While there is currently no approved norovirus vaccine, recent human clinical efficacy trials with virus-like particle–based vaccines have shown promise. Several additional norovirus vaccine platforms are also currently under development and will likely improve norovirus vaccines of the future.
Keywords: norovirus, vaccine
Abstract
Noroviruses represent the most important cause of acute gastroenteritis worldwide; however, currently no licensed vaccine exists. Widespread vaccination that minimizes overall norovirus disease burden would benefit the entire population, but targeted vaccination of specific populations such as healthcare workers may further mitigate the risk of severe disease and death in vulnerable populations. While a few obstacles hinder the rapid development of efficacious vaccines, human trials for virus-like particle (VLP)-based vaccines show promise in both immune response and protection studies, with availability of vaccines being targeted over the next 5–10 years. Ongoing work including identification of important norovirus capsid antigenic sites, development of improved model systems, and continued studies in humans will allow improvement of future vaccines. In the meantime, a better understanding of norovirus disease course and transmission patterns can aid healthcare workers as they take steps to protect high-risk populations such as the elderly and immunocompromised individuals from chronic and severe disease.
Noroviruses are the primary cause of acute gastroenteritis in the United States and worldwide, and infection is typically characterized by vomiting and/or diarrhea for 24–48 hours. Each year in the United States, noroviruses infect an estimated 21 million people and result in about 70 000 hospitalizations and 800 deaths [1]. Norovirus infections are most prevalent in the fall and winter months and can spread easily in areas where people are in close proximity, including day-care centers, schools, cruise ships, and in healthcare settings. In healthcare settings, noroviruses can cause death or severe disease outcomes in elderly and immunocompromised individuals and can result in chronic infection in transplant patients and those with immune disorders [2]. There are currently no licensed vaccines for noroviruses, although several candidates are under development, and projections indicate that an efficacious vaccine could have both economic and clinical benefits [3].
Noroviruses are nonenveloped, single-stranded, positive sense RNA viruses in the Caliciviridae family. Noroviruses are divided into 5 genogroups, and each is further subdivided into numerous genotypes. Genogroups I and II (GI and GII) are responsible for the majority of human disease and are comprised of at least 9 and 22 distinct genotypes, respectively [4]. While fluctuations in norovirus epidemiology occur by year and geographic location, GI strains generally cause approximately 10% of human disease, while the GII strains are responsible for the remaining approximately 90% [5]. A single genotype, GII.4, is responsible for more than 70% of all human outbreaks since the mid-1990s [5]. Consequently, in terms of medical relevance, GII.4 noroviruses are key strains targeted by vaccines.
Histoblood group antigens (HBGAs) serve as binding ligands and putative receptors for human norovirus docking and entry, and the presence of particular HBGAs in an individual is under specific genetic control. Upon infection, noroviruses replicate in cells in the small intestines and virus is shed in feces. Noroviruses infect via the oral route, transmitted through contact with fecal matter or aerosolized vomitus from infected people as well as contaminated surfaces, food, or water. Noroviruses are environmentally stable and have an infectious dose of between 18 and 2800 particles [6, 7], making it difficult to prevent their spread, as even small amounts of viral contamination can seed new infections.
FACTORS COMPLICATING VACCINE DESIGN
Vaccine development for noroviruses is complex (Figure 1). Noroviruses do not replicate in tissue culture and, until recently, there was no small animal model for human norovirus infection [8]. Because of the lack of a validated tissue culture model, virus neutralization cannot be directly measured after infection or vaccination. Instead, the virus-like particle (VLP)-HBGA blockade assay has been used as a surrogate to evaluate the potential neutralization response of both monoclonal antibodies and sera [9], and blockade titers in this assay correlate with human protection [10]. Both chimpanzee and gnotobiotic pig models have been developed to evaluate vaccine efficacy [11, 12]. However, studies have been halted in chimpanzees due to ethical restrictions on the use of these nonhuman primates. To date, results from only 1 vaccine efficacy study in gnotobiotic pigs have been published [11]. Because of limitations in growing virus and testing different vaccines and therapeutics outside of humans, the factors that modulate vaccine efficacy remain poorly understood.
Figure 1.
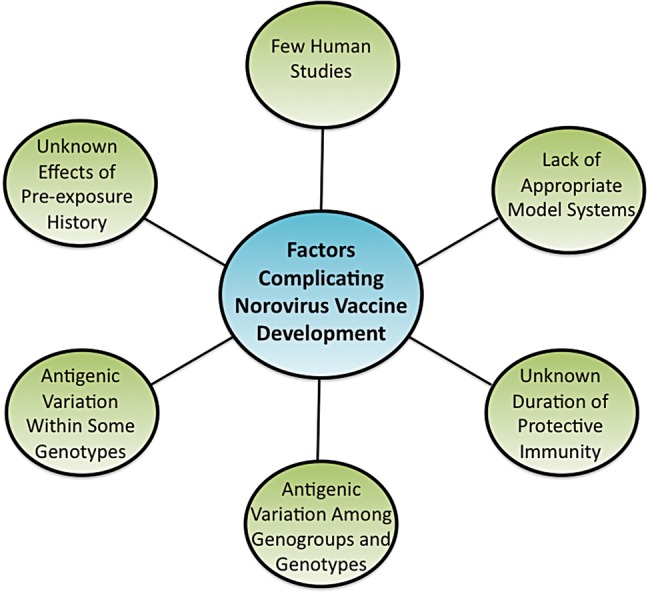
Factors that complicate norovirus vaccine design. Vaccine development for noroviruses is hampered by several factors including the limited number of challenge and vaccine efficacy studies done in humans, the absence of a cell culture system and limited animal models for testing vaccines, the limited and conflicting data on how long protective immunity lasts after infection or vaccination in humans, differing evolutionary patterns and antigenic profiles between genogroups and among genotypes within genogroups, antigenic variation within GII.4 noroviruses and possibly within other genotypes, and the unknown effects of exposures to multiple norovirus genotypes and strains during a lifetime on the immune response to newly encountered strains.
Noroviruses are highly heterogeneous viruses, and major antigenic differences exist between GI and GII noroviruses. Within the GI genogroup, antibodies from different genotypes share 5%–10% cross-reactivity, while cross-reactivity between GI and GII genotypes is <5% [13]. Furthermore, no shared neutralization epitopes have been identified between the 2 genogroups, suggesting that a multivalent GI + GII vaccine platform is needed to protect against infection with both GI and GII viruses. Although poorly characterized, there appear to be some complex cross-blockade relationships that exist within and between GI and GII genogroups, respectively, meaning that inclusion of strategically chosen representative GI and GII strains may yield a broadly protective vaccine [13]. While most noroviruses do not undergo substantial intragenotype antigenic variation, GII.4 noroviruses are a notable exception. The GII.4 genotype varies considerably over time by both mutation and RNA recombination processes, suggesting that a successful vaccine will likely need to be reformulated periodically with contemporary isolates. Antigenic variation within the GII.4 noroviruses is linked to genetic changes in potential neutralization sites on the major capsid protein P2 domain [14–17], providing discrete areas to target with vaccines. Every 2–4 years the predominant circulating GII.4 strain is replaced by a new, antigenically distinct emergent strain that is able to overcome human herd immunity [18–20]. GII.3 noroviruses also demonstrate antigenic changes over time; however, their evolutionary mechanisms differ from those of GII.4 noroviruses and are not as well studied [21]. In contrast, GI.1 and GII.2 isolates have demonstrated little if any antigenic variation over the past 30 years [22]. It is unclear whether these genogroup-specific patterns of evolution are stable or whether all strains have the potential to evolve by epochal evolution like the GII.4 genotype under the appropriate environmental conditions, leading to epidemic strain replacement over time, as seen with influenza A viruses.
The duration of protective immunity after human norovirus infection is complex. Early human challenge studies suggested that protection lasts only approximately 6 months to 2 years [23], leading to concerns regarding the feasibility of developing a successful norovirus vaccine. Others have postulated that some individuals develop long-lived immunity [14]. Although still under study, more recent work that is based on modeling of epidemiological data estimates that protective immunity after natural norovirus infection may persist for between 4 and 8 years [23], suggesting that norovirus vaccines could effectively reduce the overall severity and global disease burden. Another complicating factor is the potential for previous exposure to norovirus strains to impact the immune response to newly encountered strains. Preexposure history may complicate vaccine performance, as most individuals are exposed to multiple GI and GII norovirus infections over their lifetime, resulting in complex patterns of cross-immunity and potentially short- or long-term cross-protection. Although controversial, repeated exposures may lead to deceptive imprinting, where the immune response is increasingly directed at nonneutralizing epitopes, as has been described for other viruses such as human immunodeficiency virus (HIV) and influenza [24]. However, this has not yet been thoroughly investigated after norovirus infections or vaccination.
Available results from 2 human vaccine efficacy studies are encouraging; however, our ability to compare and contrast vaccine platform performance is currently limited. The high heterogeneity of antigenic profiles between genogroups, between some genotypes, and within the GII.4 genogroup suggest that broad vaccine-induced protection against multiple norovirus groups may be difficult but possible, especially if multivalent vaccines are used to broaden reactivity and blockade responses. One study found that immunization of mice against multiple strains broadens the serum blockade response against both homotypic VLPs and VLPs that represent strains not included in the vaccine formulation [13]. Likewise, researchers examining the strain cross-reactivity induced by a bivalent GI.1/GII.4 consensus VLP vaccine in rabbits found that heterotypic strain cross-reactivity was induced [25]. Together, these studies suggest that a multivalent vaccine approach may induce broad protection in humans; however, this remains to be evaluated in human trials.
VACCINE STRATEGIES FOR NOROVIRUSES
The first human clinical efficacy trials evaluated norovirus VLP vaccines. The multivalent human papillomavirus (HPV) vaccines Gardasil and Cervarix are VLP-based vaccines that have been shown to be highly effective at preventing HPV infections and precancerous lesions for strains included in the vaccine [26]. Successes of HPV multivalent VLP vaccines provide a template strategy for use with other viruses, including noroviruses. Takeda Pharmaceutical Company conducted human trials on a monovalent GI.1 VLP-based vaccine that is delivered intranasally [27–29]. All participants in phase 1 studies were found to have significant increases in immunoglobulin (Ig) A and/or IgG memory B-cell responses at the highest vaccine dose of 100 μg VLP [28]. In a follow-up human challenge study, participants were given 2 vaccine doses, 3 weeks apart, and then challenged with 10× ID50 (the dose required to infect ∼50% of participants) Norwalk virus 3 weeks after the second vaccine dose [29]. Results from this study showed that both gastrointestinal disease and Norwalk infection were reduced in the vaccinated group compared with the control group by 47% and 26%, respectively [29]. Long-term efficacy trials are currently underway to evaluate duration of protection for this vaccine.
Takeda Pharmaceutical is also developing a bivalent VLP-based norovirus vaccine that contains both GI.1 Norwalk and GII.4 components. The GI.1 component is identical to that used in the monovalent vaccine trial, and the GII.4 component is based on a consensus sequence from 3 GII.4 outbreak strains that first circulated widely in 2002 and 2006 [25]. Phase 1 and 2 human clinical trials with the intramuscular bivalent vaccine formulation were conducted with a group of 98 healthy participants aged 18–50 years and was found to be well tolerated [30]. While Takeda Pharmaceutical reported that the primary composite study endpoint was not met, study results were encouraging. No severe vomiting and diarrhea were reported in any of the vaccinated recipients, compared with 4 individuals who exhibited these symptoms in the placebo group after challenge [30]. Furthermore, results demonstrated a 52% reduction of vomiting and diarrhea of any severity in the vaccinated group compared with the control group after challenge [30]. Continued clinical trials and long-term efficacy studies are currently planned and underway to further evaluate this vaccine.
Other vaccine approaches under development include additional multivalent VLP-based vaccines [31], multivalent alpha-virus replicon particles (VRPs) that allow formation of norovirus VLPs [13], delivery of VLPs by edible vaccine [32], P particle–based vaccines [33], and polyvalent norovirus P domain–GST complexes [34]. These platforms have not been evaluated in human efficacy studies, but mouse and human immune induction studies provide clues as to which platforms may be most promising for further development and eventual human challenge trials. Notably, while live-attenuated vaccines have been successful for rotavirus and influenza, the lack of a culture system for human norovirus makes development of a live-attenuated norovirus vaccine untenable and dependent on future basic scientific discoveries.
Work with a multivalent VRP vaccine demonstrated that VRPs are an efficient way to deliver norovirus VLPs; this vaccine was able to induce mucosal and cellular immune responses, including more broadly blocking immune responses to the heterologous strain VLPs compared with single-strain VRPs in mice [13]. These results were corroborated by work with a trivalent norovirus/rotavirus combination vaccine that contained both GII.4 and GI.3 norovirus representatives and rotavirus VP6 that was able to broadly block VLPs representing both homotypic (GII.4 VA387 and GI.3) and heterotypic (GII.4 NO and GI.1) norovirus strains [31]. Though in their infancy, edible norovirus vaccines may have potential. A study with 24 participants who either ingested transgenic potatoes expressing norovirus VLPs or wild-type potatoes demonstrated that 95% and 20% of those who ate the transgenic potatoes developed significant increases is IgA-specific antibody-secreting cells and strain-specific serum IgG responses, respectively [32].
P particles, which are 24-mer particles comprised of the P domain of the norovirus capsid, have also been evaluated for their ability to induce immune responses in mice. While P particles were found to stimulate both cellular and humoral immune responses [33], 1 study demonstrated that VLPs, not P particles, induced cross-reactive B- and T-cell responses and primed T cells for production of interferon-γ [35]. Another study found that some P particles do not present surface blockade epitopes as efficiently as VLPs. Taken together, these data suggest that VLPs may be a more promising vaccine strategy than P particles [36].
Polyvalent complexes address issues of low immunogenicity associated with smaller antigens such as P particles by creating a platform that results in large complexes that induce immune induction more efficiently than individual smaller particles. Furthermore, these complexes are able to incorporate multiple antigens into 1 particle, potentially allowing for a multivalent vaccine approach in 1 particle. Sera from mice immunized with polyvalent complexes that incorporated multiple norovirus genotypes (GII.4 and GII.9) induced more efficient blocking ability against both GII.4 P particles and GII.9 VLPs compared with immunizations with GII.4 and GII.9 P particles, suggesting their potential utility as a multivalent vaccine platform [34].
Differing approaches are being taken to address GII.4 antigenic variation, including incorporation of a VLP that represents the predominant circulating strain [31] and design of a GII.4 consensus VLP meant to broaden the GII.4 neutralization response [25]. The recent identification of 3 evolving GII.4 blockade epitopes (designated A, D, and E) that are linked to antigenic changes in GII.4 strains has provided more focused vaccine targets [15, 16, 36] (Figure 2). In addition to modulating antigenicity, epitope D is located in the HBGA interaction site, and evolution in this epitope impacts specificity of HBGA binding, potentially altering the population susceptible to infection over time [36]. Importantly, these discrete epitopes retain their unique antigenic characteristics when moved between GII.4 strains. Although speculative, a reformulation strategy where the GII.4 vaccine component is changed based on population-wide monitoring of these epitopes could be developed. Chimeric VLPs that incorporate epitopes from both the emergent strain and circulating strain could be built to broaden the neutralization response against multiple GII.4 strains including novel variants. Importantly, no putative neutralization epitopes have been mapped in other GI or GII genotypes, making this a basic objective for improving vaccine design in the future.
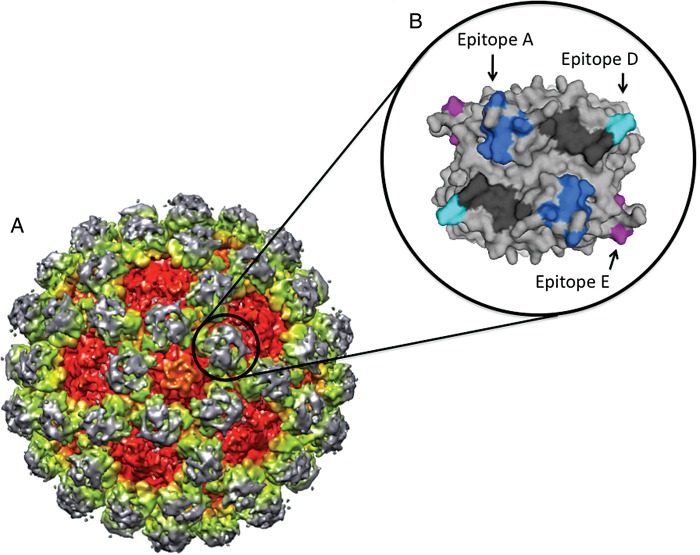
Figure 2.
GII.4 potential neutralization sites. A, A cryo electron microscopy image shows the virion structure for a GII norovirus. Approximate positions are shown for the capsid protein shell domain (red), the P1 subdomain (yellow/green), and the P2 subdomain (gray). The black circle indicates a single P2 dimer. B, A single GII.4 norovirus capsid P2 dimer (top view) is shown. Evolving surface-exposed blockade epitopes A (dark blue), D (light blue), and E (purple) are shown. Histoblood group antigen interaction sites are shown in black. These blockade epitopes represent potential vaccine and drug targets for GII.4 noroviruses.
VACCINE PROTECTION OF AT-RISK POPULATIONS
Populations that are at the highest risk for severe disease include infants and very young children, the elderly, and the immunocompromised [37]. More than 60% of norovirus outbreaks occur in long-term care facilities that serve elderly populations [5], making protection of this group a top priority. Though a licensed norovirus vaccine is not yet available, recent work has estimated the benefits and costs of widespread norovirus vaccination in terms of clinical and economic outcomes in the United States. These projections vary based on age group, vaccine cost, vaccine efficacy rate, and duration of protection [3]. In patients aged ≥65 years, a vaccine with 50% efficacy and a 12-month duration of protection would result in prevention of an estimated 214 cases, 22 outpatients visits, and 4 hospitalizations per 10 000 vaccinations; in children aged 0–4 years, an estimated 1133 norovirus cases, 189 outpatient visits, and 4 hospitalizations would be averted under the same conditions, suggesting that a vaccine would be beneficial to these high-risk populations [3]. In addition, clinicians who work with immunocompromised populations, such as transplant patients, HIV-infected individuals, cancer patients undergoing chemotherapy, and those with primary immunodeficiencies, should be aware that chronic norovirus infections can develop and persist in these patient populations for months to years [38]. Because the elderly and immunocompromised are notoriously poor responders to vaccines [39, 40], vaccination of people who interact with these populations may be critical to reduction of the risk of exposure and spread. Healthcare workers and their family members should be vaccinated to protect against secondary spread.
Recent work shows that approximately 7.2% of norovirus outbreaks occur in childcare centers and school environments [5] and that children are reported to be most efficient at transmitting virus compared with those in other age groups [23]. Together, these data suggest that children and students in day-care and educational environments may be important populations to target with a vaccine [5]. However, vaccine outcomes in young infants and toddlers may be dramatically different from those in adults due to limited exposure histories, presence of maternal antibodies, and maturing immune systems in infants. Vaccine safety and efficacy trials in young children will be critical for illuminating these differences.
Other at-risk populations include military personnel, food handlers, and travelers. Outbreaks can spread rapidly in military barracks and vessels [41]; thus, vaccination of military personnel could reduce disease burden and preserve scheduled operations and security in this environment. Noroviruses are the leading cause of foodborne illness, resulting primarily from contamination by food handlers during food preparation and processing but also during food production [42]. Vaccination of food handlers, farm workers, and food-processing facility workers may prevent outbreaks in restaurants, cruise ships, cafeterias, at catered events, and from grocery items. Travelers and travel industry workers are another group that may benefit from a vaccine, as 2.6% of outbreaks occur in vacation settings such as on cruise ships [5].
CONCLUSIONS
Predictions suggest that a norovirus vaccine with a 50% efficacy rate that is protective for 12 months could prevent 1 million to 2.2 million norovirus cases per year in the United States [3], reducing disease severity and burdens in high-risk populations such as young children and the elderly. Depending on vaccine cost and duration of protection, a norovirus vaccine will either save money (up to $2.1 billion) or result in a cost per case averted similar to other currently available vaccines (<$1500) [3]. As licensed norovirus vaccines become available, it will be necessary to continue to improve vaccine efficacy. The high antigenic heterogeneity of the norovirus family, the factors that regulate short- vs long-term immunity, and the ability of some strains to evolve quickly in response to human herd immunity represent considerable challenges for the vaccine industry (Figure 1). Despite this, epidemiological and immunological data generated by those who study noroviruses provide a basis on which to design and reformulate vaccines (Figure 3), including several recent advances that will likely contribute to the improvement of vaccines. Important antigenic sites have been identified for GII.4 noroviruses, allowing for more targeted vaccine approaches, and recent comparative studies on multiple vaccine platforms are beginning to elucidate which approaches generate the most robust immune responses. In addition, ongoing and potential future vaccine efficacy studies using the new mouse model and gnotobiotic pigs will inform development and improvements to human vaccines. Furthermore, continued work using available human challenge models for GI.1, GII.2, and GII.4 strains will provide numerous opportunities to test vaccine and immune outcomes in immunocompetent and at-risk populations, a strength for improving vaccine design over the next decade.
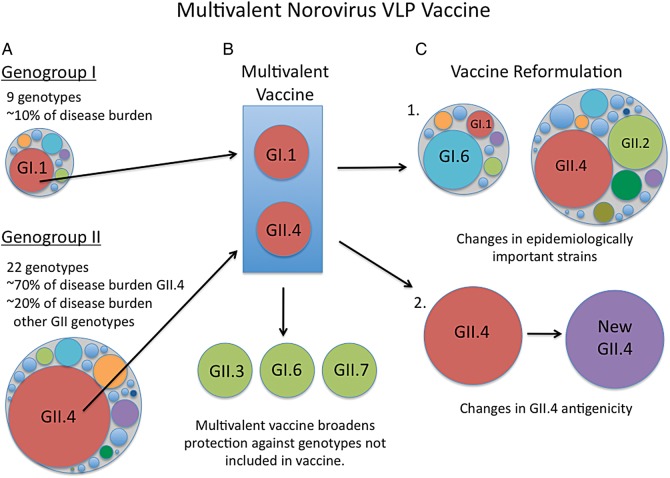
Figure 3.
Design of a multivalent norovirus virus-like particle vaccine. A, Large circles represent the 2 major norovirus genogroups that infect humans, GI and GII. Smaller circles within the larger circles represent the 9 (G1) and 22 (GII) individual genotypes within each genogroup. B, Heterogeneity of GI and GII viruses necessitates a multivalent vaccine to maximize protective coverage of multiple genotypes. Multivalent vaccines that contain virus-like particles (VLPs) representing GI.1 and GII.4 components (red circles) cover norovirus genotypes responsible for approximately 80% of outbreaks. However, a multivalent approach likely broadens the immune response to potentially protect against some heterologous norovirus genotypes (green circles) as well. Heterologous genotypes (green) are shown as examples and are not representative of published data. C, Continued work on norovirus VLP vaccines should consider that there will likely be epidemiological changes over time where relative changes in disease burden by different genotypes occur and strain replacement occurs every 2–4 years for GII.4 noroviruses. Thus, norovirus vaccines will need to be reformulated over time C.1: in response to changes in epidemiologically important viruses; and C.2: in response to changing antigenicity of GII.4 noroviruses. Changes in epidemiologically important norovirus genotypes in C.1 are shown as examples and are not representative of published data.
Notes
Financial support. This work was supported by the National Institutes of Health, Allergy, and Infectious Diseases [AI056351] and by an institutional training grant T32-AI007419 from the National Institutes of Health.
Potential conflicts of interests. K. D., L. C. L., and R. S. B. are paid by grant funding from the National Institutes of Health, Allergy, and Infectious Diseases (AI056351). K. D. is also paid by grant funding from institutional training grant T32-AI007419 from the National Institutes of Health. In addition, K. D., L. C. L., and R. S. B. have a patent, Methods and Compositions for Norovirus Blockade Epitopes, pending.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Hall AJ, Lopman BA, Payne DC, et al. Norovirus disease in the United States. Emerg Infect Dis. 2013;19:1198–205. doi: 10.3201/eid1908.130465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mattner F, Sohr D, Heim A, Gastmeier P, Vennema H, Koopmans M. Risk groups for clinical complications of norovirus infections: an outbreak investigation. Clin Microbiol Infect. 2006;12:69–74. doi: 10.1111/j.1469-0691.2005.01299.x. [DOI] [PubMed] [Google Scholar]
- 3.Bartsch SM, Lopman BA, Hall AJ, Parashar UD, Lee BY. The potential economic value of a human norovirus vaccine for the United States. Vaccine. 2012;30:7097–104. doi: 10.1016/j.vaccine.2012.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kroneman A, Vega E, Vennema H, et al. Proposal for a unified norovirus nomenclature and genotyping. Arch Virol. 2013;158:2059–68. doi: 10.1007/s00705-013-1708-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vega E, Barclay L, Gregoricus N, Shirley SH, Lee D, Vinje J. Genotypic and epidemiologic trends of norovirus outbreaks in the United States, 2009–2013. J Clin Microbiol. 2013;52:147–55. doi: 10.1128/JCM.02680-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Teunis PF, Moe CL, Liu P, et al. Norwalk virus: how infectious is it? J Med Virol. 2008;80:1468–76. doi: 10.1002/jmv.21237. [DOI] [PubMed] [Google Scholar]
- 7.Atmar RL, Opekun AR, Gilger MA, et al. Determination of the human infectious dose—50% for Norwalk virus. J Infect Dis. 2013 doi: 10.1093/infdis/jit620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taube S, Kolawole AO, Hohne M, et al. A mouse model for human norovirus. MBio. 2013;4:4 e00450–13. doi: 10.1128/mBio.00450-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harrington PR, Lindesmith L, Yount B, Moe CL, Baric RS. Binding of Norwalk virus-like particles to ABH histo-blood group antigens is blocked by antisera from infected human volunteers or experimentally vaccinated mice. J Virol. 2002;76:12335–43. doi: 10.1128/JVI.76.23.12335-12343.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reeck A, Kavanagh O, Estes MK, et al. Serological correlate of protection against norovirus-induced gastroenteritis. J Infect Dis. 2010;202:1212–8. doi: 10.1086/656364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Souza M, Costantini V, Azevedo MS, Saif LJ. A human norovirus-like particle vaccine adjuvanted with ISCOM or mLT induces cytokine and antibody responses and protection to the homologous GII.4 human norovirus in a gnotobiotic pig disease model. Vaccine. 2007;25:8448–59. doi: 10.1016/j.vaccine.2007.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bok K, Parra GI, Mitra T, et al. Chimpanzees as an animal model for human norovirus infection and vaccine development. Proc Natl Acad Sci U S A. 2011;108:325–30. doi: 10.1073/pnas.1014577107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.LoBue AD, Lindesmith L, Yount B, et al. Multivalent norovirus vaccines induce strong mucosal and systemic blocking antibodies against multiple strains. Vaccine. 2006;24:5220–34. doi: 10.1016/j.vaccine.2006.03.080. [DOI] [PubMed] [Google Scholar]
- 14.Lindesmith LC, Donaldson EF, LoBue AD, et al. Mechanisms of GII.4 norovirus persistence in human populations. PLoS Med. 2008;5:e31. doi: 10.1371/journal.pmed.0050031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lindesmith LC, Debbink K, Swanstrom J, et al. Monoclonal antibody-based antigenic mapping of norovirus GII.4-2002. J Virol. 2012;86:873–83. doi: 10.1128/JVI.06200-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Debbink K, Donaldson EF, Lindesmith LC, Baric RS. Genetic mapping of a highly variable norovirus GII.4 blockade epitope: potential role in escape from human herd immunity. J Virol. 2012;86:1214–26. doi: 10.1128/JVI.06189-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allen DJ, Noad R, Samuel D, Gray JJ, Roy P, Iturriza-Gomara M. Characterisation of a GII-4 norovirus variant-specific surface-exposed site involved in antibody binding. Virol J. 2009;6:150. doi: 10.1186/1743-422X-6-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lindesmith LC, Donaldson EF, Baric RS. Norovirus GII.4 strain antigenic variation. J Virol. 2011;85:231–42. doi: 10.1128/JVI.01364-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lindesmith LC, Costantini V, Swanstrom J, et al. Emergence of a norovirus GII.4 strain correlates with changes in evolving blockade epitopes. J Virol. 2013;87:2803–13. doi: 10.1128/JVI.03106-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Debbink K, Lindesmith LC, Donaldson EF, et al. Emergence of new pandemic GII.4 Sydney norovirus strain correlates with escape from herd immunity. J Infect Dis. 2013;208:1877–87. doi: 10.1093/infdis/jit370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boon D, Mahar JE, Abente EJ, et al. Comparative evolution of GII.3 and GII.4 norovirus over a 31-year period. J Virol. 2011;85:8656–66. doi: 10.1128/JVI.00472-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Swanstrom J, Lindesmith LC, Donaldson EF, Yount B, Baric RS. Characterization of blockade antibody responses in GII.2.1976 SMV infected subjects. J Virol. 2013;88:829–37. doi: 10.1128/JVI.02793-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simmons K, Gambhir M, Leon J, Lopman B. Duration of immunity to norovirus gastroenteritis. Emerg Infect Dis. 2013;19:1260–7. doi: 10.3201/eid1908.130472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tobin GJ, Trujillo JD, Bushnell RV, et al. Deceptive imprinting and immune refocusing in vaccine design. Vaccine. 2008;26:6189–99. doi: 10.1016/j.vaccine.2008.09.080. [DOI] [PubMed] [Google Scholar]
- 25.Parra GI, Bok K, Taylor R, et al. Immunogenicity and specificity of norovirus consensus GII.4 virus-like particles in monovalent and bivalent vaccine formulations. Vaccine. 2012;30:3580–6. doi: 10.1016/j.vaccine.2012.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pomfret TC, Gagnon JM, Jr., Gilchrist AT. Quadrivalent human papillomavirus (HPV) vaccine: a review of safety, efficacy, and pharmacoeconomics. J Clin Pharm Ther. 2011;36:1–9. doi: 10.1111/j.1365-2710.2009.01150.x. [DOI] [PubMed] [Google Scholar]
- 27.El-Kamary SS, Pasetti MF, Mendelman PM, et al. Adjuvanted intranasal Norwalk virus-like particle vaccine elicits antibodies and antibody-secreting cells that express homing receptors for mucosal and peripheral lymphoid tissues. J Infect Dis. 2010;202:1649–58. doi: 10.1086/657087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramirez K, Wahid R, Richardson C, et al. Intranasal vaccination with an adjuvanted Norwalk virus-like particle vaccine elicits antigen-specific B memory responses in human adult volunteers. Clin Immunol. 2012;144:98–108. doi: 10.1016/j.clim.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 29.Atmar RL, Bernstein DI, Harro CD, et al. Norovirus vaccine against experimental human Norwalk virus illness. N Engl J Med. 2011;365:2178–87. doi: 10.1056/NEJMoa1101245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takeda Highlights Data from Clinical Trial of Investigational Norovirus Vaccine Candidate. 2013. https://www.takeda.com/news/2013/20131007_6021.html . Accessed 10 March 2014.
- 31.Tamminen K, Lappalainen S, Huhti L, Vesikari T, Blazevic V. Trivalent combination vaccine induces broad heterologous immune responses to norovirus and rotavirus in mice. PLoS One. 2013;8:e70409. doi: 10.1371/journal.pone.0070409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tacket CO, Mason HS, Losonsky G, Estes MK, Levine MM, Arntzen CJ. Human immune responses to a novel Norwalk virus vaccine delivered in transgenic potatoes. J Infect Dis. 2000;182:302–5. doi: 10.1086/315653. [DOI] [PubMed] [Google Scholar]
- 33.Fang H, Tan M, Xia M, Wang L, Jiang X. Norovirus P particle efficiently elicits innate, humoral and cellular immunity. PLoS One. 2013;8:e63269. doi: 10.1371/journal.pone.0063269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang L, Huang P, Fang H, et al. Polyvalent complexes for vaccine development. Biomaterials. 2013;34:4480–92. doi: 10.1016/j.biomaterials.2013.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tamminen K, Huhti L, Koho T, et al. A comparison of immunogenicity of norovirus GII-4 virus-like particles and P-particles. Immunology. 2012;135:89–99. doi: 10.1111/j.1365-2567.2011.03516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lindesmith LC, Beltramello M, Donaldson EF, et al. Immunogenetic mechanisms driving norovirus GII.4 antigenic variation. PLoS Pathog. 2012;8:e1002705. doi: 10.1371/journal.ppat.1002705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koo HL, DuPont HL. Noroviruses as a potential cause of protracted and lethal disease in immunocompromised patients. Clin Infect Dis. 2009;49:1069–71. doi: 10.1086/605558. [DOI] [PubMed] [Google Scholar]
- 38.Bok K, Green KY. Norovirus gastroenteritis in immunocompromised patients. N Engl J Med. 2012;367:2126–32. doi: 10.1056/NEJMra1207742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen WH, Kozlovsky BF, Effros RB, Grubeck-Loebenstein B, Edelman R, Sztein MB. Vaccination in the elderly: an immunological perspective. Trends Immunol. 2009;30:351–9. doi: 10.1016/j.it.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duchini A, Goss JA, Karpen S, Pockros PJ. Vaccinations for adult solid-organ transplant recipients: current recommendations and protocols. Clin Microbiol Rev. 2003;16:357–64. doi: 10.1128/CMR.16.3.357-364.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Delacour H, Dubrous P, Koeck JL. Noroviruses: a challenge for military forces. J R Army Med Corps. 2010;156:251–4. doi: 10.1136/jramc-156-04-10. [DOI] [PubMed] [Google Scholar]
- 42.Hall AJ, Eisenbart VG, Etingue AL, Gould LH, Lopman BA, Parashar UD. Epidemiology of foodborne norovirus outbreaks, United States, 2001–2008. Emerg Infect Dis. 2012;18:1566–73. doi: 10.3201/eid1810.120833. [DOI] [PMC free article] [PubMed] [Google Scholar]