Abstract
Vascular Endothelial Growth Factor-C (VEGF-C) is a secreted growth factor essential for lymphangiogenesis. VEGF-C functions in both physiological and pathological lymphangiogenesis, particularly in tumor metastasis, making it an attractive therapeutic target. Members of two families of cell surface receptors transduce VEGF-C signals, Neuropilin-2 (Nrp2) and VEGF-receptor (VEGFR)-2/3. Nrp2 is a promising target for inhibition since it is highly expressed in lymphatic vessels. Here we describe a microplate-based assay for discovery of VEGF-C/Nrp2 inhibitors. We optimize this assay for use in screening an inhibitor library and identify three novel Nrp2/VEGF-C binding inhibitors from the NIH Clinical Collection small molecule library.
Keywords: Neuropilin, VEGF, ligand, receptor, assay, inhibitor
Neuropilin-2 (Nrp2) is highly expressed in lymphatic endothelial cells where it is important for physiological lymphangiogenesis and can contribute to pathological tumor lymphangiogenesis [1-3]. Additionally, expression of Nrp2 and its ligand, vascular endothelial growth factor-C (VEGF-C), are induced in a variety of cancer types and their expression is directly correlated with metastasis to regional lymph nodes and advanced stage disease. Thus, blocking Nrp2 activation by VEGF-C represents a promising anti-lymphangiogenesis therapeutic strategy. Mature VEGF-C, produced through proteolytic removal of N- and C-terminal pro-peptides, binds domains b1b2 of Nrp2 [4]. A number of C-terminal arginine containing molecules have been identified as inhibitors of Nrp/ligand binding [5]. However, these compounds have limited potency and thus identification of inhibitors that target secondary binding sites are of significant interest [6]. To identify novel small molecule inhibitors of VEGF-C binding to Nrp2-b1b2, we developed and optimized an assay that utilizes Nrp2-affinity plates and an alkaline phosphatase (AP) fusion of VEGF-C (AP-VEGF-C) for detection of competitive inhibitors of ligand binding. We used this assay to screen the National Institutes of Health (NIH) Clinical Collection, provided through the NIH Molecular Libraries Roadmap Initiative, and we report the identification of three inhibitors of VEGF-C binding to Nrp2.
To make Nrp2-b1b2-affinity plates, we expressed domains b1b2 of human Nrp2 (Nrp2-b1b2, residues 276-595) in Escherichia coli (E. coli) strain Rosetta Gami-2(DE3) (Novagen/EMD Millipore, Billerica, MA) as a hexa-histidine fusion protein from pET28b (Novagen/EMD Millipore). Nrp2-b1b2 was purified in a two-step process via immobilized metal affinity chromatography (IMAC) (HIS-Select HF Nickel Affinity Gel, Sigma-Aldrich, St. Louis, MO) followed by heparin affinity chromatography (HiTrap Heparin HP, GE Healthcare Life Sciences, Pittsburgh, PA) [7]. Purified Nrp2-b1b2 was then diluted to 50 μg/mL with 50 mM Na2CO3 pH 10.4 and immediately added to 96-well protein high-bind microplates (Plate #9018, Corning, Corning, NY) and incubated for 1 h at 37°C. Bovine serum albumin (BSA) (Simga-Aldrich) was used to coat control wells. The plate was then aspirated, washed 5× 100 μ L with PBS-T (phosphate buffered saline, 0.1% Tween 20), and stored with 100 μ L PBS-T at 4°C. To detect VEGF-C binding to Nrp2-affinity plates, we produced mature mouse VEGF-C (residues 108-223) fused at its N-terminus to the reporter gene alkaline phosphatase (AP) (AP-VEGF-C). APVEGF-C was expressed via PEI mediated transient transfection of Chinese hamster ovary suspension cells (CHO-S) [8, 9] from the pAPtag-5 vector (GenHunter Corporation, Nashville, TN). AP-VEGF-C conditioned media was then buffer exchanged into assay buffer (50 mM NaCl, 200 mM Tris pH 7.4) and concentrated to an activity of 7 × 10−1 u/mL. AP-VEGF-C binding to Nrp2-b1b2-affinity plates was detected by adding 100 μL of 1X AP substrate and the evolution of para-nitrophenol phosphate was monitored by measuring 405 nm absorption on a microplate reader. Prior to analysis the absorbance of the control wells (BSA-coated) was used to background correct the data.
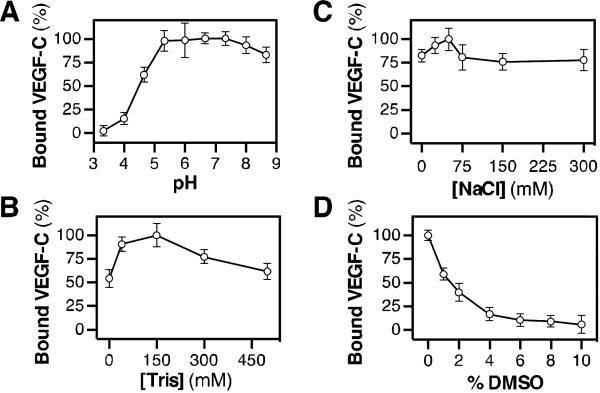
The assay conditions for screening, including pH and buffer, salt, and DMSO concentration, were optimized. First, we analyzed AP-VEGF-C binding to Nrp2-b1b2-affinity plates over a pH range of 3 to 9 (Fig. 1A). 100 mM citrate was used to buffer samples with a pH less than 6.5 and 100 mM Tris was used to buffer samples with a pH greater than 6.5. The highest AP-VEGF-C binding was observed as a stable plateau between pH = 5.5 and pH = 8. Reduction of pH below 5 eventually resulted in a complete loss of binding. The observed pH sensitivity of AP-VEGFC/Nrp2-b1b2 binding is consistent with the known requirement for a C-terminal arginine in Nrp ligands [7, 10]. In light of the observed sensitivity of AP-VEGF-C/Nrp2-b1b2 binding to extremes in pH, we determined the effect of buffer concentration on binding (Fig. 1B). AP-VEGF-C binding to Nrp2-b1b2-affinity plates was analyzed at physiological pH = 7.4 with Tris concentrations between 0 mM and 500 mM. The assay tolerated a range of Tris concentrations, with the highest binding observed at 150 mM. Additionally, we determined the effect of ionic strength on AP-VEGF-C/Nrp2-b1b2 binding (Fig. 1C). Binding was measured in 200 mM Tris pH 7.4 over a range of NaCl concentrations, from 0 mM to 300 mM. The highest AP-VEGF-C binding was observed at 50 mM NaCl and greater than 75% binding was observed for all other NaCl concentrations tested. Collectively, we used these data to define the assay buffer as 50 mM NaCl, 200 mM Tris pH 7.4 for producing the highest signal-to-noise while maintaining tight control over pH.
Figure 1. Assay optimization.
(A) The pH sensitivity of AP-VEGF-C/Nrp2-b1b2 binding was analyzed over a pH range of 3 to 9. (B) The effect of increasing Tris concentrations on APVEGF-C/Nrp2-b1b2 was determined. (C) AP-VEGF-C/Nrp2-b1b2 binding was analyzed for a range of NaCl concentrations. (D) DMSO tolerance was determined by titrating DMSO with APVEGF-C and analyzing binding to Nrp2-b1b2-affinity plates.
As a final optimization step, we determined the effect of dimethylsulfoxide (DMSO), a common solvent used in small molecule libraries, on AP-VEGF-C binding to Nrp2-b1b2-affinity plates (Fig. 1D). AP-VEGF-C was prepared in assay buffer and binding to Nrp2-b1b2 was analyzed in the presence of increasing concentrations of DMSO, from 0% to 10% (v/v). DMSO markedly inhibited AP-VEGF-C/Nrp2-b1b2 binding with 60% of AP-VEGF-C binding lost at 2% DMSO. Therefore, in order to maintain robust assay signal, a maximum of 2% DMSO was not exceeded in subsequent experiments.
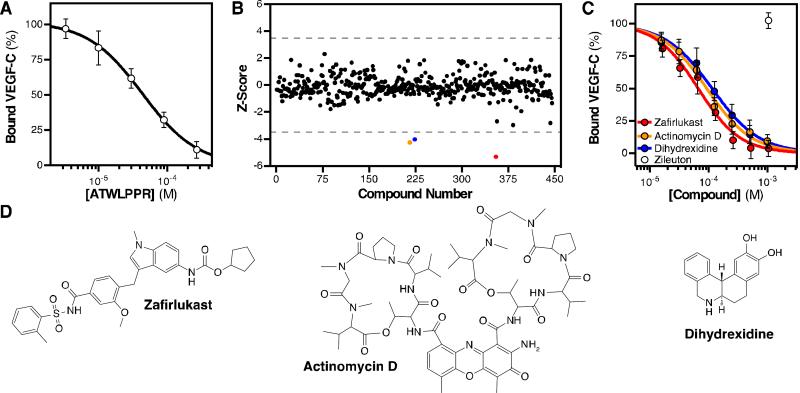
With the optimized assay conditions, we then validated our approach by testing the ability of the Nrp-binding inhibitory peptide, ATWLPPR [11-13], which was initially identified as a competitive inhibitor of Nrp1 ligand binding, to inhibit AP-VEGF-C binding to Nrp2-b1b2 (Fig. 2A). Serial dilutions of ATWLPPR from 300 μM to 3 μM were prepared in assay buffer containing AP-VEGFC and 2% DMSO. ATWLPPR fully inhibited AP-VEGF-C/Nrp2-b1b2 binding with an IC50 = 43 μM. This is consistent with the previously published potency of this peptide against Nrp1 (IC50 = 60 μM) and confirms that the core ligand binding pocket engaged by ATWLPPR is conserved between Nrp1 and Nrp2 [11]. These data confirm the functionality of our assay in characterizing inhibitors of VEGF-C/Nrp2-b1b2 binding.
Figure 2. Screen for small molecule inhibitors.
(A) The ability of the peptide ATWLPPR to inhibit AP-VEGF-C/Nrp2-b1b2 binding was determined. ATWLPPR inhibited binding with an IC50 = 43 μM. (B) The NIH Clinical Collection small molecule library was screened for inhibitors of AP-VEGF-C/Nrp2-b1b2 binding. Three compounds exceeded the hit criteria (dashed line), reducing binding by more than 3.5 standard deviations from the mean. (C) Hits were validated by titrating each compound with AP-VEGF-C and measuring Nrp2-b1b2 binding. Zafirlukast, Actinomycin D, and Dihydrexidine inhibited AP-VEGF-C/Nrp2-b1b2 binding with IC50 = 66, 92, and 113 μM, respectively. Zileuton was unable to inhibit binding. (D) Chemical structure of the identified hit compounds.
C-terminal arginine-like compounds have established efficacy for inhibition of VEGF/Nrp binding [14]. However, these compounds suffer from limited potency and thus we aimed to identify non-C-terminal arginine-like inhibitors. The NIH Clinical Collection is a diverse small molecule library of approximately 450 drug-like compounds that have a history of clinical testing with known safety profiles. We screened the NIH Clinical Collection for inhibitors of VEGF-C/Nrp2-b1b2 binding (Fig. 2B, PubChem BioAssay AID: 16941), testing each compound at 200 μM (2% DMSO). The quality of the results were evaluated by two commonly used parameters in high-throughput screens: signal-to-noise (S/N) and Z-factor (Z), the latter takes into account both the assay dynamic range and signal variability. By these two parameters our assay was defined as high quality with an average across-plate S/N = 32 and Z = 0.55 [15]. The data were Z-score normalized (rescaled to set the mean signal (μ) = 0 with a standard deviation (μ) = 1) [16], thus allowing direct comparison of each compound’s activity across different plates. The Z-score for each compound was plotted and a stringent “hit” criteria was defined as differing by more than 3.5 standard deviations from the mean. This selection criteria restricted hits to <1% of the screened compounds and included only inhibitors (Fig. 2B, dashed line). Importantly, all hit compounds were tested for the ability to directly alter the activity of AP, resulting in a false positive, and no effect was observed.
Three compounds exceeded the criteria for a hit: Zafirlukast (μ = -5.3), dihydrexidine HCl (μ = −4.3), and Actinomycin D (μ = −4.0). Each hit was confirmed by preparing a concentrated compound stock (50 mM) in 100% DMSO and measuring the inhibitory potency against APVEGF-C/Nrp2-b1b2 binding in triplicate (Fig. 2C). All hit compounds showed greater than 90% inhibition of AP-VEGF-C/Nrp2-b1b2 binding and their relative potencies were consistent with the initial screen. Zafirlukast (Santa Cruz Biotechnology, Dallas, TX) was the most potent inhibitor with an IC50 = 66 μM, dihydrexidine hydrochloride (Tocris Bioscience, Bristol, UK) was the weakest inhibitor (IC50 = 113 μM), and Actinomycin D (Tocris Bioscience) fell in between these two compounds (IC50 = 92 μM). Zileuton (Santa Cruz Biotechnology), a drug structurally distinct from Zafirlukast but also a leukotriene receptor antagonist, was tested alongside the compounds and showed no ability to inhibit AP-VEGF-C/Nrp2-b1b2, indicating the specific activity of these compounds.
Here we report the development of a VEGF-C/Nrp2 binding assay that we used to screen a small molecule compound library for inhibitors. We identified three novel inhibitors of VEGFC/Nrp2 binding. Intriguingly, both Actinomycin D and Zafirlukast are used to treat disease states, tumor angiogenesis and asthma, respectively, where anti-angiogenesis has been suggested to be an important beneficial secondary effect of treatment [17, 18]. In both cases, a direct contribution of Nrp inhibition in the biological activities of these compounds is an important area for future studies. Additionally, the development of this assay opens the door for screening other large chemically diverse small molecule libraries to identify more potent inhibitors of VEGF-C function. Notably, none of the three molecules identified contain a C-terminal arginine-like moiety (Fig. 2D), thus providing novel lead compounds for optimization and combinatorial approaches towards the production of a potent and selective Nrp2 inhibitor.
Acknowledgements
This work was supported by National Institutes of Health grants R01GM094155 (C.W.V.K.) and T32HL072743 (M.W.P.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stacker SA, Achen MG, Jussila L, Baldwin ME, Alitalo K. Lymphangiogenesis and cancer metastasis. Nature reviews. Cancer. 2002;2:573–583. doi: 10.1038/nrc863. [DOI] [PubMed] [Google Scholar]
- 2.Ellis LM. The role of neuropilins in cancer. Mol Cancer Ther. 2006;5:1099–1107. doi: 10.1158/1535-7163.MCT-05-0538. [DOI] [PubMed] [Google Scholar]
- 3.Parker MW, Guo HF, Li X, Linkugel AD, Vander Kooi CW. Function of members of the neuropilin family as essential pleiotropic cell surface receptors. Biochemistry. 2012;51:9437–9446. doi: 10.1021/bi3012143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karpanen T, Heckman CA, Keskitalo S, Jeltsch M, Ollila H, Neufeld G, Tamagnone L, Alitalo K. Functional interaction of VEGF-C and VEGF-D with neuropilin receptors. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2006;20:1462–1472. doi: 10.1096/fj.05-5646com. [DOI] [PubMed] [Google Scholar]
- 5.Parker MW, Linkugel AD, Vander Kooi CW. Effect of C-Terminal Sequence on Competitive Semaphorin Binding to Neuropilin-1. Journal of molecular biology. 2013 doi: 10.1016/j.jmb.2013.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo HF, Li X, Parker MW, Waltenberger J, Becker PM, Vander Kooi CW. Mechanistic Basis for the Potent Anti-Angiogenic Activity of Semaphorin 3F. Biochemistry. 2013 doi: 10.1021/bi401034q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vander Kooi CW, Jusino MA, Perman B, Neau DB, Bellamy HD, Leahy DJ. Structural basis for ligand and heparin binding to neuropilin B domains. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:6152–6157. doi: 10.1073/pnas.0700043104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Longo PA, Kavran JM, Kim MS, Leahy DJ. Transient mammalian cell transfection with polyethylenimine (PEI) Methods in enzymology. 2013;529:227–240. doi: 10.1016/B978-0-12-418687-3.00018-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aricescu AR, Lu W, Jones EY. A time- and cost-efficient system for high-level protein production in mammalian cells, Acta crystallographica. Section D. Biological crystallography. 2006;62:1243–1250. doi: 10.1107/S0907444906029799. [DOI] [PubMed] [Google Scholar]
- 10.Parker MW, Xu P, Guo HF, Vander Kooi CW. Mechanism of Selective VEGF-A Binding by Neuropilin-1 Reveals a Basis for Specific Ligand Inhibition. PloS one. 2012;7:e49177. doi: 10.1371/journal.pone.0049177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Starzec A, Vassy R, Martin A, Lecouvey M, Di Benedetto M, Crepin M, Perret GY. Antiangiogenic and antitumor activities of peptide inhibiting the vascular endothelial growth factor binding to neuropilin-1. Life sciences. 2006;79:2370–2381. doi: 10.1016/j.lfs.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 12.Starzec A, Ladam P, Vassy R, Badache S, Bouchemal N, Navaza A, du Penhoat CH, Perret GY. Structure-function analysis of the antiangiogenic ATWLPPR peptide inhibiting VEGF(165) binding to neuropilin-1 and molecular dynamics simulations of the ATWLPPR/neuropilin-1 complex. Peptides. 2007;28:2397–2402. doi: 10.1016/j.peptides.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 13.Parker MW, Hellman LM, Xu P, Fried MG, Vander Kooi CW. Furin processing of semaphorin 3F determines its anti-angiogenic activity by regulating direct binding and competition for neuropilin. Biochemistry. 2010;49:4068–4075. doi: 10.1021/bi100327r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jarvis A, Allerston CK, Jia H, Herzog B, Garza-Garcia A, Winfield N, Ellard K, Aqil R, Lynch R, Chapman C, Hartzoulakis B, Nally J, Stewart M, Cheng L, Menon M, Tickner M, Djordjevic S, Driscoll PC, Zachary I, Selwood DL. Small molecule inhibitors of the neuropilin-1 vascular endothelial growth factor A (VEGF-A) interaction. J Med Chem. 2010;53:2215–2226. doi: 10.1021/jm901755g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang JH, Chung TD, Oldenburg KR. A Simple Statistical Parameter for Use in Evaluation and Validation of High Throughput Screening Assays. Journal of biomolecular screening. 1999;4:67–73. doi: 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]
- 16.Malo N, Hanley JA, Cerquozzi S, Pelletier J, Nadon R. Statistical practice in high-throughput screening data analysis. Nature biotechnology. 2006;24:167–175. doi: 10.1038/nbt1186. [DOI] [PubMed] [Google Scholar]
- 17.Lee CG, Ma B, Takyar S, Ahangari F, Delacruz C, He CH, Elias JA. Studies of vascular endothelial growth factor in asthma and chronic obstructive pulmonary disease. Proceedings of the American Thoracic Society. 2011;8:512–515. doi: 10.1513/pats.201102-018MW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blumberg N. Tumor angiogenesis factor. Speculations on an approach to cancer chemotherapy. The Yale journal of biology and medicine. 1974;47:71–81. [PMC free article] [PubMed] [Google Scholar]