for certain tumor types.
Acknowledgments
The author would like to thank Dr. Robert A. Anders for helpful comments regarding this manuscript. Research in the author’s laboratory is supported by the Dermatology Foundation, Melanoma Research Alliance, NIH (R01 CA142779), Bristol Myers Squibb, and Stand Up to Cancer (SU2C-AACR-DT10 Grant number 114578).
Footnotes
Conflicts of Interest: Bristol Myers Squibb (research funding and advisory board member)
References
- 1.Di Caro G, Bergomas F, Grizzi F, Doni A, Bianchi P, Malesci A, et al. Occurrence of tertiary lymphoid tissue is associated to T cell infiltration and predicts better prognosis in early stage colorectal carcinomas. Clin Can Res. 2014 doi: 10.1158/1078-0432.CCR-13-2590. (This issue.) [DOI] [PubMed] [Google Scholar]
- 2.Galon J, Mlecnik B, Bimndea G, Angell HK, Berger A, Lagorce C, et al. Towards the introduction of the ‘Immunoscore’ in the classification of malignant tumors. J Pathol. 2014;232:199–209. doi: 10.1002/path.4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bindea G, Mlecnik B, Fridman W, Pages F, Galon J. Natural immunity to cancer in humans. Curr Opin Immunol. 2010;22:215–222. doi: 10.1016/j.coi.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 4.Fridman WH, Pages F, Sautes-Fridman C, Galon J. The immune contexture in human tumors: impact on clinical outcome. Nat Rev Can. 2012;12:298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 5.Mlecnik B, Tosolini M, Kirilovsky A, Berger A, Bindea G, Meatchi T, et al. Histopathologic-based prognostic factors of colorectal cancers are associated with the state of the local immune reaction. J Clin Oncol. 2011;29:610–8. doi: 10.1200/JCO.2010.30.5425. [DOI] [PubMed] [Google Scholar]
- 6.Dieu-Nosjean MC, Antione M, Danel C, Heudes D, Wislez M, Poulot V, et al. Long term survival for patients with non-small-cell lung cancer with intratumoral lymphoid structures. J Clin Oncol. 2008;26:4410–7. doi: 10.1200/JCO.2007.15.0284. [DOI] [PubMed] [Google Scholar]
- 7.Chaisemartin L, Goc J, Damotte D, Validire P, Magdeleinat P, Alifano M, et al. Characterization of chemokines and adhesion molecules associated with T cell presence in tertiary lymphoid structures in human lung cancer. Cancer Res. 2011;71:6391–9. doi: 10.1158/0008-5472.CAN-11-0952. [DOI] [PubMed] [Google Scholar]
- 8.Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, et al. Sci Transl Med. 2012;4:127ra37. doi: 10.1126/scitranslmed.3003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ascierto PA, Capone M, Urba WJ, Bifulco CB, Botti G, Lugli A, et al. The additional facet of immunoscore: immunoprofiling as a possible predictive tool for cancer treatment. J Transl Med. 2013;11:1–4. doi: 10.1186/1479-5876-11-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gajewski TF, Schreiber H, Fu Y. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14:1014–22. doi: 10.1038/ni.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng L, Edil B, Nguyen T, Yager A, Bever K, Sharma R, et al. Novel tertiary lymphoid aggregates induced in pancreatic adenocarcinoma by an allogenic GM-CSF secreting pancreatic tumor vaccine as a neoadjuvant treatment. ASCO Annual meeting; 2011. Abstract #157. [Google Scholar]
- 12.Thomas NE, Busam KJ, From L, Kricker A, Armstrong BK, Anton-Culver H, et al. Tumor-infiltrating lymphocyte grade in Primary Melanomas is independently associated with melanoma-specific survival in the population-based genes, envorinoment, and melanoma study. J Clin Oncol. 2013;31:4252–9. doi: 10.1200/JCO.2013.51.3002. [DOI] [PMC free article] [PubMed] [Google Scholar]