Abstract
Purpose
To determine if MRI BI-RADS criteria or radiologist perception correlate with presence of invasive cancer after initial core biopsy of ductal carcinoma in situ (DCIS).
Materials and Methods
Retrospective search spanning 2000-2007 identified all core-biopsy diagnoses of pure DCIS that coincided with preoperative MRI. Two radiologists fellowship-trained in breast imaging categorized lesions according to ACR MRI-BIRADS lexicon and estimated likelihood of occult invasion. Semi-quantitative signal enhancement ratio (SER) kinetic analysis was also performed. Results were compared to histopathology.
Results
51 consecutive patients with primary core biopsy-proven DCIS and concurrent MRI were identified. Of these, 13 patients (25%) had invasion at excision. Invasion correlated significantly with presence of a mass for both readers (p=0.012, 0.001), rapid initial enhancement for Reader 1 (p=0.001), and washout kinetics for Reader 2 (p=0.012). Significant correlation between washout and invasion was confirmed by SER (p=0.006) when threshold percent enhancement was sufficiently high (130%), corresponding to rapidly enhancing portions of the lesion. Radiologist perception of occult invasion was strongly correlated to true presence of invasion.
Conclusion
These results provide evidence that certain BI-RADS MRI criteria, as well as radiologist perception, correlate with occult invasion after an initial core biopsy of DCIS.
Keywords: breast, carcinoma, ductal carcinoma in situ, invasive ductal carcinoma, invasive lobular carcinoma
INTRODUCTION
Ductal carcinoma in situ (DCIS) is a pre-invasive form of breast cancer. It is rarely symptomatic. The advent of screening mammography, which has allowed visualization of the characteristic microcalcifications associated with the disease, has led to a dramatic increase in the incidence of DCIS (1), which presently accounts for 20% of screen-detected cancers (2). The true reservoir of DCIS is likely larger than current detection rates would suggest, as autopsy series data estimate a median prevalence of 9% among women who have never been diagnosed with breast cancer during life (3).
The standard method of diagnosis for DCIS is core biopsy, a fast and minimally-invasive alternative to open surgery with high diagnostic accuracy for DCIS (4). This approach has provided a manageable course of action for the large number of indicated biopsies prompted by microcalcifications found at mammographic screening. Yet when microcalcifications are sampled by biopsy rather than excised, a certain degree of disease underestimation of concurrent invasive cancer is unavoidable. Overall underestimation rates vary considerably by choice of biopsy device, and are lowest with vacuum-assisted core biopsy needles (5, 6). Even when a biopsy result is concordant with imaging for presence or absence of malignancy, there is the additional question of what the final surgical pathology reveals in relation to the biopsy. In particular, we are interested in cases where invasive cancer is found after an initial biopsy result of DCIS. One of the largest series of 1,326 stereotactic biopsies revealing DCIS reported by Jackman et al revealed a DCIS underestimation rate (invasion found at surgery) of 20.4% by 14-guage large-core biopsy and 11.2% by directed 14- or 11-guage vacuum-assisted biopsy (7).
The early identification of invasive cancer concurrent with DCIS is clinically significant and can result in changes to surgical management. Under current treatment guidelines, a sentinel node biopsy would not typically be performed for DCIS (8), but would for invasive carcinoma. Furthermore, growing evidence suggests that hormonal therapy may slow or stabilize certain hormone-receptor-positive invasive breast cancers (9-11). Similar interest in pre-invasive breast cancer has lead to prospective pilot studies investigating hormone treatment in DCIS (12, 13). These investigations and others like them are often performed in a neoadjuvant setting and may eventually allow an expectant management option in certain low-risk forms of DCIS. The safe implementation of any strategy that postpones surgery for DCIS, however, is critically dependent on the exclusion of invasion, a goal not reliably achieved by current methods.
Among breast imaging modalities, MRI has the greatest potential for reliably excluding invasion in patients diagnosed with pure DCIS. Breast MRI has proven to be highly sensitive for detecting and evaluating breast cancer of various types, including DCIS. Recent studies have shown MRI sensitivity ranges between 88 - 92% for detection of DCIS (14, 15), and 91-95% for the detection of invasive ductal carcinoma (16, 17). There is very little literature, however, regarding the separation of DCIS from invasive cancer on breast MRI (18-20).
The purpose of this study is to determine whether BI-RADS MRI criteria and/or radiologist perception correlate with the presence of invasive cancer after an initial core biopsy of pure DCIS. To address this question, we retrospectively identified a cohort of women with an initial diagnosis of pure DCIS who underwent preoperative MRI, and asked two board-certified radiologists with fellowship training in breast imaging to assign BI-RADS criteria and probability of invasion to each case without access to surgical outcomes. Results were compared to surgical pathology.
MATERIALS AND METHODS
The study protocol was reviewed and approved by the institutional human subjects review board. A comprehensive search of pathology and radiology archives at our institution was performed in order to identify all women with a core biopsy diagnosis of pure DCIS between January 1, 2000, and December 31, 2007, including cases referred from outside institutions which were reviewed by our institution’s pathology department, who also had an ipsilateral breast MRI breast prior to definitive excision. Stereotactic biopsies were routinely performed with a directed 11-gauge vacuum-assisted core biopsy device (Mammotome, Devicor Medical Products, Cincinnati, OH) for an average of 9 cores. The few lesions detectable by ultrasound were sampled under ultrasound guidance with a 14-gauge spring-loaded core biopsy needle for an average of 5 cores. In order to avoid the possibility of disease progression, MRI and surgery must have occurred within 6 months of core biopsy. Patients with intervening treatment between MRI and surgical excision were excluded, as were those with a diagnosis of invasive/microinvasive disease on core biopsy, a history of ipsilateral invasive cancer in the past 2 years, or recent (within 6 months) ipsilateral breast surgery. If more than one biopsy-proven DCIS lesion was present in the same patient, the initial index lesion was used for analysis.
MRI Procedure
All imaging was performed on a 1.5 Tesla Signa system (GE Medical Systems, Milwaukee, WI) in the prone position using a dedicated breast radiofrequency coil. Although efforts were made to scan premenopausal patients during days 7 through 14 of their menstrual cycle, exceptions were made when such timing would delay the patient’s surgery. For the period 2000-06, unilateral breast acquisitions were obtained in the sagittal plane using a 4-channel breast coil (MRI Devices, Waukesha, WI). In 2007, axial bilateral scans were obtained using an 8-channel breast coil (Sentinelle Vanguard, Toronto, Canada). The standard clinical breast MRI protocol consisted of a fat-suppressed T2-weighted fast spin echo sequence and a three dimensional fat-suppressed T1-weighted fast gradient recalled echo sequence obtained before and twice after a bolus power intravenous injection of 0.1 mmol/kg gadopentetate dimeglumine (Magnevist; Bayer HealthCare Pharmaceuticals, Wayne, New Jersey) followed by saline flush in accordance with methodology of the original ACRIN 6657 protocol (21).
Two board-certified radiologists with fellowship training in breast imaging were given the location and preliminary biopsy result of the primary lesion as marked by biopsy clip or the estimated biopsy location, and asked to categorize the lesion. Readers were blinded to the surgical results, but had patient demographics available to them. In order to isolate the performance of MRI, breast ultrasound and mammography were not made available to the readers. Both radiologists were provided a standard report form based on current American College of Radiology BI-RADS-MRI criteria (22). In addition to providing a description of morphology and kinetics, the radiologist was asked to grade background breast fibroglandular density, background enhancement, and probability of occult invasion. For probability of occult invasion, readers were instructed to take into account all factors available to them on the exam and in their experience, including lesion kinetics, size, morphology, multiplicity, and background enhancement. Probability of occult invasion could be rated as “none,” “possible,” “probable,” or “definite.” Kinetics, as rated by the radiologists, were qualitatively assessed by comparing early and late post-contrast images and were categorized according to BI-RADS-MRI criteria. For non-mass-like enhancement, an additional variable was added to address the degree of enhancement confluence, ranging from sparse to relatively solid. Hence “enhancement confluence” was defined as the percent density of enhancement within the most densely enhancing square centimeter of the lesion of interest, and was subjectively assessed by the readers into 5 categories of <10%, 10-25%, 25-50%, 51-75%, and >75%. The radiologists were provided with visual training examples of each category prior to reader studies. Enhancement confluence could range from < 10% for sparse non-mass-like enhancement intercalated with non-enhancing breast parenchyma, to > 75% for near-solid enhancement.
In addition to the qualitative assessment of kinetics performed by the radiologists, kinetics were also assessed using a semi-quantitative, semi-automated method utilizing signal enhancement ratio (SER) developed by Hylton et al (23). SER is defined as (S1-S0)/(S2-S0) where S0 is precontrast, S1 is early post-contrast, and S2 is late post-contrast signal in any given voxel. Regions-of-interest (ROI’s) were manually drawn around the primary target as marked by biopsy clip or estimated biopsy location by an experienced research assistant under the supervision of a radiologist. SER was calculated on a per-voxel basis using the ACRIN 6657 protocol {Hylton, 2012 #29}. A minimum enhancement threshold was set based on the initial percent enhancement (PE), defined as 100*(S1-S0)/S0. For the purposes of this study, moderate early enhancement was set empirically at a minimum threshold PE of 70, and rapid early enhancement was set at 130. Delayed kinetic characteristics were described per BI-RADS criteria as “persistent”, “plateau”, or “washout”. Plateau delayed enhancement was defined as late post-contrast signal within +/- 10% of early post-contrast signal, or SER between 0.9-1.1. Similarly, persistent delayed enhancement was defined as SER < 0.9, and washout delayed enhancement was defined as SER > 1.1. For each lesion ROI, the relative amount of persistent, plateau, and washout enhancement was described as a percentage of the total lesion enhancement that rose above the set threshold PE value (70 or 130). MRI morphologic and kinetic descriptors were compared to reported final histopathology at surgical excision(s) of the lesion.
Histopathology
Standard surgical pathology procedure involves careful marking of specimen margins in order to preserve anatomic orientation. Gross specimens are routinely sectioned in the sagittal plane. For standard clinical practice, DCIS size is usually documented as the size of the largest focus of disease rather than total diameter of disease extent (which may contain intervening normal tissue). For the purposes of DCIS measurement, we focused on those cases documenting the full disease extent.
The presence, type, size, and grade of any invasive component was recorded from the definitive surgical resection following the MRI. In some cases, immunohistochemical studies were needed to properly evaluate the case and establish the diagnosis of invasion or microinvasion. In cases where the patient underwent additional surgical re-excision for the purpose of clearing positive margins, both pathology reports were considered in determining presence of invasion. Estrogen and progesterone receptor staining was performed at the time of the core biopsy in most cases.
Statistical analysis
Summary statistics in frequency and percentages of subjects were calculated for all of the patient demographic variables as well as clinical impression scores. Fisher’s exact probability model was applied to each BI-RADS descriptor under each reader to evaluate the association of the predictive to outcome variables. Analyses of BIRADS characteristics were lesion-based. For kinetic analysis, the normality of the data distribution was first tested by the Shapiro-Wilk normality test. Significance was then determined using Student’s t-test for normally distributed data, Wilcoxon rank-sum otherwise. Reader agreement was evaluated using Kappa statistics. All analyses were performed by SAS (SAS V9.3, SAS Institute, Cary, NC, USA) and STATA (Stata/IC V11.2, College Station, TX, USA) software. A threshold of 5% was used to establish statistical significance.
RESULTS
Over the 8-year period, 357 cases of pure DCIS were identified by core biopsy performed or reviewed by our institution. Of these, 51 patients also had a preoperative MRI and satisfied all other entrance criteria. The majority of patients were premenopausal (61%) with a mean age at diagnosis of 48 years (range: 24-71) (Table 1). Primary mode of presentation was microcalcifications on mammography (41; 80%), although some presented with palpable mass and/or nipple discharge (10; 20%). In most cases, patients were referred to MRI for surgical planning/evaluation of disease extent, and/or because of suspected invasion. Estrogen receptor (ER) and progesterone receptor (PR) status was positive in 22 women (43%), negative in 11 (22%), and mixed or unknown in the remainder. Most patients proceeded to lumpectomy (29; 57%) as their initial surgery. The majority of patients obtained the breast MRI either before, or more than 20 days after core biopsy (37; 73%), although 3 patients obtained MRI within 10 days of biopsy (the shortest interval was 5 days). The full range of time between core biopsy and MRI was (-18) to 84 days.
Table 1.
Demographic data for 51 patients with pure DCIS diagnosed by core-biopsy
| Demographic variable | Number (Percent) |
|---|---|
| Menopausal status | |
| Premenopausal | 31 (61%) |
| Perimenopausal | 2 (4%) |
| Postmenopausal | 13 (25%) |
| Unknown | 5 (10%) |
| Age | |
| 20-29 | 1 (2%) |
| 30-39 | 6 (12%) |
| 40-49 | 25 (49%) |
| 50-59 | 15 (29%) |
| 60 + | 4 (8%) |
| Personal breast cancer history | |
| Negative | 42 (82%) |
| Positive | 5 (10%) |
| Unknown | 4 (8%) |
| Presentation | |
| Calcifications | 41 (80%) |
| Nipple discharge &/or palpable mass | 10 (20%) |
| Surgery | |
| Lumpectomy | 29 (57%) |
| Mastectomy | 22 (43%) |
| Hormone receptor status | |
| ER+/PR+ | 22 (43%) |
| ER+/PR- | 4 (8%) |
| ER-/PR+ | 4 (8%) |
| ER-/PR- | 11 (21%) |
| Unknown | 10 (20%) |
| Time between biopsy and MRI | |
| ≤ 10 days | 3 (6%) |
| 11-20 days | 11 (22%) |
| > 20 days | 31 (61%) |
| Biopsy performed after MRI | 6 (12%) |
ER: Estrogen receptor status; PR: Progesterone receptor status.
Pathologic Findings
Of the 51 lesions, 4 lesions (8%) had no evidence of residual DCIS or invasion on final surgical histopathology, including a case of a 2.4 cm region of lobular carcinoma in situ (LCIS) and 3 lesions revealing only proliferative fibrocystic change. The remaining 47 lesions demonstrated DCIS. Of these, only 26 cases described full DCIS extent, which resulted in a median DCIS diameter of 3.1 cm (range: 0.2-15.0 cm).
Of the 51 original lesions, 13 (25%) were found to contain invasion at definitive surgical excision, all in association with DCIS. The DCIS in these cases was usually high grade (77%). All 13 lesions contained invasive ductal components, two also had invasive lobular components. Three of the invasive cases originally presented with a palpable mass, one with nipple discharge, and the remainder as microcalcifications.
Most invasive tumor consisted of small nests of invasive tumor among a background of DCIS. Median size of invasive carcinoma was 0.6 cm (range: <0.1-4.0 cm, Table 2). The majority of the 13 invasive tumors (8 cases; 62%) measured less than 1 cm, two of which had microinvasion only (foci measuring < 0.1 cm). Three tumors were greater than 2 cm, specifically measuring 2.5 cm, 4 cm, and 4 cm. On further analysis of these cases, two represented an admixture of invasive disease and DCIS, one of which could only be diagnosed after immunohistological studies (one of the 4 cm cases). The final 4 cm tumor was identified in an 8.1 cm region of DCIS on re-excision, in a patient whose imaging studies were compromised by foreign body injections into the breasts and pectoralis muscles.
Table 2.
Characteristics of the invasive cancer cases discovered at definitive excision, after original diagnosis of pure DCIS by core-biopsy
| Invasive Tumor Size a (cm) | DCIS Tumor Size (cm) | Invasive Tumor Grade a | Invasive Pathology Type | Original Presentation | Lymph Node Status |
|---|---|---|---|---|---|
| <0.1 | 4.5 | - | Microinvasiveb ductal | Nipple discharge | (-) |
| <0.1 | 4.1 | - | Microinvasiveb ductal | Calcifications | (-) |
| 0.2 | 3.8 | II | Ductal | Calcifications | (-) |
| 0.2 | 0.5* | II | Ductal | Calcifications | (-) |
| 0.2 | 2* | - | Ductal | Palpable Mass | (+) |
| 0.3 | 6 | I | Ductal | Calcifications | (-) |
| 0.6* | 0.9 | II | Ductal & lobular | Calcifications | (-) |
| 0.8 | 2.8 | I | Ductal | Palpable Mass | (-) |
| 1.1 | 2 | II | Ductal | Calcifications | (-) |
| 1.3* | 9 | II | Ductal | Calcifications | (+) |
| 2.5 | 2.5 | II | Mixed lobular & ductal | Calcifications | (+) |
| 4 c | 6 | II | Ductal | Palpable Mass | (+) |
| 4 d | 8.1 | I | Ductal | Calcifications | (+) |
Several foci present, largest focus diameter reported.
Scarff-Bloom-Richardson grade
“Microinvasive” is defined as foci of invasion through the basement membrane measuring less than 1 mm.
Pathology required immunohistochemical studies to properly evaluate the case and establish the diagnosis of microinvasion throughout the region.
Imaging was compromised by foreign body injections into the breasts and pectoralis muscles.
All 13 patients with invasion underwent sentinel or axillary lymph node sampling at the time of the original surgery (9 patients) or as a separate surgery (4 patients). In some cases where sentinel lymph node sampling was included in the original surgery, pre-operative notes imply that the pre-operative MRI played a major factor in this decision. Five of the patients with invasion (38%) were found to have nodal metastases.
Radiographic Findings
On preoperative breast MRI, lesion type correlated significantly to presence of invasion at surgical excision for both readers (Table 3, p=0.009 & 0.002). MRI lesion types were primarily classified as non-mass-like enhancement or masses (Figure 1), although some cases revealed no residual enhancement above background. The BI-RADS descriptor “focus” (less than 5 mm of enhancement) was not used for any of the lesions.
Table 3.
MRI lesion type compared to final surgical pathology
| Variable | No invasion at pathology | Invasion at pathology | p-value |
|---|---|---|---|
| Reader 1 | |||
| No lesion | 91% (10/11) | 9% (1/11) | |
| NMLE | 77% (27/35) | 23% (8/35) | 0.009 |
| Mass | 20% (1/5) | 80% (4/5) | |
| Reader 2 | |||
| No lesion | 100% (3/3) | 0% (0/3) | |
| NMLE | 88% (29/33) | 12% (4/33) | 0.002 |
| Mass | 40% (6/15) | 60% (9/15) |
NMLE: Non-mass-like enhancement
Figure 1.
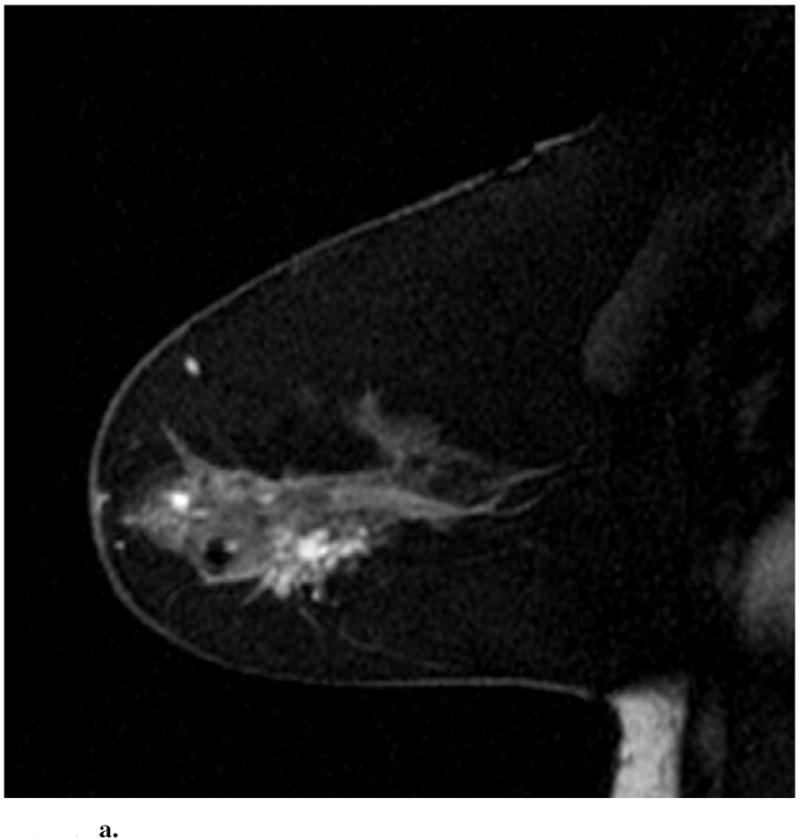
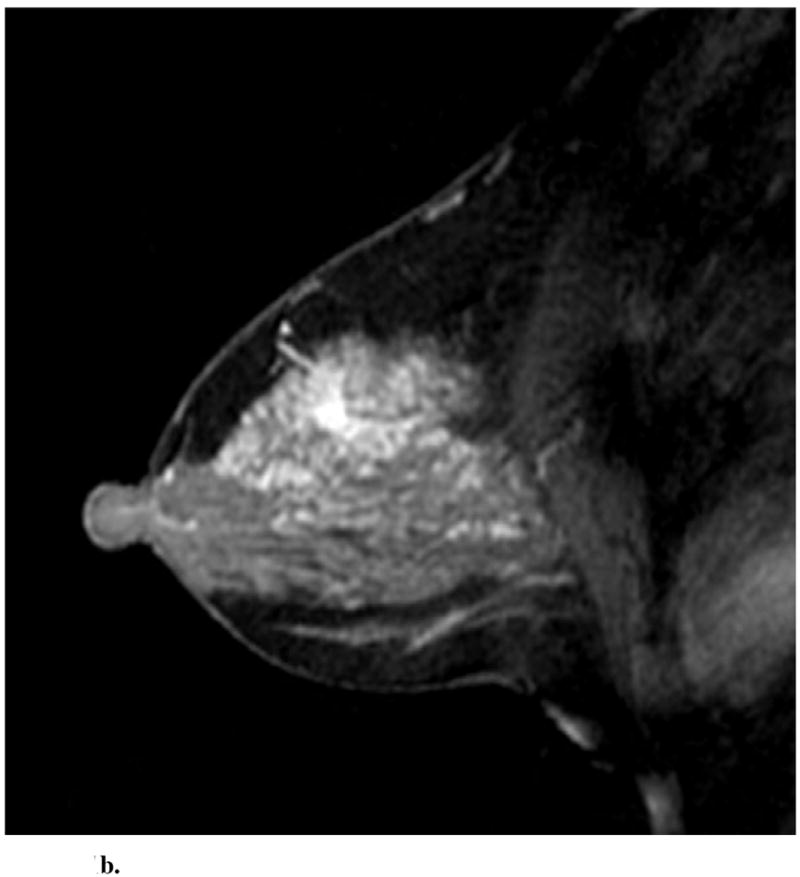
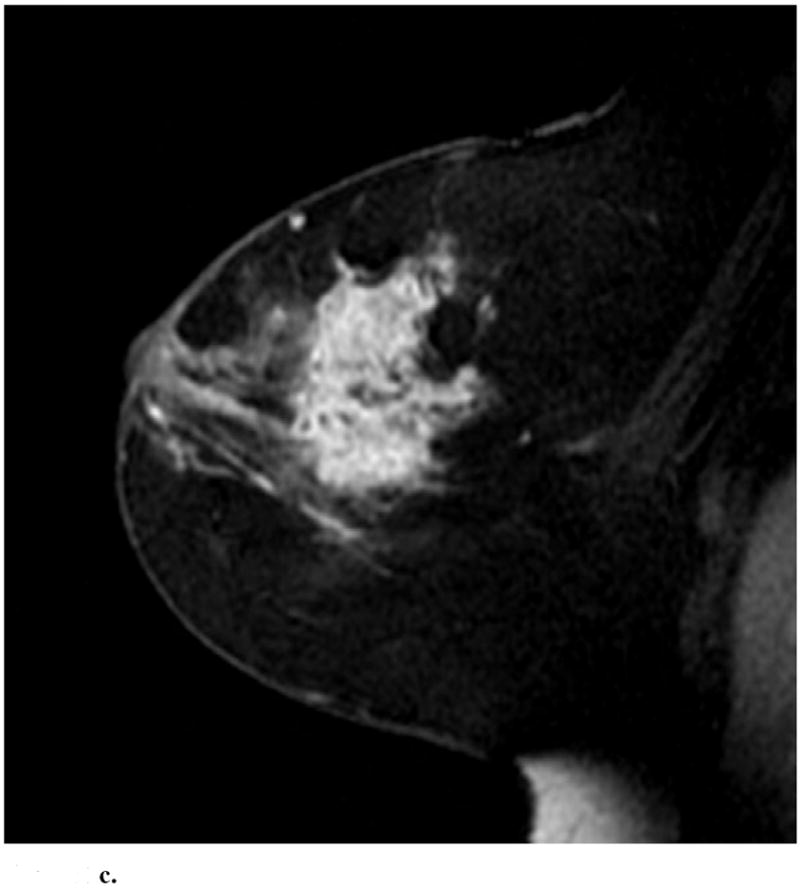
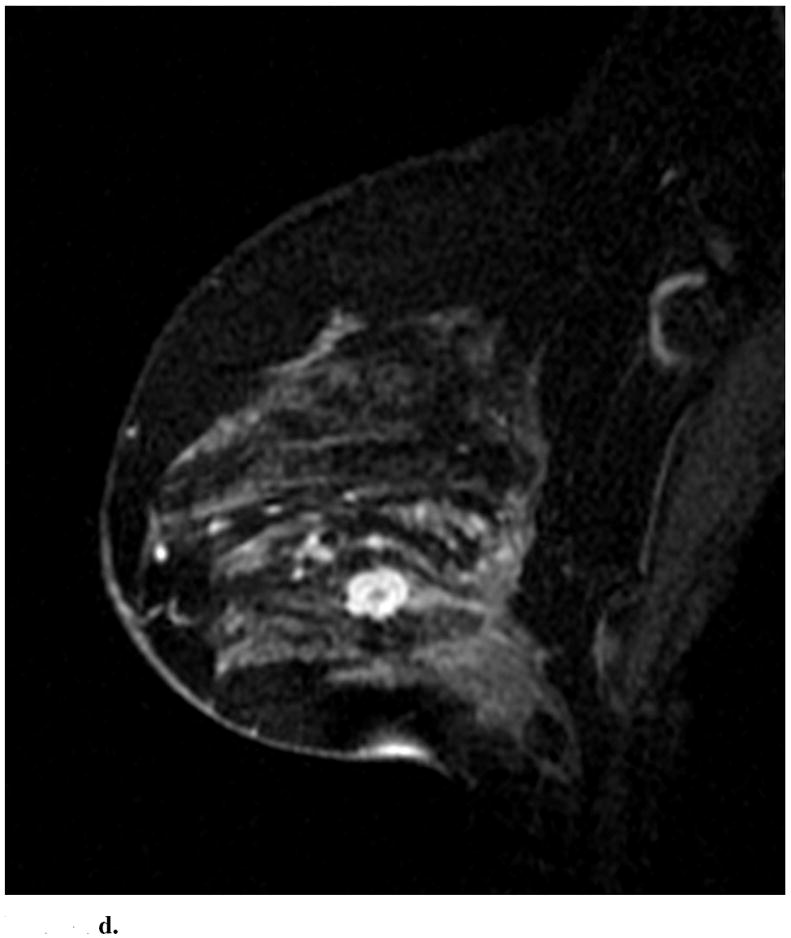
Sagittal images from immediate postcontrast T1 3D-GRE sequence in 4 different patients found to have invasion in addition to DCIS. (a): Clumped ductal non-mass-like enhancement. A 2.5 cm mixed lobular and invasive ductal carcinoma was found at pathology. (b): Clumped segmental non-mass like enhancement. A 0.3 mm invasive ductal carcinoma was found at pathology. (c): Heterogeneous regional non-mass-like enhancement per Reader 1 and a lobular mass with irregular margins and heterogeneous enhancement per Reader 2. Microinvasion was found at pathology. (d): Lobular mass. Foci measuring up to 0.6 cm containing invasive lobular and ductal carcinoma were found at pathology.
As would be expected, the majority of the 51 original lesions demonstrated non-mass-like enhancement (Table 3: 35 & 33 for Reader 1 & 2), some of which did reveal invasion at surgery (23% & 12%). No enhancement above background was seen in 11 and 3 cases, for Reader 1 and Reader 2 respectively. Some of these cases revealed invasion for one reader (9% & 0%). In contrast, although relatively few lesions were classified as masses (5 & 15), the majority of these did in fact contain invasion (80% & 60%).
The presence of a mass was confirmed to strongly associate with invasion for both readers when specifically comparing presence of each variable to the presence of invasion (Table 4). All other significant variables were significant only for one of the two readers, including non-mass-like enhancement (negative correlation with invasion), highly confluent enhancement (positive correlation), rapid uptake and washout (positive correlation), and persistent delayed enhancement (negative correlation). No significant correlation was found for background breast fibroglandular density, background enhancement, or for T2 signal characteristics.
Table 4.
Variables significantly correlating with invasion
| Variable | Correlation direction | p-value | |
|---|---|---|---|
| Reader 1 | Reader 2 | ||
| Lesion type | |||
| Presence of NMLE+ | Negative for invasion | 0.730 | 0.006* |
| Presence of mass | Positive for invasion | 0.012* | 0.001* |
| NMLE | |||
| Enhancement confluence > 75% | Positive for invasion | 0.015* | 0.142 |
| Enhancing lesions, kinetics | |||
| Rapid initial kinetics | Positive for invasion | 0.001* | 0.469 |
| Persistent delayed kinetics | Negative for invasion | 0.097 | 0.039* |
| Washout delayed kinetics | Positive for invasion | 0.515 | 0.012* |
p-values reaching significance. Within each category, significance was calculated by Fisher’s exact probability method comparing presence/absence of each variable to the presence/absence of invasion on excisional histopathology.
NMLE: Non-mass-like enhancement
Because of small number of masses associated with invasion, no significant associations were found for mass descriptors. However, certain trends were present. Among invasive cases described as masses, most mass shapes were classified as lobular, round or oval, none as irregular. All mass margins associated with invasion were classified as smooth or irregular, none as spiculated. Internal enhancement of masses associated with invasion ranged widely within the available BI-RADS lexicon, with the single exclusion of dark internal septations.
The significance of both rapid early kinetics and washout delayed kinetics was tested by semi-quantitative SER whole-tumor kinetics analysis (Table 5). At a low percent enhancement (PE) threshold of 70, approximating a medium initial enhancement kinetic cut-off, no type of delayed enhancement reached significance for separating cases of invasion from non-invasion. Median values between the groups are similar. In contrast, at a PE threshold of 130, approximating a rapid initial enhancement cut-off, the percent washout (and only the percent washout) of lesion region-of-interest significantly correlated with presence of invasion (p=0.006), with median values of 9% among non-invasive cases and 36% among invasive cases.
Table 5.
SER kinetics compared to final surgical pathology
| Variable | No invasion at pathology Median (IQR) |
Invasion at pathology Median (IQR) |
p-value |
|---|---|---|---|
| At PE = 70% | |||
| % Washout | 9 (21) | 14 (11) | 0.261 |
| % Plateau | 26 (15) | 29 (9) | 0.252 |
| % Persistent | 60 (36) | 54 (9) | 0.697 |
| At PE=130% | |||
| % Washout | 9 (37) | 36 (23) | 0.006* |
| % Plateau | 34 (51) | 44 (23) | 0.486 |
| % Persistent | 18 (35) | 21 (25) | 0.383 |
PE: percent enhancement defining the minimum enhancement threshold
IQR: interquartile range, the range between the 25th and 75th percentile
p value reaching significance. All p values were obtained by Wilcoxon rank-sum test for non-normal data distributions.
Finally, radiologists rated perceived risk of invasion for each preoperative MRI. As radiologist perceived risk increases, the probability of true invasion also generally increases (Table 6), although there is variability between the readers. The kappa- statistic measure of interrater agreement (κ) for possibility of invasion revealed fair agreement (0.27, 95% CI: 0.01-0.53). Overall, radiologist perceived risk of invasion is significantly correlated with true presence of invasion for both readers (p=0.009, 0.002).
Table 6.
Reader perception of invasion compared to final surgical pathology
| Reader perceived risk of invasion | Percentage with invasion at pathology | p-value |
|---|---|---|
| Reader 1 | ||
| None | 4% (1/23) | 0.002 |
| Possible | 44% (8/18) | |
| Probable | 33% (3/9) | |
| Definite | 100% (1/1) | |
| Reader 2 | ||
| None | 0% (0/17) | 0.001 |
| Possible | 29% (6/21) | |
| Probable | 50% (6/12) | |
| Definite | 100% (1/1) |
DISCUSSION
The results of this study provide evidence that BI-RADS MRI criteria, as well as the radiologist perception, can correlate with the presence of invasive cancer after an initial core biopsy of pure DCIS. In this retrospective study, BI-RADS lesion type correlated strongly with presence of invasion for both readers. The strongest predictor was the presence of a mass, regardless of mass features, which correlated highly with presence of invasion at surgical excision for both readers. Rapid early enhancement, increased confluence of enhancement in non-mass-like enhancement, and washout also correlated with invasion, but only for one or the other reader. Semi-quantitative kinetics analysis demonstrated a significant correlation between washout and subsequent invasion when PE threshold is raised to a sufficiently high value.
These results are in keeping with what is generally expected of the MRI appearance of invasive breast cancers. Findings such as rapid early enhancement, washout, and presence of a mass are consistent with known associated features of invasive breast carcinoma (24). Older MRI studies of DCIS have noted the correlation of an invasive component to spiculated enhancement, presumably representing a mass (18). Recent studies comparing DCIS with and without invasion have reported a similar high rate of occult invasion among DCIS lesions presenting as masses on MRI, regardless of mass characteristics (19, 20). Of note, these two studies have not found kinetics to be useful for differentiating DCIS from invasive disease. DCIS itself is known to have highly variable kinetics (25). Under subjective reader analysis, we agree that the utility of ‘worst curve’ kinetics for determination of occult invasion is marginal. Semi-quantitative kinetics analysis, however, identified a very strong association between invasion and one particular combination of early and late kinetics (p=0.006): that of very rapid contrast uptake with subsequent washout. As the semi-quantitative whole-tumor kinetics analysis is substantively different from ‘worst curve’ estimation, it may hold promise for future differentiation between DCIS lesions that do and do not harbor invasion, the basis of further study.
Interestingly, a number of DCIS-proven lesions did not demonstrate a discrete lesion (6% for Reader 2, 22% for Reader 1). In reviewing these cases, the variability between readers may in large part be due to whether enhancement was assigned to a discrete lesion or to background. This issue is more problematic among DCIS lesions, which classically present as non-mass-like enhancement that may or may not rise above background enhancement levels (26, 27). As readers were specifically given the latitude to base perception of invasion on all factors available to them on the MRI scans, including severity of background enhancement, these factors were taken into account within the radiologist perception score. Thus three cases were rated as “possible” invasion despite lack of discrete enhancement above background at the biopsy site (two for Reader 1 and one for Reader 2). In one of these cases, an invasive cancer was indeed present. The impact of background enhancement on reader confidence highlights the need for some overall background enhancement assessment and its impact on exam sensitivity, particularly in DCIS cases.
Because it is retrospective, this study was subject to inherent limitations and biases that could be addressed with a prospective design. During this 8-year period, we encountered moderate variability in MRI protocol and quality characteristics, including a transition from sagittal unilateral to axial bilateral scanning, and long acquisition times. The lack of a contralateral breast for symmetry comparison is especially limiting in the evaluation of DCIS, which can appear very similar to background. As a retrospective study, the results are intrinsically affected by selection bias. Preoperative breast MRI for DCIS remains controversial, and the small subset of women selected for a breast MRI often had a clinical presentation that was suspicious for invasion, despite a core-biopsy diagnosis of pure DCIS. This would be expected to cause an oversampling of invasion, and an overestimation of the prevalence of occult invasion after a core biopsy showing DCIS (25% in this study). However, the rate of occult invasion found in the current study is in the range reported by others (28), and the percentage of DCIS cases presenting without clinical symptoms in this study (80%) is comparable to that observed for DCIS in general (29), mitigating some of these factors.
Another limitation of this study is the substantial inter-reader variability. The few reported studies of inter- and intra- reader variability using MRI morphology descriptors have found only poor to moderate agreement for most lesion characteristics, and only fair agreement on final lesion assessment and recommendations (κ=0.23-0.37) (30, 31). Similarly, fair agreement between readers has been noted by others when assessing early and delayed kinetics (κ= 0.27-0.38) (31, 32). These studies show that BI-RADS criteria can have substantive inter-reader variability, which we expect to be similar or greater in general clinical practice. Although our study was not specifically designed to study reader agreement, our experience is in keeping with these observations. Any clinically useful criteria for differentiating DCIS with and without occult invasion must perform satisfactorily within such an environment. In a setting of substantial inter-reader variability, only the presence of a mass was sufficiently robust to demonstrate significant and consistent correlation with occult invasion.
Invasive disease underestimation at core-biopsy has significant ramifications in treatment planning, influencing both current surgical management decisions and the feasibility of any non-surgical options that may become available in the future. As prospective pilot studies are already underway investigating the neoadjuvant hormone effects in DCIS (13), there is need for an imaging algorithm that will allow a reasonable estimate for risk of invasion prior to initiation of treatment options that may delay surgery. These pilot trials require a high degree of confidence that enrolled DCIS patients are without invasive disease before pursuing alternative therapies.
In conclusion, the results of this study provide evidence that certain BI-RADS MRI criteria, as well as the overall radiologist perception, correlate with the presence of invasive cancer after an initial core biopsy of pure DCIS. In particular, the presence of a mass should be considered as exclusion criteria for planned pilot trials delaying definitive surgical therapy of DCIS patients. The role of semi-quantitative whole-tumor kinetics analysis for evaluating occult invasion should be further studied. Results also suggest that attention should be paid not only to specific BI-RADS criteria, but also to the radiologist perception of possible invasion as a synthesis of the overall MRI exam. In sum, this study supports the premise that MRI has the potential to improve stratification of patients into low and high risk groups for occult invasive disease, with ramifications for treatment planning.
Acknowledgments
This work was supported by NIH-funded grants R01 CA116182 and 1 T32 EB001631-04. Grateful acknowledgement is made to Shoujun Zhao, MD, PhD, for his dedication of time and effort into this study.
References
- 1.Ernster VL, Barclay J, Kerlikowske K, Grady D, Henderson C. Incidence of and treatment for ductal carcinoma in situ of the breast. JAMA. 1996:913–8. [PubMed] [Google Scholar]
- 2.Ernster VL, Ballard-Barbash R, Barlow WE, et al. Detection of ductal carcinoma in situ in women undergoing screening mammography. J Natl Cancer Inst. 2002:1546–54. doi: 10.1093/jnci/94.20.1546. [DOI] [PubMed] [Google Scholar]
- 3.Welch HG, Black WC. Using autopsy series to estimate the disease “reservoir” for ductal carcinoma in situ of the breast: how much more breast cancer can we find? Ann Intern Med. 1997:1023–8. doi: 10.7326/0003-4819-127-11-199712010-00014. [DOI] [PubMed] [Google Scholar]
- 4.Verkooijen HM Group CBARLCS. Diagnostic accuracy of stereotactic large-core needle biopsy for nonpalpable breast disease: results of a multicenter prospective study with 95% surgical confirmation. Int J Cancer. 2002:853–9. doi: 10.1002/ijc.10419. [DOI] [PubMed] [Google Scholar]
- 5.Liberman L, Drotman M, Morris EA, et al. Imaging-histologic discordance at percutaneous breast biopsy. Cancer. 2000:2538–46. doi: 10.1002/1097-0142(20001215)89:12<2538::aid-cncr4>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 6.Ciatto S, Houssami N, Ambrogetti D, et al. Accuracy and underestimation of malignancy of breast core needle biopsy: the Florence experience of over 4000 consecutive biopsies. Breast Cancer Res Treat. 2007:291–7. doi: 10.1007/s10549-006-9289-6. [DOI] [PubMed] [Google Scholar]
- 7.Jackman RJ, Burbank F, Parker SH, et al. Stereotactic breast biopsy of nonpalpable lesions: determinants of ductal carcinoma in situ underestimation rates. Radiology. 2001:497–502. doi: 10.1148/radiology.218.2.r01fe35497. [DOI] [PubMed] [Google Scholar]
- 8.Intra M, Rotmensz N, Veronesi P, et al. Sentinel node biopsy is not a standard procedure in ductal carcinoma in situ of the breast: the experience of the European institute of oncology on 854 patients in 10 years. Ann Surg. 2008:315–9. doi: 10.1097/SLA.0b013e31815b446b. [DOI] [PubMed] [Google Scholar]
- 9.Mathew J, Agrawal A, Asgeirsson KS, et al. Primary endocrine therapy in locally advanced breast cancers--the Nottingham experience. Breast Cancer Res Treat. 2009:403–7. doi: 10.1007/s10549-008-9930-7. [DOI] [PubMed] [Google Scholar]
- 10.Tan SM, Cheung KL, Willsher PC, Blamey RW, Chan SY, Robertson JF. Locally advanced primary breast cancer: medium-term results of a randomised trial of multimodal therapy versus initial hormone therapy. Eur J Cancer. 2001:2331–8. doi: 10.1016/s0959-8049(01)00298-2. [DOI] [PubMed] [Google Scholar]
- 11.Hoff PM, Valero V, Buzdar AU, et al. Combined modality treatment of locally advanced breast carcinoma in elderly patients or patients with severe comorbid conditions using tamoxifen as the primary therapy. Cancer. 2000:2054–60. doi: 10.1002/(sici)1097-0142(20000501)88:9<2054::aid-cncr11>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 12.Chen Y-Y, DeVries S, Anderson J, et al. Pathologic and biologic response to preoperative endocrine therapy in patients with ER-positive ductal carcinoma in situ. BMC Cancer. 2009:285. doi: 10.1186/1471-2407-9-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hwang ES, Kinkel K, Esserman LJ, Lu Y, Weidner N, Hylton NM. Magnetic resonance imaging in patients diagnosed with ductal carcinoma-in-situ: value in the diagnosis of residual disease, occult invasion, and multicentricity. Annals of surgical oncology. 2003;10:381–88. doi: 10.1245/aso.2003.03.085. [DOI] [PubMed] [Google Scholar]
- 14.Menell JH, Morris EA, Dershaw DD, Abramson AF, Brogi E, Liberman L. Determination of the presence and extent of pure ductal carcinoma in situ by mammography and magnetic resonance imaging. The breast journal. 2005:382–90. doi: 10.1111/j.1075-122X.2005.00121.x. [DOI] [PubMed] [Google Scholar]
- 15.Kuhl CK, Schrading S, Bieling HB, et al. MRI for diagnosis of pure ductal carcinoma in situ: a prospective observational study. Lancet. 2007;370:485–92. doi: 10.1016/S0140-6736(07)61232-X. [DOI] [PubMed] [Google Scholar]
- 16.Bluemke DA, Gatsonis CA, Chen MH, et al. Magnetic resonance imaging of the breast prior to biopsy. JAMA : the journal of the American Medical Association. 2004;292:2735–42. doi: 10.1001/jama.292.22.2735. [DOI] [PubMed] [Google Scholar]
- 17.Berg WA, Gutierrez L, NessAiver MS, et al. Diagnostic accuracy of mammography, clinical examination, US, and MR imaging in preoperative assessment of breast cancer. Radiology. 2004:830–49. doi: 10.1148/radiol.2333031484. [DOI] [PubMed] [Google Scholar]
- 18.Soderstrom CE, Harms SE, Copit DS, et al. Three-dimensional RODEO breast MR imaging of lesions containing ductal carcinoma in situ. Radiology. 1996;201:427–32. doi: 10.1148/radiology.201.2.8888235. [DOI] [PubMed] [Google Scholar]
- 19.Huang Y-T, Cheung Y-C, Lo Y-F, Ueng S-H, Kuo W-L, Chen S-C. MRI findings of cancers preoperatively diagnosed as pure DCIS at core needle biopsy. Acta Radiologica. 2011;52:1064–68. doi: 10.1258/ar.2011.110213. [DOI] [PubMed] [Google Scholar]
- 20.Goto M, Yuen S, Akazawa K, et al. The role of breast MR imaging in pre-operative determination of invasive disease for ductal carcinoma in situ diagnosed by needle biopsy. European radiology. 2011 doi: 10.1007/s00330-011-2357-2. [DOI] [PubMed] [Google Scholar]
- 21.Hylton NM, Blume JD, Bernreuter WK, et al. Locally advanced breast cancer: MR imaging for prediction of response to neoadjuvant chemotherapy--results from ACRIN 6657/I-SPY TRIAL. Radiology. 2012;263:663–72. doi: 10.1148/radiol.12110748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Committee B-R, College of Radiology BI-RADS Committee A, College of Radiology A. ACR BI-RADS Breast Imaging and Reporting Data System: Breast Imaging Atlas. 2003 [Google Scholar]
- 23.Hylton NM. Vascularity assessment of breast lesions with gadolinium-enhanced MR imaging. Magnetic resonance imaging clinics of North America. 1999;7:411–20. x. [PubMed] [Google Scholar]
- 24.Schnall MD, Blume J, Bluemke DA, et al. Diagnostic architectural and dynamic features at breast MR imaging: multicenter study. Radiology. 2006;238:42–53. doi: 10.1148/radiol.2381042117. [DOI] [PubMed] [Google Scholar]
- 25.Jansen SA, Newstead GM, Abe H, Shimauchi A, Schmidt RA, Karczmar GS. Pure ductal carcinoma in situ: kinetic and morphologic MR characteristics compared with mammographic appearance and nuclear grade. Radiology. 2007;245:684–91. doi: 10.1148/radiol.2453062061. [DOI] [PubMed] [Google Scholar]
- 26.Mossa-Basha M, Fundaro GM, Shah BA, Ali S, Pantelic MV. Ductal carcinoma in situ of the breast: MR imaging findings with histopathologic correlation. Radiographics : a review publication of the Radiological Society of North America, Inc. 2010;30:1673–87. doi: 10.1148/rg.306105510. [DOI] [PubMed] [Google Scholar]
- 27.Orel SG, Mendonca MH, Reynolds C, Schnall MD, Solin LJ, Sullivan DC. MR imaging of ductal carcinoma in situ. Radiology. 1997;202:413–20. doi: 10.1148/radiology.202.2.9015067. [DOI] [PubMed] [Google Scholar]
- 28.Houssami N, Ciatto S, Ellis I, Ambrogetti D. Underestimation of malignancy of breast core-needle biopsy: concepts and precise overall and category-specific estimates. Cancer. 2007:487–95. doi: 10.1002/cncr.22435. [DOI] [PubMed] [Google Scholar]
- 29.Barreau B, de Mascarel I, Feuga C, et al. Mammography of ductal carcinoma in situ of the breast: review of 909 cases with radiographic-pathologic correlations. European journal of radiology. 2005;54:55–61. doi: 10.1016/j.ejrad.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 30.Ikeda DM, Hylton NM, Kinkel K, et al. Development, standardization, and testing of a lexicon for reporting contrast-enhanced breast magnetic resonance imaging studies. Journal of magnetic resonance imaging : JMRI. 2001;13:889–95. doi: 10.1002/jmri.1127. [DOI] [PubMed] [Google Scholar]
- 31.Swami A, Raza S, Sivarajah R, Chikarmane SA, Gautam S, Birdwell RL. Interobserver Variability in Use of the BI-RADS Lexicon for Breast MRI. RSNA 2009 Scientific Assembly and Annual Meeting Program, SSA01-05; 2009. p. 292. [Google Scholar]
- 32.Stoutjesdijk MJ, Fütterer JJ, Boetes C, van Die LE, Jager G, Barentsz JO. Variability in the description of morphologic and contrast enhancement characteristics of breast lesions on magnetic resonance imaging. Investigative radiology. 2005;40:355–62. doi: 10.1097/01.rli.0000163741.16718.3e. [DOI] [PubMed] [Google Scholar]


