Abstract
Cannabis contains the psychoactive component delta9-tetrahydrocannabinol (delta9-THC), and the non-psychoactive components cannabidiol (CBD), cannabinol, and cannabigerol. It is well-known that delta9-THC and other cannabinoid CB1 receptor agonists are neuroprotective during global and focal ischemic injury. Additionally, delta9-THC also mediates psychological effects through the activation of the CB1 receptor in the central nervous system. In addition to the CB1 receptor agonists, cannabis also contains therapeutically active components which are CB1 receptor independent. Of the CB1 receptor-independent cannabis, the most important is CBD. In the past five years, an increasing number of publications have focused on the discovery of the anti-inflammatory, anti-oxidant, and neuroprotective effects of CBD. In particular, CBD exerts positive pharmacological effects in ischemic stroke and other chronic diseases, including Parkinson’s disease, Alzheimer’s disease, and rheumatoid arthritis. The cerebroprotective action of CBD is CB1 receptor-independent, long-lasting, and has potent anti-oxidant activity. Importantly, CBD use does not lead to tolerance. In this review, we will discuss the therapeutic possibility of CBD as a cerebroprotective agent, highlighting recent pharmacological advances, novel mechanisms, and therapeutic time window of CBD in ischemic stroke.
Keywords: cannabinoids, cannabidiol, ischemic stroke, neuroprotective effect
1. Introduction
Cannabis contains over 60 different terpeno-phenol compounds that have been identified so far but the role and importance of many of these has yet to be fully understood. Select structures are shown in Figure 1. Delta9-tetrahydrocannabinol (delta9-THC) is the most psychoactive component that was isolated in 1964 by Gaoni and Mechoulam at the Weizmann Institute in Rehovot (Israel). It has been demonstrated to produce hypothermia, learning and memory impairment, impairment of the prepulse inhibition of the startle reflex, catalepsy-like immobilisation, aggressive behaviour, analgesia, hypoactivity and enhancement of preference for high fat diet [1,2,3,4,5,6,84,85,86,87,88]. These effects are at least partly caused by binding to cannabinoid receptor type 1 (CB1) within the brain. So far, two types of cannabinoid receptors have been identified: type1 (CB1 receptor) and type2 (CB2 receptor). CB1 receptors are mainly expressed in the central and the peripheral nervous system. CB2 receptors are found in cells of the immune system, such as lymphocytes and neutrophils, as well as in resident inflammatory cells within the CNS [7,8,9].
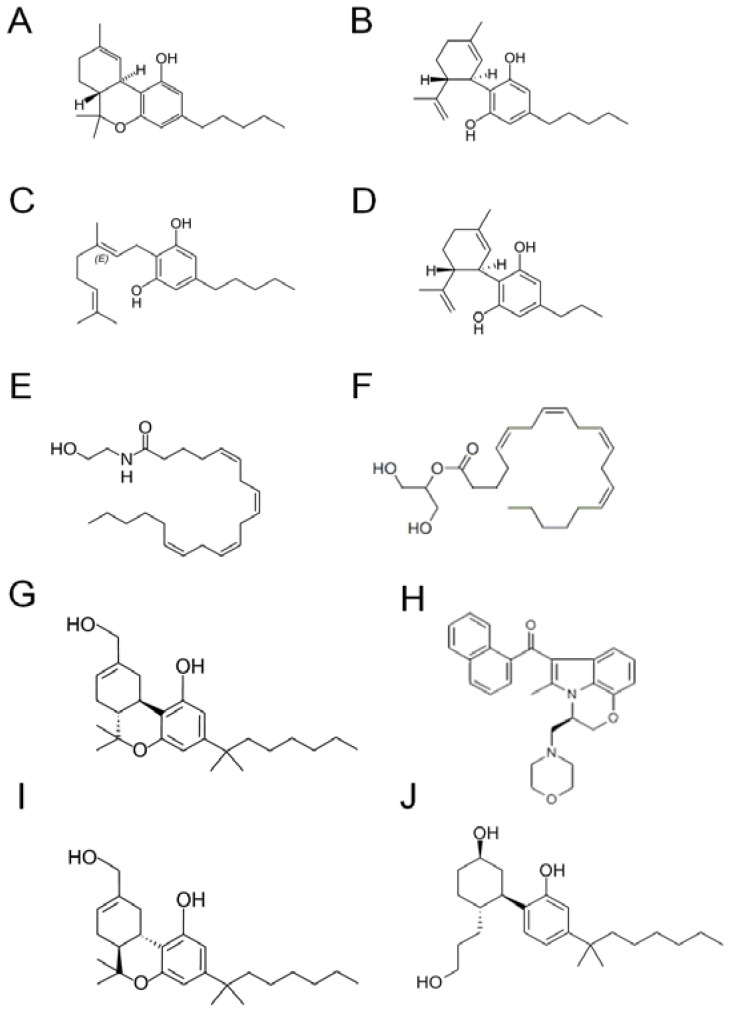
Figure 1.
Cannabinoid structures. (A) Delta9-tetrahydrocannabinol (delta9-THC); (B) cannabidiol (CBD); (C) cannabigerol (CBG); (D) cannabidivarin (CBDV); (E) anandamide (AEA); (F) 2-arachidonyl glycerol (2-AG); (G) HU210; (H) WIN55, 212-2; (I) HU211; (J) CP55940; (A)–(D) natural cannabinoids; (E) and (F) endocannabinoids; (G)–(J) synthetic cannabinoids.
On the other hand, cannabidiol (CBD), cannabigerol (CBG), cannabidivarin (CBDV) are known as non-psychoactive components of cannabis. These compounds have shown anti-inflammatory, immunosuppressive, analgesic, anxiolytic and anti-cancer effects [10]. In particular, much attention has been focused on CBD, which constitutes up to 3–4% of the cannabis extract. CBD was first isolated in 1940 by Adams and coworkers, but its structure and stereochemistry were only determined in 1963 by Mechoulam and Shvo. CBD has very low affinity (in the micromolar range) for the CB1 receptor as well as for the CB2 receptor and was found to be an anticonvulsant in animal models of epilepsy and in humans with epilepsy. Moreover, CBD has anti-spasmodic, anxiolytic, antinausea and anti-rheumatoid arthritis properties [11]. CBD has also been shown to be protective against N-methyl-D-aspartate and beta-amyloid peptide toxicity [12], as well as global and focal ischemic injury [13,14]. More recently, CBD has been found to have diverse additional functions, such as the neuroprotective effect induced through directly interacting with mitochondria-dependent Ca2+ regulation [79], the enhancement of adenosine signaling by inhibiting its uptake [15], blocking new cannabinoid receptor GPR55 [16], and inhibition of high-mobility group box1 activity.
In this review we will discuss the recent development of “CBD pharmacology” in ischemic stroke, focusing on the therapeutic potential of non-psychoactive cannabinoid CBD highlighting recent pharmacological advances, new mechanisms of action, and the therapeutic time window for its use in ischemic stroke.
2. Pharmacology of CBD in Ischemic Stroke
2.1. Cannabinoid Receptors and Endocannabinoid System
Accumulating data now suggest that cannabinoid CB1 receptors contribute to neuroprotection through anti-excitotoxicity [14], the phosphatidylinositol-3 kinase/Akt pathway [17], and hypothermia [18]. We have reported that the neuroprotective and hypothermic effects of delta9-THC were related to CB1 receptors [89]. CBD has also been described as protective against global and focal ischemic injury [14,18]. Several groups have recently shown that CBD can interact with cannabinoid CB1 and CB2 receptors. Pertwee and Ross showed that CBD can antagonize the CB1 agonists such as WIN55,212 and CP55940 by acting at prejunctional sites that was unlikely to be cannabinoid CB1 or CB2 receptors [19]. Castillo and coworkers recently demonstrated that CBD implicated the neuroprotective effect mediated by CB2 and adenosine receptors in an in vitro model of newborn hypoxic-ischemic brain damage in mice [21]. In addition, Thomas and coworkers noted that CBD displayed unexpected high potency as an antagonist of CB1 and CB2 receptor agonists [20]. Interestingly, the neuroprotective effect of CBD showed a dose dependent bell shaped curve in mice subjected to middle cerebral artery occlusion (MCAO) [22]. Intraperitoneal injection of CBD 1 or 3 mg/kg but not 10 mg/kg during 4 h MCAO prevented cerebral infarction 24 hours after cerebral ischemia. Other groups have demonstrated that 5 mg/kg was the most effective CBD dose, and that there was a dose-dependent bell-shaped curve for CBD’s effects on the electroencephalographic flattening in gerbils subjected to cerebral ischemia [13]. CBD has been also reported to inhibit anandamide amidase [23] and the reuptake of anandamide [24], suggesting CBD may induce an increase of anandamide signaling within the ischemic brain. Anandamide and other CB1 receptor agonists are known to reduce the release of a variety of neurotransmitters including glutamate via CB1 receptor [25,26,27,28,29]. In our previous study, delta9-THC significant reduced the release of glutamate during MCAO, but CBD did not affect glutamate release [30], suggesting that CBD might have other mechanism or stimulate other receptors besides the cannabinoid receptors. The mechanism of this biphasic effect of CBD is still unclear, but CBD may induce cerebroprotective effect through modulating endogenous cannabinoid system.
2.2. Serotonin 5-HT1A Receptor-Dependent Mechanism
In 1974, an interactive study comparing CBD and THC in healthy volunteers demonstrated for the first time that CBD could act as an anxiolityc drug [31]. Experimentally, the anxiolytic properties of CBD have been demonstrated in different animal models such as the conditioned emotional response, the Vogel conflict test, and the elevated plus-maze [32,33]. Recently, it has been shown that CBD might exert anxiolytic effects by activating post-synaptic 5-HT1A receptors [34]. 5-HT1A receptors have been shown to play critical roles in the pathophysiology of depression, aggression, and anxiety. It appears to have a role in vasodilatation and neuroprotection [35,36,37,38,39,40]. We previously applied a new approach to the investigation of the neuroprotective mechanism of CBD by examining the effects of a CB1 receptor antagonist, a vanilloid-receptor (VR1) antagonist, and a 5-HT1A receptor antagonist in a 4h MCA occlusion model in mice. Interestingly, the neuroprotective effect of CBD was inhibited by the 5-HT1A receptor antagonist, WAY100135. Furthermore, the increased cerebral blood flow induced by CBD was also in part reduced by WAY100135. In contrast, the CB1 receptor antagonist and VR1 did not inhibit the effect of CBD [22]. Russo and co-workers demonstrated that CBD, but not delta9-THC, displaced the 5HT1A agonist, [3H]-8-hydroxy-2-di-n-propylaminotetralin ([3H]-8-OH-DPAT) from the cloned human 5HT1A receptor in a dose-dependent manner, and they have concluded that CBD was an agonist of the 5HT1A receptor [41]. More recently, Magen and co-workers supported that CBD (5 mg/kg, i.p.) induced activation of 5-HT1A receptors located in forebrain regions including the hippocampus, and improved cognitive and locomotor function which were impaired by bile-duct ligation [42]. Taken together, these data indicate that CBD may activate the 5-HT1A receptor which leads to the improvement of cognitive and functional impairment after cerebral ischemia.
2.3. Potent Anti-Oxidant Mechanism
In 1998, Hampson and co-workers noted that the nonpsychoactive marijuana constituent CBD prevented both glutamate neurotoxicity and ROS-induced cell death with a stronger effect than either of the dietary antioxidants, α-tocopherol or ascorbate [43]. Surprisingly, CBD protected neurons with comparable efficacy to the potent antioxidant, butylated hydroxytoluene (BHT). In same research group, CBD and delta9-THC suppressed the oxidation potential measured by cyclic voltammetry and CBD was more effective than delta9-THC, suggesting that cannabidiol may be a neuroprotective antioxidant [14]. We have assessed both CBD and delta9-THC for antioxidant activity using the original 1,1-diphenyl-2-picryhydrazyl (DPPH) radical method described by Brand-Williams et al. [44]. Our results showed that CBD exhibited stronger antioxidative power (EC50 = 89.2 μM) than delta9-THC (EC50 = 464.2 μM) [45]. In an in vivo study, Hamelink and co-workers found that CBD protected against hippocampal-entorhinal-cortical neurodegeneration caused by ethanol exposure in rats. They showed that this effect of CBD attributed its anti-oxidative action [46]. These data suggest that CBD may be a very useful therapeutic agent for oxidative disorders after ischemic stroke.
2.4. Cerebroprotective Effect without the Development of Tolerance
Repeated treatment with delta9-THC and other CB1 receptor agonists result in the development of tolerance to its most acute behavioral and pharmacological effects [47,48,49,50]. Delta9-THC has been shown to lead to tolerance of hypoactivity, hypothermia, anti-nociception, catalepsy and pentobarbital-induced sleep prolongation [51,52,53]. In fact, 14 day-repeated treatment with delta9-THC (10 mg/kg) led to tolerance of the hypothermic and also neuroprotective effects. Additionally, the expression levels of CB1 receptor were down-regulated in mice with repeated doses of 10 mg/kg [45]. On the other hand, repeated treatment with CBD did not lead to development of tolerance in the cerebroprotective effect on infarction. CBD induced an increase in cerebral blood flow (CBF) even after 14 days of repeated treatment. Since these effects of CBD were in part inhibited by WAY100135, CBD may not be full 5-HT1A agonist. It is known that, 8-OH-DPAT, a complete 5HT1A agonist, induces tolerance [54], while a 5-HT1A receptor partial agonist such as buspirone does not [55]. For that reason, the neuroprotective effect of CBD may be mediated through partial agonistic effect on the 5-HT1A receptor.
2.5. Potent Anti-Inflammatory Effect
Emerging data now support the evidence of the anti-inflammatory action of CBD. For example, CBD suppressed interleukin-1 and inducible nitric oxide synthease induced by beta-amyloid [56]. CBD suppressed tumor necrosis factor α production in lipopolysaccharide-treated mice via A2A adenosine receptor activation, and this effect was reversed with an A2A adenosine-receptor antagonist, and abolished in A2A receptor knockout mice [15]. In addition, CBD inhibited neutrophil migration induced by formyl-methionyl-leucyl-phenylalanine in CB1 and CB2 receptor independent pathways which are antagonized by the endogenous compound N-arachdonoyl-L-serine [57]. We have reported that CBD had a cerebroprotective action via a cannabinoid receptor-independent myeloperoxidase inhibiting mechanism in a 4h MCA occlusion model in mice [30]. In this model, the neuroprotective effect of delta9-THC was only evident with pre-ischemic treatment via inhibition of the release of glutamate in in vivo microdialysis-HPLC measurements and hypothermia in CB1 receptor dependent mechanism. On the other hand, CBD was cerebroprotective even when administered 6 hours after cerebral ischemia. In addition, we demonstrated that CBD, but not delta9-THC, inhibited myeloperoxidase activity in neutrophils and prevented the decrease in cerebral blood flow due to the failure of cerebral microcirculation after reperfusion. In a more recent study, we also found that the anti-inflammatory action of CBD was associated with inhibition of plasma high-mobility group box1 leaked from dying cells and released from damaged cells and/or monocytes/macrophages [58]. These results suggest that CBD may prevent post-ischemic injury progressively induced by ischemic stroke.
3. Therapeutic Time Window of CBD and Other Cannabinoids in Ischemic Stroke
Focal cerebral ischemia induces a complex series of mechanisms. The pattern of excitory amino acid efflux in different models of cerebral ischemia derives from the finding that a massive release of glutamate in the ischemic early phase is considered to play a major role in inducing ischemic and post-ischemic cell death [59]. In fact, antagonists of glutamate receptors reduce the ischemic penumbra [60,61], and inhibitors of glutamate release exhibit cerebroprotective activity against ischemia/ reperfusion-evoked injury [62].
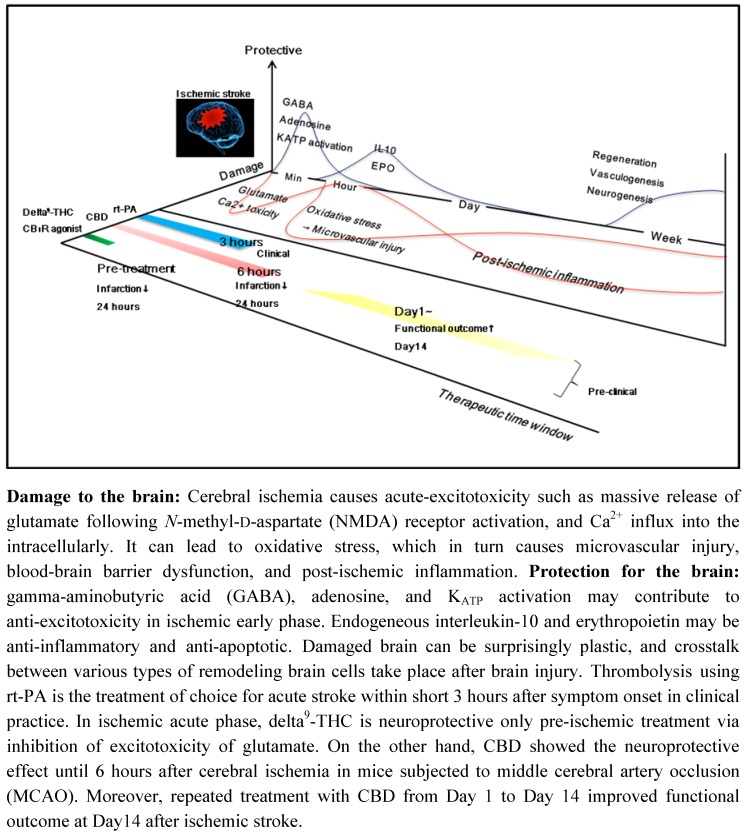
Figure 2.
Therapeutic time window of CBD and delta9-THC in pre-clinical study.
Within seconds to minutes after an excitotoxic injury oxidative stress is evident and this in turn causes microvascular injury, blood-brain barrier dysfunction, and post-ischemic inflammation over the next 24 hours. Moreover, the initial ischemic event activates migroglia and astrocytes which react by secreting cytokines, chemokines, and matrix metalloproteases. These inflammatory mediators lead to an upregulation of intracellular adhesion molecule-1, E-selectin and P-selectin on endothelial cells, allowing blood derived inflammatory cells, mainly neutrophils, to infiltrate the ischemic brain area [63]. Damaged brain can be surprisingly plastic, and crosstalk between various types of remodeling brain cells take place after brain injury [64,65,66]. GABA, adenosine, and KATP activation may contribute to anti-excitotoxicity in the ischemic early phase. Endogeneous interleukin-10 and erythropoietin may lead to anti-inflammation and anti-apoptosis. In the late phase after cerebral ischemia, the generation of new blood vessels facilitates highly coupled neurorestorative processes including neurogenesis and synaptogenesis [67,68]. In fact, neurogenesis after stroke has been demonstrated in the adult human brain [69]. Thus, cerebral ischemia induces complex mechanisms progressively such as the processes of cell destruction and of cell protection/regeneration, which suggest that multifunctional molecule and compounds without interfering with beneficial endogenous mechanisms may become a candidate to prolong the therapeutic time window after ischemic stroke. In the search for therapies for stroke, to date over 1,026 drugs have been tested in animal models, of which 114 underwent further clinical evaluation [70]. Recombinant tissue plasminogen activator (rt-PA) remains the only agent shown to improve stroke outcome in clinical trials. However, patients are eligible for thrombolysis by using rt-PA only if they come to medical attention within a short time (3 hours) after symptom onset. This restriction is considered necessary due to the risk of hemorrhage and potential damage caused by ischemia/reperfusion injury. Research is now underway to expand the therapeutic time window for the use of thrombolytic therapy after cerebral ischemia.
3.1. CBD and Delta9-THC
We have previously reported that CBD (3 mg/kg) has a potent and long-lasting neuroprotective effect when administered both pre- and post-ischemia, whereas only pre-ischemic treatment with delta9-THC (10 mg/kg) reduced the infarction size when administered 24 hours after 4 h MCAo in mice. The neuroprotective effect of delta9-THC and other cannabinoids is related to the CB1 receptor-mediated inhibition of voltage-sensitive Ca2+ channels, which reduces Ca2+ influx, glutamate release, and excitotoxicity [12]. In fact, delta9-THC inhibited the massive release of glutamate, but this neuroprotective effect of delta-9THC was inhibited by the CB1 receptor antagonist SR141716 [45], which suggest that delta9-THC is neuroprotective as a pre-ischemic treatment via inhibition of glutamate excitotoxicity. Actually, treatment with delta9-THC immediately after reperfusion did not prevent cerebral infarction. On the other hand, CBD was neuroprotective even when administered 6 hours after cerebral ischemia (2 hours after reperfusion). In addition, CBD significantly inhibited the myeloperoxidase activity of neutrophils at 1 hour and 20 hours after reperfusion and suppressed the decrease in CBF due to the failure of cerebral microcirculation after reperfusion. In addition, CBD decreased the number of Iba1- and GFAP-positive cells and improved neurological score and motor coordination at 3 days after cerebral ischemia. We concluded that the therapeutic time window of CBD was 6 hours after onset of ischemia in early treatment. CBD had a potent and long-lasting neuro- protective effect and prevented progressive post-ischemic injury [58]. In a more recent study, we reported that repeated treatment with CBD from 1 day or 3 days after cerebral ischemia improved the functional deficits, such as neurological score and motor coordination, and survival rates. In addition, both groups did not increase the HMGB1 level in plasma, and decreased the number of Iba1 expressing HMGB1 positive cells and TUNEL positive cells, which suggest that CBD may be cerebroprotective not only during the early phase, but also during the chronic phase after ischemic stroke [71]. However, treatment with CBD from Day 5 did not improve the functional outcome Day 14 after cerebral ischemia. As described above, CBD has a potent anti-inflammatory effect, such as inhibition of glial activation. Activated microglia and reactive astrocytes have also a role of beneficial in ischemic brain. For example, microglia can produce neurotorophic factors such as brain-derived neurotorophic factor, insulin-like growth factor 1 [95]. And, reactive astrocytes can also release many growth factors such as nerve growth factor [96]. Taken together, a treatment with CBD in ischemic early phase may implicate the neuroprotective action through inhibition of acute inflammatory reaction, whereas ischemic delayed treatment with CBD may interfere with beneficial endogenous mechanisms.
3.2. Synthetic CB1 Receptor Agonist, HU210 and R(+)-WIN55,212-2
Leker and co-workers investigated the therapeutic time window of the synthetic CB1 receptor agonist, HU210 in rats subjected to permanent middle cerebral artery occlusion (PMCAO). They demonstrated that administration of HU-210 (45 μg/kg) at 1, 2, or 4 but not 6 hours after PMCAO resulted in reduced motor disability and infarct volumes compared with vehicle controls 72 hours after cerebral ischemia and thus, therapeutic time window appeared to extend to 4 hours after PMCAO and the salutary effects of HU-210 were only partially abolished by the CB1 receptor antagonist SR141716 and warming [18]. They concluded that the neuroprotective effects of HU-210 were dependent on potent hypothermia via CB1 receptor dependent mechanism. Another research group noted that 40 min before the induction of global cerebral ischemia, treatment with R(+)-WIN55,212-2 (1 mg/kg), a synthetic aminoalkylindole cannabinoid CB1 receptor agonist, induced a dose-dependent increase in neuronal survival that reached maximal levels (56% of sham-operated controls) in a global cerebral ischemia model in rats. Treatment with R(+)-WIN55,212-2 (1 mg/kg) 30 min before ischemia, but not 60–120 min after ischemia, reduced the infarct size 24 hours after ischemic stroke onset [72], suggesting that pre-ischemic treatment with R(+)-WIN55,212-2 is protective in cerebral ischemia.
3.3. A Synthetic Non-Competitive NMDA Antagonist, HU-211, Dexanabinol
Dexanabinol (HU-211) is a synthetic, nonpsychotropic cannabinoid [90]. It has been shown to act as a noncompetitive N-methyl-D-aspartate receptor antagonist [91], as well as having antioxidant [92], anti-inflammatory effects [93,94]. The therapeutic time window of nonpsychotropic cannabinoid, HU-211 in closed head injury was first reported by Mechoulam’s research group in 1993 [73]. HU-211 dissolved in middle-chain triglycerides (MCT) oil at a dose of 25 mg/kg was given intraperitoneally immediately and 1, 2, or 3 h after impact. The drug was found to be very effective in improving motor function recovery when given 2 h after the injury. In addition, Shohami and co-workers have shown the long-term effect of HU-211 on motor and memory functions after closed head injury in the rat [74]. HU-211 (5 mg/kg) was administered intravenously at 4 or 6 h after closed head injury. Cognitive functions were evaluated using the Morris water maze, with rats trained either before or after closed head injury. The data showed that HU-211 was a potent cerebroprotective agent, with a therapeutic window of about 4 h. Moreover, this compound HU-211 was also used for cerebral ischemia. Belayev and co-workers have reported that HU-211 (4 mg/kg, i.v.) significantly improved neurological deficits and reduced brain damage 60 min after forebrain ischemia in rats, but neuroprotection was no longer significant after 3 h [75]. Leker’s research group demonstrated that treatment with HU-211, dexanabinol (4.5 mg/kg) 1 h and 3 h but not 6 h after permanent middle cerebral artery occlusion in rats improved motor function and reduced infarct volume 24 h post ischemia [76].
4. Therapeutic Possibility of CBD
Cannabinoids may play a role in neuroprotection in disorders such as stroke, Parkinson’s disease, traumatic brain injury and epilepsy. These disorders may be caused by the generation of free radicals, reactive oxygen species, and pro-inflammatory cytokines. Emerging data suggest that natural- and synthetic-cannabinoids show a potent anti-oxidant activity and anti-inflammatory reaction in in vivo and in vitro studies. Among cannabis compounds, CBD may represent a very promising agent with the highest prospect for therapeutic use. An Israeli pharmaceutical company called Pharmos was conducting human clinical trials using a synthetic dextrocannabinid, dexanabinol, which has neuroprotective properties through inhibition of NMDA glutamate receptors, as well as anti-inflammatory and antioxidant activities like CBD, in patients with severe traumatic brain injury [80] and had been granted orphan drug status by the FDA [81]. However, in December 2004, Pharmos announced that no efficacy was observed as measured by the primary clinical outcome endopoint in Phase III TBI trials. After this announcement, SAPHIR and Pharmos investigators noted that there was a potential for selection bias that may creep into well-designed randomized clinical trials as a result of factors outside the control of investigators [82]. In 2003, a clinical trial investigating the protective properties of CBD in neurodegeneration was also performed by GW pharmaceuticals, with the results still pending.
A cannabis based-extract with approx 1:1 ratio of delta9-THC and CBD (Sativex) is marketed in Canada for the symptomatic relief of neuropathic pain in adults with multiple sclerosis. Health Canada has issued a Qualifying Notice that enabled full approval in early 2005. Three years ago, a randomized controlled trial of cannabis-based medicine (CBM) was performed in patients with multiple sclerosis in the UK. The results showed that CBM improved the symptoms of spasticity in multiple sclerosis and that delta9-THC plus CBD was better tolerated than delta9-THC as a single molecule [77]. More recently, a randomized placebo-controlled double blind clinical trial of Sativex was performed in patients with painful diabetic neuropathy. This trial assessing the efficacy of Sativex in patient with depression has shown it to be no more efficacious than placebo. Patients with depression had significantly greater baseline pain scores that improved regardless of intervention [78].
More recent clinical trials in 2010 showed that adjunctive treatment with cannabidiol was safe, well tolerated and effective in the treatment of psychosis in patients with Parkinson’s disease [83]. Unfortunately, there are currently no clinical studies to investigate the cerebroprotective effect of CBD after stroke.
5. Conclusions
In the last 10 years, it has been possible to demonstrate that CBD has the following unique therapeutic profile: 1) a cannabinoid receptor-independent mechanism, 2) long-lasting cerebro- protective effect after ischemic stroke, and lack of development of tolerance. Moreover, CBD has almost no side effects, including psychotropic activity. Preliminary studies highlight the fact that the multifunctional actions of CBD may lead to benefits in more complex systems within the brain after ischemic stroke. CBD offers new therapeutic possibilities for treating ischemic stroke, although further clinical studies are needed to definitively evaluate the clinical values of CBD.
Acknowledgements
Part of this study was supported by a program for developing the supporting system for upgrading education and research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (20590552) and Advanced Materials Institute of Fukuoka University.
References
- 1.Wiley J.L., Martin B.R. Cannabinoid pharmacology: implications for additional cannabinoid receptor subtypes. Chem. Phys. Lipids. 2002;121:57–63. doi: 10.1016/s0009-3084(02)00146-9. [DOI] [PubMed] [Google Scholar]
- 2.Martin B.R., Compton D.R., Thomas B.F., Prescott W.R., Little P.J., Razdan R.K., Johnson M.R., Melvin L.S., Mechoulam R., Ward S.J. Behavioral, biochemical, and molecular modeling evaluations of cannabinoid analogs. Pharmacol. Biochem. Behav. 1991;40:471–478. doi: 10.1016/0091-3057(91)90349-7. [DOI] [PubMed] [Google Scholar]
- 3.Fujiwara M. Characteristics of abnormal behavior induced by delta9-tetrahydrocannabinol in rats. Nippon. Yakurigaku. Zasshi. 2001;117:35–41. doi: 10.1254/fpj.117.35. [DOI] [PubMed] [Google Scholar]
- 4.Mishima K., Egashira N., Hirosawa N., Fujii M., Matsumoto Y., Iwasaki K., Fujiwara M. Characteristics of learning and memory impairment induced by delta9-tetrahydrocannabinol in rats. Jpn. J. Pharmacol. 2001;87:297–308. doi: 10.1254/jjp.87.297. [DOI] [PubMed] [Google Scholar]
- 5.Nagai H., Egashira N., Sano K., Ogata A., Mizuki A., Mishima K., Iwasaki K., Shoyama Y., Nishimura R., Fujiwara M. Antipsychotics improve delta9-tetrahydrocannabinol-induced impairment of the prepulse inhibition of the startle reflex in mice. Pharmacol. Biochem. Behav. 2006;84:330–336. doi: 10.1016/j.pbb.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 6.Egashira N., Matsuda T., Koushi E., Mishima K., Iwasaki K., Shoyama Y., Fujiwara M. Involvement of 5-hydroxytriptamine1A receptors in delta9-tetrahydrocannabinol-induced catalepsy-like immobilization in mice. Eur. J. Pharmacol. 2006;550:117–122. doi: 10.1016/j.ejphar.2006.08.051. [DOI] [PubMed] [Google Scholar]
- 7.Klein T.W., Neuton C.A., Friedman H. Cannabinoids and the immune system. Pain. Res. Manag. 2001;6:95–101. doi: 10.1155/2001/326867. [DOI] [PubMed] [Google Scholar]
- 8.Maresz K., Carrier E.J., Ponomarev E.D., Hilard C.J., Dittel B.N. Modulation of the cannabinoid CB2 receptor in microglial cells in response to inflammatory stimuli. J. Neurochem. 2005;95:437–445. doi: 10.1111/j.1471-4159.2005.03380.x. [DOI] [PubMed] [Google Scholar]
- 9.Pertwee R.G. Reseptors and channels targeted by synthetic cannabinoid receptor agonists and antagonists. Curr. Med. Chem. 2010;17:1360–1381. doi: 10.2174/092986710790980050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Izzo A.A., Borrelli F., Capasso R., Di Marzo V., Mechoulam R. Non-psychotropic plant cannabinoids: new therapeutic opportunities from an ancient herb. Trends. Pharmacol. Sci. 2009;30:515–527. doi: 10.1016/j.tips.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 11.Mechoulam R., Parker L.A., Gallily R. Cannabidiol: an overview of some pharmacological aspects. J. Clin. Phamacol. 2002;42:11–19. doi: 10.1002/j.1552-4604.2002.tb05998.x. [DOI] [PubMed] [Google Scholar]
- 12.Iuvone T., Esposito G., Esposito R., Santamaria R., Di Rosa M., Izzo A.A. Neuroprotective effect of cannabidiol, a non-psychoactive component from Cannabis sativa, on beta-amyloid-induced toxicity in PC12 cells. J. Neurochem. 2004;89:134–141. doi: 10.1111/j.1471-4159.2003.02327.x. [DOI] [PubMed] [Google Scholar]
- 13.Braida D., Pegorini S., Arcidiacono M.V., Consalez G.G., Croci L., Sala M. Post-ischemic treatment with cannabidiol prevents electroencephalographic flattening, hyperlocomotion and neuronal injury in gerbils. Neurosci. Lett. 2003;346:61–64. doi: 10.1016/S0304-3940(03)00569-X. [DOI] [PubMed] [Google Scholar]
- 14.Hampson A.J., Grimaldi M., Lolic M., Wink D., Rosenthal R., Axelrod J. Neuroprotective antioxidants from marijuana. Ann. N.Y. Acad. Sci. 2000;899:274–282. [PubMed] [Google Scholar]
- 15.Carrier E.J., Auchampach J.A., Hillard C.J. Inhibition of an equilibrative nucleoside transporter by cannabidiol: a mechanism of cannabinoid immunosuppression. Proc. Natl. Acad. Sci. USA. 2006;103:7895–7900. doi: 10.1073/pnas.0511232103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ryberg E., Larsson N., Sjögren S., Hjorth S., Hermansson N.O., Leonova J., Elebring T., Nilsson K., Drmota T., Greasley P.J. The orphan receptor GPR55 is a novel cannabinoid receptor. Br. J. Pharmacol. 2007;152:1092–1101. doi: 10.1038/sj.bjp.0707460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Molina-Holgado E., Vela J.M., Arevalo-Martin A., Almazan G., Molina-Holgado F., Borrell J., Guaza C. Cannabinoids promote oligodendrocyte progenitor survival: involvement of cannabinoid receptors and phosphotidylinositol-3 kinase/Akt signaling. J. Neurosci. 2002;22:9742–9753. doi: 10.1523/JNEUROSCI.22-22-09742.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leker R.R., Gai N., Mechoulam R., Ovadia H. Drug-induced hypothermia reduces ischemic damage: effects of the cannabinoid HU-210. Stroke. 2003;34:2000–2006. doi: 10.1161/01.STR.0000079817.68944.1E. [DOI] [PubMed] [Google Scholar]
- 19.Pertwee R.G., Ross R.A. Cannabinoid receptors and their liganads. Prostaglandins. Leukot. Essent. Fatty. Acids. 2002;66:101–121. doi: 10.1054/plef.2001.0341. [DOI] [PubMed] [Google Scholar]
- 20.Thomas A., Baillie G.L., Phillips A.M., Razdan R.K., Ross R.A., Pertwee R.G. Cannabidiol displays unexpectedly high potency as an antagonist of CB1 and CB2 receptor agonist. Br. J. Pharmacol. 2007;150:613–623. doi: 10.1038/sj.bjp.0707133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Castillo A., Tolón M.R., Fernández-Ruiz J., Romero J., Martinez-Orgado J. The neuroprotective effect of cannabidiol in an in vitro model of newborn hypoxic-ischemic brain damage in mice is mediated by CB(2) and adenosine receptors. Neurobiol. Dis. 2010;37:434–440. doi: 10.1016/j.nbd.2009.10.023. [DOI] [PubMed] [Google Scholar]
- 22.Mishima K., Hayakawa K., Abe K., Ikeda T., Egashira N., Iwasaki K., Fujiwara M. Cannabidiol prevents cerebral infarction via a serotonergic 5-hydroxytriptamine1A receptor-dependent mechanism. Stroke. 2005;36:1077–1082. doi: 10.1161/01.STR.0000163083.59201.34. [DOI] [PubMed] [Google Scholar]
- 23.Watanabe K., Kayano Y., Yamamoto I., Yoshimura H. Inhibition of anandamide amidase activity in mouse brain microsomes by cannabinoids. Biol. Pharm. Bull. 1996;19:1109–1111. doi: 10.1248/bpb.19.1109. [DOI] [PubMed] [Google Scholar]
- 24.Bisogno T., Hanus L., De Petrocellis L., Tchilibon S., Ponde D.E., Brandi I., Moriello A.S., Davis J.B., Mechoulam R., Di Marzo V. Molecular targets for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br. J. Pharmacol. 2001;134:845–852. doi: 10.1038/sj.bjp.0704327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang C.C., Lo S.W., Hsu K.S. Presynaptic mechanisms underlying cannabinoid inhibition of excitatory synaptic transmission in rat striatal neurons. J. Physiol. 2001;532:731–748. doi: 10.1111/j.1469-7793.2001.0731e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lévénés C., Daniel H., Soubrié P., Crépel F. Cannabinoids decrease excitatory synaptic transmission and impair long-term depression in rat cerebellar Purkinje cells. J. Physiol. 1998;510:867–879. doi: 10.1111/j.1469-7793.1998.867bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shen M., Piser T.M., Sybold V.S., Thayer S.A. Cannabinoid receptor agonists inhibit glutamaterigic synaptic transmission in rat hippocampal cultures. J. Neurosci. 1996;16:4322–4334. doi: 10.1523/JNEUROSCI.16-14-04322.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Köfalvi A., Vizi E.S., Ledent C., Sperlágh B. Cannabinoids inhibit release of [3H]glutamate from rodent hippocampal synaptosomes via a novel CB1 receptor-independent action. Eur. J. Neurosci. 2003;18:1973–1978. doi: 10.1046/j.1460-9568.2003.02897.x. [DOI] [PubMed] [Google Scholar]
- 29.Fowler C.J. Plant-derived, synthetic and endogenous cannabinoids as neuroprotective agents. Non-psychoactive cannabinoids ‘entourage’ compounds and inhibitors N-acyl ethanolamine breakdown as therapeutic strategies to avoid psychotropic effects. Brain. Res. Brain. Res. Rev. 2003;41:26–43. doi: 10.1016/S0165-0173(02)00218-7. [DOI] [PubMed] [Google Scholar]
- 30.Hayakawa K., Mishima K., Nozako M., Hazekawa M., Irie K., Fujioka M., Orito K., Abe K., Hasebe N., Egashira N., Iwasaki K., Fujiwara M. Delayed treatment with cannabidiol has a cerebroprotective action via a cannabinoid receptor-independent myeloperoxidase-inhibiting mechanism. J. Neurochem. 2007;102:1488–1496. doi: 10.1111/j.1471-4159.2007.04565.x. [DOI] [PubMed] [Google Scholar]
- 31.Karnoil I.G., Shirakawa I., Kasinski N., Pfeferman A., Carlini E.A. Cannabidiol interferes with the effects of delta9-tetrahydrocannabinol in man. Eur. J. Pharmacol. 1974;28:172–177. doi: 10.1016/0014-2999(74)90129-0. [DOI] [PubMed] [Google Scholar]
- 32.Zuardi A.W. Cannabidiol: from an inactive cannabinoid to a drug with wide spectrum of action. Rev. Bras. Psiquiatr. 2008;30:271–280. doi: 10.1590/s1516-44462008000300015. [DOI] [PubMed] [Google Scholar]
- 33.Moreira F.A., Aguiar D.C., Campos A.C., Lisboa S.F., Terzian A.L., Resstel L.B., Guimarães F.S. Antiaversive effects of cannabinoids: is the periaqueductal gray involved? Neural. Plast. 2009 doi: 10.1155/2009/625469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Campos A.C., Guimarães F.S. Involvement of 5HT1A receptors in the anxiolytic-like effects of cannabidiol injected into the dorsolateral periaqueductal gray of rats. Psychopharmacology (Berl). 2008;199:223–230. doi: 10.1007/s00213-008-1168-x. [DOI] [PubMed] [Google Scholar]
- 35.Gardner C.R. Resent developments in 5HT-related pharmacology of animal models of anxiety. Pharmacol. Biochem. Behav. 1986;24:1479–1485. doi: 10.1016/0091-3057(86)90215-7. [DOI] [PubMed] [Google Scholar]
- 36.Eltze M., Boer R., Sanders K.H., Kolassa N. Vasodilatation elicited by 5-HT1A receptor agonists in constant-pressure-perfused rat kidney is mediated by blockade of alpha 1A-adrenoceptors. Eur. J. Pharmacol. 1991;202:33–44. doi: 10.1016/0014-2999(91)90250-t. [DOI] [PubMed] [Google Scholar]
- 37.Verbeuren T.J. Vasodilator effect of tertatolol in isolated perfused rat kidneys: involvement of endothelial 5-HT1A receptors. Cardiology. 1993;1:5–9. doi: 10.1159/000176004. [DOI] [PubMed] [Google Scholar]
- 38.Semkova I., Wolz P., Krieglstein J. Neuroprotective effect of 5-HT1A receptor agonist, BAY X 3702 demonstrated in vitro and in vivo. Eur. J. Pharmacol. 1998;359:251–260. doi: 10.1016/S0014-2999(98)00634-7. [DOI] [PubMed] [Google Scholar]
- 39.Hill R.H., Svensson E., Dewael Y., Grillner S. 5-HT inhibits N-type but not L-type Ca (2+) channels via 5-HT1A receptors in lamprey spinal neurons. Eur. J. Neurosci. 2003;18:2919–2924. doi: 10.1111/j.1460-9568.2003.03051.x. [DOI] [PubMed] [Google Scholar]
- 40.Madhavan L., Freed W.J., Anantharam V., Kanthasamy A.G. 5-hydroxytryptamine 1A receptor activation protects against N-methyl-D-aspartate-induced apoptotic cell death in striatal and mesencephalic cultures. J. Pharmacol. Exp. Ther. 2003;304:913–923. doi: 10.1124/jpet.102.044370. [DOI] [PubMed] [Google Scholar]
- 41.Russo E.B., Burnett A., Hall B., Parker K.K. Agonistic properties of cannabidiol at 5-HT1a receptors. Neurochem. Res. 2005;30:1037–1043. doi: 10.1007/s11064-005-6978-1. [DOI] [PubMed] [Google Scholar]
- 42.Magen I., Avraham Y., Ackerman Z., Vorobiev L., Mechoulam R., Berry E.M. Cannabidiol ameliorates cognitive and motor impairments in bile-duct ligated mice via 5-HT1A receptor activation. Br. J. Pharmacol. 2010;159:950–957. doi: 10.1111/j.1476-5381.2009.00589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hampson A.J., Grimaldi M., Axelrod J., Wink D. Cannabidiol and (-) Delta9-tetrahydrocannabinol are neuroprotective antioxidants. Proc. Natl. Acad. Sci. USA. 1998;95:8268–8273. doi: 10.1073/pnas.95.14.8268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brand-Williams W., Cuvelier M.E., Berset C. Use of a free radical method to evaluate antioxidant activity. Lebnsm-Wiss. Technol. 1995;28:25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- 45.Hayakawa K., Mishima K., Nozako M., Ogata A., Hazekawa M., Liu A.X., Fujioka M., Abe K., Hasebe N., Egashira N., Iwasaki K., Fujiwara M. Repeated treatment with cannabidiol but not Delta9-tetrahydrocannabinol has a neuroprotective effect without the development of tolerance. Neuropharmacology. 2007;52:1079–1087. doi: 10.1016/j.neuropharm.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 46.Hamelink C., Hampson A., Wink D.A., Eiden L.E., Eskay R.L. Comparison of cannabidiol, antioxidants, and diuretics in reversing binge ethanol-induced neurotoxicity. J. Pharmacol. Exp. Ther. 2005;314:780–788. doi: 10.1124/jpet.105.085779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abood M.E., Martin B.R. Neurobiology of marijuana abuse. Trend. Pharmacol. Sci. 1992;13:201–206. doi: 10.1016/0165-6147(92)90064-d. [DOI] [PubMed] [Google Scholar]
- 48.Sim L.J., Hampson R.E., Deadwyler S.A., Childers S.R. Effects of chronic treatment with D9-tetrahydrocannabinol on cannabinoid-stimulated [35S]GTPgS autoradiography in rat brain. J. Neurosci. 1996;1:8057–8066. doi: 10.1523/JNEUROSCI.16-24-08057.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Paquett J.J., Wang H.Y., Bakshi K., Olmstead M.C. Cannabinoid-induced tolerance is associated with a CB1 receptor G protein coupling switch that is prevented by ultra-low dose rimonabant. Behav. Pharmacol. 2007;18:767–776. doi: 10.1097/FBP.0b013e3282f15890. [DOI] [PubMed] [Google Scholar]
- 50.Blair R.E., Deshpande L.S., Sombati S., Elphick M.R., Martin B.R., DeLorenzo R.J. Prolonged exposure to WIN55, 212-2 causes downregulation of the CB1 receptor and the development of tolerance to its anticonvulsant effects in the hippocampal neuronal culture of acquired epilepsy. Neuropharmacology. 2009;57:208–218. doi: 10.1016/j.neuropharm.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Watanabe K., Narimatsu S., Yamamoto I., Yoshimura H. Difference in tolerance development o hypothermia and pentobarbital-induced sleep prolongating effect of 11-hydroxy-delta 8-tetrahydrocannabinol and 11-oxo-delta 8-tetrahydrocannabinol in mice. Eur. J. Pharmacol. 1982;77:53–56. doi: 10.1016/0014-2999(82)90535-0. [DOI] [PubMed] [Google Scholar]
- 52.Watanabe K., Yamamoto I., Yoshimura H. Development of tolerance and cross-tolerance to the cataleptogenic effects of delta 8-tetrahydrocannabinol and 11-hydroxy-delta 8-tetrahydrocannabinol in mice. Eur. J. Pharmacol. 1983;94:349–351. doi: 10.1016/0014-2999(83)90427-2. [DOI] [PubMed] [Google Scholar]
- 53.Sim-Selly I.J., Martin B.R. Effect of chronic administration of R-(+)-[2,3-dyhydro-5methyl-3-[(morpholinyl)methyl]pyrrolo[1,2,3-de]-1,4-benzoxazinyl]-(1-naphthalenyl-9-methanone mesylate (WIN55,212-2) or delta(9)-tetrahydrocannabinol on cannabinoid receptor adaptation in mice. J. Pharmacol. Exp. Ther. 2002;303:36–44. doi: 10.1124/jpet.102.035618. [DOI] [PubMed] [Google Scholar]
- 54.Renyi L., Moller K.A., Ensler K., Evenden J. The non-competitive NMDA receptor antagonist (+)MK801 counteracts the long-lasting attenuation of the hypothermic response induced by acute doses of 8-OH-DPAT in the rat. Neuropharmacology. 1992;31:1265–1268. doi: 10.1016/0028-3908(92)90055-t. [DOI] [PubMed] [Google Scholar]
- 55.Young A.H., McShane R., Park S.B., Cowen P.J. Buspirone-induced hypothermia in nomal male volunteers. Biol. Psychiatr. 1993;34:665–666. doi: 10.1016/0006-3223(93)90161-6. [DOI] [PubMed] [Google Scholar]
- 56.Esposito G., Scuderi C., Savani C., Steardo L., Jr., De Filippis D., Cottone P., Iuvone T., Cuomo V., Steardo L. Cannabidiol in vivo blunts beta-amyloid induced neuroinflammation by suppressing IL-1beta and iNOS expression. Br. J. Pharmacol. 2007;151:1272–1279. doi: 10.1038/sj.bjp.0707337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McHugh D., Tanner C., Mechoulam R., Pertwee R.G., Ross R.A. Inhibition of human neutrophil chemotaxis by endogenous cannabinoids and psytocannabinoids: evidence for a site distinct from CB1 and CB2. Mol. Pharmacol. 2008;73:441–450. doi: 10.1124/mol.107.041863. [DOI] [PubMed] [Google Scholar]
- 58.Hayakawa K., Mishima K., Irie K., Hazekawa M., Mishima S., Fujioka M., Orito K., Egashira N., Katsurabayashi S., Takasaki K., Iwasaki K., Fujiwara M. Cannabidiol prevents a post-ischemic injury progressively induced by cerebral ischemia via a high-mobility group box1-inhibiting mechanism. Neuropharmacology. 2008;55:1280–1286. doi: 10.1016/j.neuropharm.2008.06.040. [DOI] [PubMed] [Google Scholar]
- 59.Bullock R., Zauner A., Myseros J.S., Marmarou A., Woodward J.J., Young H.F. Evidence for prolonged release of excitatory amino acids in severe human head trauma. Relationship to clinical events. Ann. N. Y. Acad. Sci. 1995;765:290–297. doi: 10.1111/j.1749-6632.1995.tb16586.x. [DOI] [PubMed] [Google Scholar]
- 60.Obrenovitch T.P., Richards D.A. Extracellular neurotransmitter changes in cerebral ischaemia. Cerebrovasc. Brain. Metab. Rev. 1995;7:1–54. [PubMed] [Google Scholar]
- 61.Obrenovitch T.P. The ischaemic penumbra: twenty years on. Cerebrovasc. Brain. Metab. Rev. 1995;7:297–323. [PubMed] [Google Scholar]
- 62.Martinez-Tica J.F., Zornow M.H. Effects of adenosine agonists and an antagonist on excitatory transmitter release from the ischemic rabbit hippocampus. Brain. Res. 2000;872:110–115. doi: 10.1016/s0006-8993(00)02483-5. [DOI] [PubMed] [Google Scholar]
- 63.Lakhan S.E., Kirchgessner A., Hofer M. Inflammatory mechanisms in ischemic stroke: therapeutic approaches. J. Transl. Med. 2009;7:97. doi: 10.1186/1479-5876-7-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen Y., Swanson R.A. Astrocytes and brain injury. J. Cereb. Blood. Flow. Metab. 2003;23:137–149. doi: 10.1097/01.WCB.0000044631.80210.3C. [DOI] [PubMed] [Google Scholar]
- 65.Chopp M., Zhang Z.G., Jiang Q. Neurogenesis, angiogenesis, and MRI induces of functional recovery from stroke. Stroke. 2007;38:827–831. doi: 10.1161/01.STR.0000250235.80253.e9. [DOI] [PubMed] [Google Scholar]
- 66.Lo E.H. A new penumbra: transitioning from injury into repair after stroke. Nat. Med. 2008;14:497–500. doi: 10.1038/nm1735. [DOI] [PubMed] [Google Scholar]
- 67.Sun Y., Jin K., Xie L., Childs J., Mao X.O., Logvinova A., Greenberg D.A. VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. J. Clin. Invest. 2003;11:1843–1851. doi: 10.1172/JCI17977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang Z.G., Zhang L., Jiang Q., Zhang R., Davies K., Powers C., Bruggen N., Chopp M. VEGF enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain. J. Clin. Invest. 2000;106:829–838. doi: 10.1172/JCI9369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Minger S.L., Ekonomou A., Carta E.M., Chinoy A., Perry R.H., Ballard C.G. Endogeneous neurogenesis in the human brain following cerebral ischemia. Regen. Med. 2007;2:69–74. doi: 10.2217/17460751.2.1.69. [DOI] [PubMed] [Google Scholar]
- 70.Pérez de la Ossa N., Dávalos A. Neuroprotection in cerebral infarction: the opportunity of new studies. Cerebrovasc. Dis. 2007;24:153–156. doi: 10.1159/000107391. [DOI] [PubMed] [Google Scholar]
- 71.Hayakawa K., Irie K., Sano K., Watanabe T., Higuchi S., Enoki M., Nakano T., Harada K., Ishikane S., Ikeda T., Fujioka M., Orito K., Iwasaki K., Mishima K., Fujiwara M. Therapeutic time window of cannabidiol treatment on delayed ischemic damage via high-mobility group box1-inhibiting mechanism. Biol. Pharm. Bull. 2009;32:1538–1544. doi: 10.1248/bpb.32.1538. [DOI] [PubMed] [Google Scholar]
- 72.Nagayama T., Sinor A.D., Simon R.P., Chen J., Graham S.H., Jin K., Greenberg D.A. Cannabinoids and neuroprotection in global and focal cerebral ischemia and in neuronal cultures. J. Neurosci. 1999;19:2987–2995. doi: 10.1523/JNEUROSCI.19-08-02987.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shohami E., Novikov M., Mechoulam R. A nonpsychotropic cannabinoid, HU-211, has cerebroprotective effects after closed head injury in the rat. J. Neurotrauma. 1993;10:109–119. doi: 10.1089/neu.1993.10.109. [DOI] [PubMed] [Google Scholar]
- 74.Shohami E., Novikov M., Bass R. Long-term effect of HU-211, a novel non-competitive NMDA antagonist, on motor and memory functions after closed head injury in the rat. Brain. Res. 1995;674:55–62. doi: 10.1016/0006-8993(94)01433-I. [DOI] [PubMed] [Google Scholar]
- 75.Belayev L., Busto R., Watson B.D., Ginsberg M.D. Post-ischemic administration of HU211, a novel non-competitive NMDA antagonist, protects against blood-brain barrier disruption in photochemical cortical infarction in rats: a quantitative study. Brain. Res. 1995;702:266–270. doi: 10.1016/0006-8993(95)01127-9. [DOI] [PubMed] [Google Scholar]
- 76.Lavie G., Teichner A., Shohami E., Ovadia H., Leker R.R. Long term cerebroprotective effects of dexanabinol in a model of focal cerebral ischemia. Brain. Res. 2001;901:195–201. doi: 10.1016/s0006-8993(01)02356-3. [DOI] [PubMed] [Google Scholar]
- 77.Collin C., Davies P., Mutiboko I.K., Ratcliffe S. Sativex Spasticity in MS Study Group. Eur. J. Neurol. 2007;14:290–296. doi: 10.1111/j.1468-1331.2006.01639.x. [DOI] [PubMed] [Google Scholar]
- 78.Selvarajah D., Gandhi R., Emery C.J., Tesfaya S. Randamized placebo-controlled double-blind clinical trial of cannabis-based medicinal product (Sativex) in painful diabetic neuropathy: depression is a major confounding factor. Diabetes Care. 2010;33:128–130. doi: 10.2337/dc09-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ryan D., Drysdale A.J., Lafourcade C., Pertwee R.G., Platt B. Cannabidiol targets mitochondria to regulate intracellular Ca2+ levels. J. Neurosci. 2009;29:2053–2063. doi: 10.1523/JNEUROSCI.4212-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Knoller N., Levi L., Shoshan I., Reichenthal E., Razon N., Rappaport Z.H., Biegon A. Dexanabinol (HU-211) in the treatment of severe closed head injury: a randomized, placebo-controlled, phase Ⅱ clinical trial. Crit. Care. Med. 2002;30:548–554. doi: 10.1097/00003246-200203000-00009. [DOI] [PubMed] [Google Scholar]
- 81.Mechoulam R., Hanus L. The cannabinoids: an overview. Therapeutic implications in vomiting and nausea after cancer chemotherapy, in appetite promotion, in multiple sclerosis and in neuroprotection. Pain. Res. Manag. 2001;6:67–73. doi: 10.1155/2001/183057. [DOI] [PubMed] [Google Scholar]
- 82.Slieker F.J., Kompanje E.J., Murray G.D., Ohman J., Stocchetti N., Teasdale S.G., Maas A.I. SAPHIR and Pharmos TBI Investigators. Neurosurgery. 2008;62:1321–1329. doi: 10.1227/01.neu.0000333304.79931.4d. [DOI] [PubMed] [Google Scholar]
- 83.Tomillero A., Moral M.A. Gateways to clinical trials. Methods. Find. Exp. Clin. Pharmacol. 2010;32:193–215. doi: 10.1358/mf.2010.32.3.1492017. [DOI] [PubMed] [Google Scholar]
- 84.Fujiwara M., Egashira N. New perspectives in the studies on endocannabinoid and cannabis: abnormal behaviors associate with CB1 cannabinoid receptor and development of therapeutic application. J. Pharmacol. Sci. 2004;96:362–366. doi: 10.1254/jphs.fmj04003x2. [DOI] [PubMed] [Google Scholar]
- 85.Egashira N., Matsuda T., Koushi E., Mishima K., Iwasaki K., Shoyama Y., Fujiwara M. Involvement of 5-hydroxytriptamine1A receptors in Delta9-tetrahydrocannabinol-induced catalepsy-like immobilization in mice. Eur. J. Pharmacol. 2006;550:117–122. doi: 10.1016/j.ejphar.2006.08.051. [DOI] [PubMed] [Google Scholar]
- 86.Egashira N., Ishigami N., Mishima K., Iwasaki K., Oishi R., Fujiwara M. Delta9-Tetrahydrocannabinol-induced cognitive deficits are reversed by olanzapine but not haloperidol in rats. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2008;32:499–506. doi: 10.1016/j.pnpbp.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 87.Egashira N., Matsuda T., Koushi E., Higashihara F., Mishima K., Chidori S., Hasebe N., Iwasaki K., Nishimura R., Oishi R., Fujiwara M. Delta (9)-tetrahydrocannabinol prolongs the immobility time in the mouse forced swim test: involvement of cannabinoid CB (1) receptor and serotonergic system. Eur. J. Pharmacol. 2008;589:117–121. doi: 10.1016/j.ejphar.2008.03.046. [DOI] [PubMed] [Google Scholar]
- 88.Higuchi S., Irie K., Mishima S., Araki M., Ohji M., Shirakawa A., Akitake Y., Matsuyama K., Mishima K., Mishima K., Iwasaki K., Fujiwara M. The cannabinoid 1-receptor silent antagonist-2050 attenuates preference for high-fat diet and activated astrocytes in mice. J. Pharmacol. Sci. 2010;112:369–372. doi: 10.1254/jphs.09326sc. [DOI] [PubMed] [Google Scholar]
- 89.Hayakawa K., Mishima K., Abe K., Hasebe N., Takamatsu F., Yasuda H., Ikeda T., Inui K., Egashira N., Iwasaki K., Fujiwara M. Cannabidiol prevents infarction via the non-CB1 cannabinoid receptor mechanism. Neuroreport. 2004;15:2381–2385. doi: 10.1097/00001756-200410250-00016. [DOI] [PubMed] [Google Scholar]
- 90.Mechoulam R., Feigenbaum J.J., Lander N., Segal M., Järbe T.U., Hiltunen A.J., Consroe P. Enantiomeric cannabinoids: stereospecificity of psychotropic activity. Experientia. 1988;44:762–764. doi: 10.1007/BF01959156. [DOI] [PubMed] [Google Scholar]
- 91.Feigenbaum J.J., Bergmann F., Richmond S.A., Mechoulam R., Nadler V., Kloog Y., Sokolovsky M. Proc. Natl. Acad. Sci. USA. 1989;86:9584–9587. doi: 10.1073/pnas.86.23.9584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Eshhar N., Striem S., Kohen R., Tirosh O., Biegon A. Neuroprotective and antioxidant activities of HU-211, a novel NMDA receptor antagonist. Eur. J. Pharmacol. 1995;283:19–29. doi: 10.1016/0014-2999(95)00271-l. [DOI] [PubMed] [Google Scholar]
- 93.Shohami E., Gallily R., Mechoulam R., Bass R., Ben-Hur T. Cytokine production in the brain following closed head injury: dexanabinol (HU-211) is a novel TNF-alpha inhibitor and an effective neuroprotectant. J. Neuroimmunol. 1997;72:169–177. doi: 10.1016/s0165-5728(96)00181-6. [DOI] [PubMed] [Google Scholar]
- 94.Yoles E., Belkin M., Schwartz M. HU-211, a nonpsychotropic cannabinoid, produces short- and long-term neuroprotection after optic nerve axotomy. J. Neurotrauma. 1996;13:49–57. doi: 10.1089/neu.1996.13.49. [DOI] [PubMed] [Google Scholar]
- 95.Constam D.B., Philipp J., Malipiero U.V., ten Dijke P., Schachner M., Fontana A. Differential expression of transforming growth factor-beta 1, -beta 2, and -beta 3 by glioblastoma cells, astrocytes, and microglia. J. Immunology. 1992;148:1404–1410. [PubMed] [Google Scholar]
- 96.Strauss S., Otten U., Joggerst B., Piuss K., Volk B. Increased levels of nerve growth factor (NGF) protein and mRNA and reactive gliosis following kainic acid injection into the rat striatum. Neurosci. Lett. 1994;168:193–196. doi: 10.1016/0304-3940(94)90448-0. [DOI] [PubMed] [Google Scholar]