This is the first comprehensive study in sub-Saharan Africa looking at the full spectrum of central nervous system opportunistic infections in the HIV population. This study highlights that multiple pathogens are often present in the cerebrospinal fluid of individuals living with HIV.
Keywords: HIV, Zambia, cerebrospinal fluid, PCR, opportunistic infections
Abstract
Background. Knowledge of central nervous system (CNS) opportunistic infections (OIs) among people living with human immunodeficiency virus (HIV) in sub-Saharan Africa is limited.
Methods. We analyzed 1 cerebrospinal fluid (CSF) sample from each of 331 HIV-infected adults with symptoms suggestive of CNS OI at a tertiary care center in Zambia. We used pathogen-specific primers to detect DNA from JC virus (JCV), varicella zoster virus (VZV), cytomegalovirus (CMV), Epstein-Barr virus (EBV), herpes simplex virus (HSV) types 1 and 2, Mycobacterium tuberculosis, and Toxoplasma gondii via real-time polymerase chain reaction (PCR).
Results. The patients' median CD4+ T-cell count was 89 cells/µL (interquartile range, 38–191 cells/µL). Of 331 CSF samples, 189 (57.1%) had at least 1 pathogen. PCR detected DNA from EBV in 91 (27.5%) patients, M. tuberculosis in 48 (14.5%), JCV in 20 (6.0%), CMV in 20 (6.0%), VZV in 13 (3.9%), HSV-1 in 5 (1.5%), and HSV-2 and T. gondii in none. Fungal and bacteriological studies showed Cryptococcus in 64 (19.5%) patients, pneumococcus in 8 (2.4%), and meningococcus in 2 (0.6%). Multiple pathogens were found in 68 of 189 (36.0%) samples. One hundred seventeen of 331 (35.3%) inpatients died during their hospitalization. Men were older than women (median, 37 vs 34 years; P = .01), more recently diagnosed with HIV (median, 30 vs 63 days; P = .03), and tended to have a higher mortality rate (40.2% vs 30.2%; P = .07).
Conclusions. CNS OIs are frequent, potentially treatable complications of AIDS in Zambia. Multiple pathogens often coexist in CSF. EBV is the most prevalent CNS organism in isolation and in coinfection. Whether it is associated with CNS disease or a marker of inflammation requires further investigation. More comprehensive testing for CNS pathogens could improve treatment and patient outcomes in Zambia.
Neurologic complications occur in 40%–60% of persons infected with human immunodeficiency virus (HIV) [1]. Among the most serious neurologic complications of HIV are opportunistic infections (OIs) of the central nervous system (CNS). Zambia has one of the largest rates of HIV infection in the world with an overall prevalence of 12.7%, approaching 25% in some urban populations [2, 3]. Cerebrospinal fluid (CSF) is commonly obtained from patients in sub-Saharan Africa suspected of having a CNS infection. However, the available diagnostic tests are generally limited to Gram stain, India ink stain, bacterial and fungal cultures, and cryptococcal antigen (CrAg) testing for Cryptococcus neoformans. As a result, country-specific data in Africa regarding prevalence of CNS OIs are sparse, and patients remain inadequately treated.
Particularly absent from the majority of these studies is the prevalence data of CNS OIs diagnosed via polymerase chain reaction (PCR). CSF PCR is the standard diagnostic technique used in resource-rich settings to detect the presence of viral, parasitic, and specific bacterial OIs of the CNS; however, PCR is not currently used for routine CSF testing of OIs in HIV-infected patients in Zambia or in the majority of sub-Saharan Africa. We sought to determine the prevalence of CNS OIs in HIV-infected Zambian adults and analyze their impact on patient outcomes.
METHODS
Patient Population
This cross-sectional study was conducted at the University Teaching Hospital (UTH) in Lusaka, Zambia, between 1 October 2010 and 31 May 2012, and was approved by the University of Zambia School of Medicine Biomedical Research Ethics Committee and Beth Israel Deaconess Medical Center Institutional Review Board.
We enrolled consecutive HIV-infected adults who presented to the emergency room at UTH with symptoms suggesting a CNS infection requiring a lumbar puncture (LP) as part of the routine evaluation. Specific symptoms included meningismus, headache, alteration in consciousness, seizure, or a focal neurological deficit. Study staff reviewed charts to determine if an LP was done by the treating provider due to concerns of a CNS infection. Patients or healthcare proxies then provided written consent for use of excess CSF not needed for routine studies. At the time of enrollment, a study neurologist (O. K. S) documented patient demographic data, presenting symptoms, past medical history, and neurological exam. As part of hospital protocol, all patients receiving an LP have microscopy, culture, Gram stain, and India ink testing on CSF. CrAg testing, when available, is provided to patients sometimes at a fee of ∼US$10. We followed patients for the duration of their hospitalization to document the outcome of discharge or death.
CSF Testing
All CSF testing was performed in our molecular laboratory at UTH. Excess CSF obtained from each of these 331 patients was aliquoted into plain sterile tubes and stored at −20°C for molecular diagnostics. DNA was extracted in batches from 200 µL of CSF using a Qiagen MinElute kit (Qiagen, Germantown, Maryland). We performed PCR testing for Mycobacterium tuberculosis (tuberculosis), Epstein-Barr virus (EBV), JC virus (JCV), varicella zoster virus (VZV), cytomegalovirus (CMV), herpes simplex virus type 1 (HSV-1), herpes simplex virus type 2 (HSV-2), and Toxoplasma gondii. Ten microliters of DNA solution was used for each PCR reaction. Extraction control consisted of sterile molecular-grade water.
PCR primers and probes were previously described [4–7] or designed using Primer Express (Life Technologies, Grand Island, New York) (Table 1). DNA amplification was carried out on a Rotorgene 6000 (Corbett Life Sciences, Sydney, Australia) using Applied Biosystems (Roche, Branchburg, New Jersey) TaqMan Universal PCR Master Mix R. The PCR thermocycler conditions were 50°C for 2 minutes, 95°C for 10 minutes, 40 cycles of 95°C for 15 seconds, then 60°C for 1 minute and 60°C for 7 minutes.
Table 1.
Polymerase Chain Reaction Primers and Probes
| Pathogen (Target Gene, Reference) | Sense Primer 5′-3′ | Antisense Primer 5′-3′ | Probe 5′-3′ |
|---|---|---|---|
| EBV (B95–8 BNRF1a) | GGAACCTGGTCATCCTTTGC | ATGGACCGGTTAATCCGATCT | AGCGAGCAGTACGAGTGCCTGCGb |
| Tuberculosis (IS6110 [5]) | AGGCGAACCCTGCCCAG | GATCGCTGATCCGGCCA | TGTGGGTAGCAGACCTCACCTATGTGTCGA |
| JCV (T gene [4]) | AGAGTGTTGGGATCCTGTGTTTT | GAGAAGTGGGATGAAGACCTGTTT | TCATCACTGGCAAACATTTCTTCATGGC |
| CMV (DNA polymerasea) | CTTAACCACTACAGCAAAGGTACGA | ATGATAGCGGCGTTAGGTGACA | TGCCCGAAACGAATAGCGTTGCC |
| VZV (DNA polymerase [7]) | CGGCATGGCCCGTCTAT | TCGCGTGCTGCGGC | ATTCAGCAATGGAAACACACGACGCC |
| HSV-1 (gD [7]) | CGGCCGTGTGACACTATCG | CTCGTAAAATGGCCCCTCC | CCATACCGACCACACCGACGAACC |
| HSV-2 (gG [7]) | CGCTCTCGTAAATGCTTCCCT | TCTACCCACAACAGACCCACG | CGCGGAGACATTCGAGTACCAGATCG |
| Toxo (B1 [6]) | CAAGCAGCGTATTGTCGAGTAGAT | GCGTCTCTTTCATTCCCACATTTT | CAGAAAGGAACTGCATCCGTT |
Abbreviations: CMV, cytomegalovirus; EBV, Epstein-Barr virus; HSV, herpes simplex virus; JCV, JC virus; Toxo,Toxoplasma gondii; VZV, varicella zoster virus.
a In-house design with PrimerExpress (Life Technologies).
b All probes have 6FAM as reporter dye and TAMRA as quencher, except the Toxo probe, which uses nonfluorescent quencher Minor groove binder (MGB).
Positive controls consisted of genomic DNA for each pathogen (ATCC, Manassas, Virginia). Two negative controls for every PCR run consisted of an extraction control and sterile water. All samples were run in duplicate. A sample was considered positive if 2 of 2 results showed amplified products. If only 1 of 2 samples was positive after the initial run, a second PCR test was performed. If the second PCR showed amplification, the sample was considered positive. Results were not used for patient care in the context of this pilot observational study. To minimize interassay variability, samples were tested in batches, usually several weeks after the patient's initial presentation.
Statistical Analysis
Confidence intervals for death rates were estimated using methods for exact binomial confidence intervals. Wilcoxon rank-sum and Fisher exact tests were used to compare study variables between groups. Univariate and multivariate logistic regression models were utilized to assess predictors of death. A significance level of <0.10 was used to select variables for the multivariate analyses. Maximum likelihood method was used to estimate model parameters, and significance was tested using the Wald test statistic. Statistical analyses were performed using the R package version 3.0.1 and SAS software version 9.3 (SAS Institute, Cary, North Carolina). P values were 2-sided and considered statistically significant if <.05.
RESULTS
Patient Characteristics
Characteristics of the 331 patients are shown in Table 2. There was a similar sex distribution, but men were older and more recently diagnosed with HIV compared with women. Overall, 83 (25.1%) patients were diagnosed with HIV infection at the time of presentation. They were severely immunosuppressed, and the enrollment CD4+ T-cell count tended to be lower in men than in women. One hundred nineteen (36.0%) patients had a prior history of tuberculosis. Only 118 (35.6%) patients were on combination antiretroviral therapy (cART) at the time of enrollment for a median duration of 240 days (interquartile range, 33–1223 days). cART exposure tended to be higher in women than in men.
Table 2.
Patient Demographics (N = 331)
| Characteristic | Men | Women | P Value |
|---|---|---|---|
| No. | 169 (51.1%) | 162 (48.9%) | .74 |
| Age, y, median (IQR) | 37 (32–42) | 34 (30–41) | .01 |
| CD4+ T cells/µL, median (IQR) | 81 (30–156) | 99 (43–217) | .18 |
| On cART at time of hospitalization | 57 (33.7%) | 60 (37.0%) | .57 |
| Days since HIV diagnosis, median (IQR) | 30 (1–504) | 63 (1–1092) | .03 |
Abbreviations: cART, combination antiretroviral therapy; HIV, human immunodeficiency virus; IQR, interquartile range.
Infectious Pathogens Detected in CSF
Pathogens were detected in 189 (57.1%) CSF samples. The number of patients diagnosed with 1 or more pathogens is shown in Table 3. India ink testing was positive on 31 of 318 (9.7%) CSF samples tested. Of these 31 samples, 30 (96.8%) were also yeast positive on Gram stain. CrAg testing was performed on 161 samples and 41 (25.5%) were found to be positive. CrAg testing was markedly more sensitive than India ink for the diagnosis of cryptococcal meningitis (P < .0001). Sufficient testing to make a diagnosis of cryptococcal meningitis (either yeast or India ink positive or CrAg tested) was performed on 184 (55.6%) patients. Of 189 samples, 121 (64.0%) had 1 organism and 68 (36.0%) had multiple organisms detected. EBV was the most common pathogen found (27.5%) and the most common copathogen (15.1%). There were high rates of detection of Cryptococcus (19.3%) and tuberculosis (14.5%). We also detected JCV (6.0%), CMV (6.0%), VZV (3.9%), and HSV-1 (1.5%) in this patient population. Pneumococcal and meningococcal meningitis was diagnosed in 2.4% and 0.6% of patients, respectively. No cases of toxoplasmosis or HSV-2 were detected by PCR.
Table 3.
Characteristics of Patients With Cerebrospinal Fluid Pathogen(s)
| Pathogens | Individual CSF Prevalence | 1 Pathogen | No. of Cases | 2 Pathogens | No. of Cases | 3 Pathogens | No. of Cases | 4 Pathogens | No. of Cases |
|---|---|---|---|---|---|---|---|---|---|
| EBV | 91/331 (27.5%) | EBV | 41 | EBV/Crypto | 19 | EBV/TB/CMV | 4 | EBV/Crypto/TB/VZV | 1 |
| Cryptoa | 64/331 (19.3%) | Crypto | 35 | EBV/TB | 11 | EBV/TB/JCV | 1 | Crypto/TB/VZV/CMV | 1 |
| TB | 48/331 (14.5%) | TB | 20 | EBV/VZV | 3 | EBV/TB/Meningo | 1 | ||
| JCV | 20/331 (6.0%) | JCV | 9 | EBV/JCV | 2 | EBV/TB/Crypto | 1 | ||
| CMV | 20/331 (6.0%) | CMV | 6 | EBV/Pneumo | 2 | EBV/Crypto/JCV | 1 | ||
| VZV | 13/331 (3.9%) | VZV | 4 | EBV/CMV | 1 | EBV/JCV/CMV | 1 | ||
| Pneumo | 8/331 (2.4%) | HSV-1 | 2 | EBV/HSV-1 | 1 | EBV/JCV/VZV | 1 | ||
| HSV-1 | 5/331 (1.5%) | HSV-2 | 0 | Crypto/CMV | 2 | ||||
| Meningo | 2/331 (0.6%) | Toxo | 0 | Crypto/VZV | 2 | ||||
| HSV-2 | 0/331 (0%) | Pneumo | 4 | Crypto/TB | 1 | ||||
| Toxo | 0/331 (0%) | Crypto/HSV-1 | 1 | ||||||
| TB/CMV | 4 | ||||||||
| TB/JCV | 2 | ||||||||
| TB/VZV | 1 | ||||||||
| JCV/Pneumo | 2 | ||||||||
| JCV/HSV-1 | 1 | ||||||||
| CMV/Meningo | 1 | ||||||||
| Totals | 271 pathogens | 121/331 (36.6%) | 56/331 (16.9%) | 10/331 (3%) | 2/331 (0.6%) |
Abbreviations: CMV, cytomegalovirus; Crypto, Cryptococcus neoformans; CSF, cerebrospinal fluid; EBV, Epstein-Barr virus; HSV, herpes simplex virus; JCV, JC virus; Meningo, meningococcus; Pneumo, pneumococcus; TB, Mycobacterium tuberculosis; Toxo, Toxoplasma gondii; VZV, varicella zoster virus.
a One hundred eighty-four of 331 (55.6%) patients had sufficient testing.
Head computed tomography (CT) and magnetic resonance imaging (MRI) are not performed routinely in Zambia, but are available to patients who are able to pay the equivalent of US$170 and $270, respectively. Patients can receive reduced rates or exemptions if the costs are deemed prohibitive. These exams were only performed in few individuals, and were not systematically analyzed for this study. Postmortem analyses are rarely performed at UTH due to difficulty in obtaining consent from family members.
Presence of Seizures in Patients With CSF Infection
Seizures occurred in 79 of 331 (23.9%) patients, including 39 of 189 (20.6%) of those with and 40 of 142 (28.1%) without specific pathogen(s) in their CSF (P = .12).
Seizure was most commonly seen with Cryptococcus (26.6%), JCV (25.0%), or VZV (23.1%). Seizure was also found in cases with CSF detection of HSV-1 (20.0%), EBV (16.5%), CMV (15.0%), and tuberculosis (12.0%). Seizure was not a presenting symptom in any cases of pneumococcal or meningococcal meningitis.
Mortality and Clinical Presentation of Patients With CSF Infection and Seizures
Of 331 patients, 117 (35.3%) died during their hospitalization, including 68 (40.2%) men and 49 (30.2%) women (P = .07). Moreover, 78 of 189 (41.3%) patients who had a detectable pathogen in the CSF died, compared with 39 of 142 (27%) deaths in patients with no pathogen detected (P = .01).
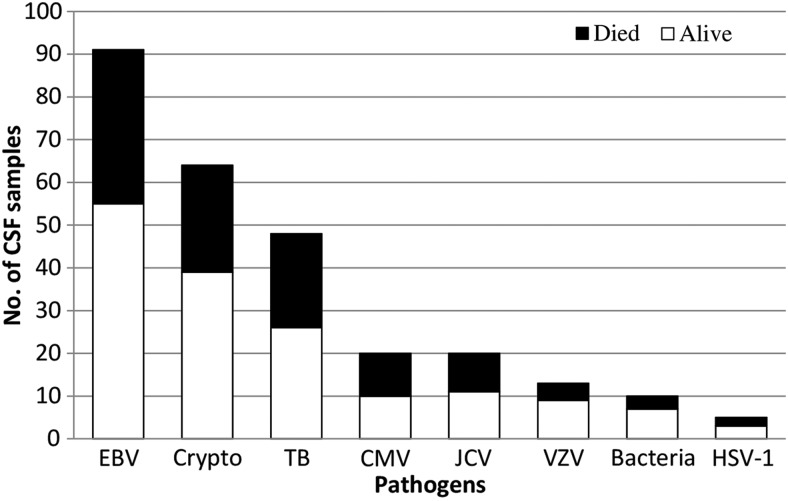
The percentage of patients diagnosed with each CSF pathogen who remained alive or died during their hospitalization is shown in Figure 1. The mortality of CSF EBV–positive patients was 39.5% (36 of 91). The clinical presentation of CSF EBV–positive patients was variable, including headache, neckache, and seizure. Mortality among patients diagnosed with cryptococcal meningitis was 39.0%.
Figure 1.
Pathogen-associated mortality. Histogram of the numbers of cerebrospinal fluid samples with detectable pathogen belonging to patients who remained alive or died during their hospitalization. Abbreviations: Bacteria, pneumococcus or meningococcus; CMV, cytomegalovirus; Crypto, Cryptococcus neoformans; CSF, cerebrospinal fluid; EBV, Epstein-Barr virus; HSV, herpes simplex virus; JCV, JC virus; TB, Mycobacterium tuberculosis; VZV, varicella zoster virus.
Mortality of patients testing PCR positive for CSF tuberculosis was 45.8% (22 of 48). Altogether, a viral infection or tuberculosis was diagnosed by CSF PCR in 150 patients, including 86 with CMV, VZV, HSV-1, or tuberculosis, for which specific treatments are available. Finally, mortality was similar between patients who developed seizures (32.9% [26/79]) and those who did not (36.1% [91/252]) (P = .69).
Table 4 summarizes the potential risk factors of death in our study population. The odds of death together with 95% confidence intervals were reported for each covariate. Longer duration of knowing HIV diagnosis, and high Glasgow Coma Scale (GCS) score were associated with lower odds of death, whereas female sex and longer duration on cART only showed a trend with lower odds of death. Conversely, tuberculosis, cryptococcal meningitis, EBV, and other pathogens showed a trend with higher odds of death. There was no difference in mortality between patients who had single or multiple pathogens in the CSF. In the multivariate analysis, only high GCS score remained significant.
Table 4.
Variables Associated With Death in Univariate and Multivariate Analysis
| Variable | Univariate Analysis |
Multivariate Analysis |
||
|---|---|---|---|---|
| Odds Ratio (95% CI) | P Value | Odds Ratio (95% CI) | P Value | |
| Categorical | ||||
| Female | 0.64 (.40–1.01) | .06 | 0.54 (.20–1.48) | .23 |
| cART status | 0.85 (.52–1.38) | .51 | ||
| Tuberculosis | 1.69 (.90–3.17) | .10 | ||
| Cryptococcal meningitis | 1.12 (.59–2.10) | .74 | ||
| EBV | 1.37 (.82–2.28) | .22 | ||
| Other pathogensa | 1.52 (.84–2.76) | .17 | ||
| Multiple pathogensb | 0.96 (.52–1.78 | .89 | ||
| Continuous | ||||
| Age | 0.91 (.72–1.16) | .44 | ||
| cART (days) | 0.67 (.43–1.04) | .08 | 1.00 (.46–2.19) | .99 |
| HIV diagnosis (days) | 0.56 (.34–.91) | .02 | 0.61 (.21–1.80) | .37 |
| CD4 count | 0.70 (.44–1.11) | .13 | ||
| Glasgow Coma Scale | 0.33 (.24–.46) | <.0001 | 0.46 (.28–.75) | .002 |
Abbreviations: cART, combination antiretroviral therapy; CI, confidence interval; EBV, Epstein-Barr virus; HIV, human immunodeficiency virus.
a Other pathogens indicate all other single pathogens other than EBV detected in the cerebrospinal fluid (CSF).
b Multiple pathogens indicate 2–4 organisms detected in CSF.
DISCUSSION
PCR uncovered the presence of multiple pathogens that would have otherwise remained undetected. There was a high prevalence of coinfection in the CSF, indicative of severe immunosuppression in this population. These findings may reflect late presentation of patients to health facilities. The prevalence of EBV, JCV, VZV, and CMV are higher than previously reported from a comparable study in sub-Saharan Africa [8].
The high prevalence of EBV was the most striking finding, particularly in the presence of other pathogens. EBV is frequently detected in the CSF with other microorganisms, although its pathogenic role remains unclear [9, 10]. In a study of bacterial meningitis in Malawi, 61% of HIV-infected patients had detectable EBV in the CSF, and it was also associated with an increased risk of death [11]. Detection of EBV DNA from a CSF sample may simply represent amplification of quiescent virus within B lymphocytes. Indeed, 30 of 91 (33.0%) EBV CSF PCR–positive samples had ≥10 red blood cells/µL CSF (10–6000), and it is possible that detection was due to blood contamination. EBV is associated with primary CNS lymphoma in HIV-infected patients. We did not obtain neuroimaging as part of this study. However, in those EBV PCR–positive patients with neuroimaging, mass lesions were not commonly seen. Our PCR assays were performed on a real-time PCR apparatus but were read as qualitative output. Measurement of EBV DNA load helps determine the pathogenesis of EBV infection [12]. Therefore, concomitant measurement of EBV copy number in the CSF and blood as well as neuroimaging will be necessary to determine whether EBV is pathogenic, a marker of inflammation, or an innocent bystander in the CNS. Management of EBV encephalitis includes supportive care with or without use of corticosteroids [13]. There has also been rare success reported with ganciclovir [14]. This medication is not available at public hospitals in Zambia.
Patients with confirmed cryptococcal meningitis were treated with amphotericin B, which is provided at no cost at UTH, but flucytosine is not available. The documented mortality rate of 39% in this population is markedly improved compared with previously documented 100% mortality at UTH prior to the availability of amphotericin B [15]. Only 161 of 331 (48.6%) patients had CrAg testing. Because the rate of detection using this test was 25.5%, compared with 9.7% with India ink staining we can extrapolate that approximately 27 individuals (8.2% of the total cohort) went undiagnosed solely because of lack of adequate testing. As untreated cryptococcal meningitis is universally lethal, if these 27 individuals had been correctly diagnosed and treated, approximately 11 would have survived based on our 39.0% mortality rate.
HIV strains and host susceptibility factors in Africa were thought to be less conducive to infection by JCV, the etiologic agent of progressive multifocal leukoencephalopathy (PML) [16]. However, the prevalence of JCV infection of the brain in our study is similar to that reported in Western countries before the widespread availability of cART [17], suggesting that prior studies lacked adequate testing. Indeed, all JCV CSF–positive patients had neurological findings consistent with PML.
Interestingly, T. gondii DNA was not detected in any CSF samples in our study population. Sensitivity of CSF PCR for T. gondii has been reported as between 33% and 100% depending on the technique, patient population, and presence of anti-Toxoplasma treatment [6, 18, 19]. Our findings are in sharp contrast with previous reports from Europe and the United States, where CNS toxoplasmosis was the most common CNS OI among untreated HIV-infected patients [20]. This is likely because T. gondii seroprevalence has been documented as low as 4% in Zambian HIV-infected patients [21] and because cotrimoxazole prophylaxis is broadly implemented in Zambia for prevention of Pneumocystis jiroveci pneumonia and CNS toxoplasmosis. Additionally, lower rates of raw meat consumption and other regional dietary habits may account for the low seroprevalence. HSV-2 was not detected in any CSF samples. Seroprevalence of HSV-2 in an urban Zambian population has previously been documented at 47.2% [22]. Further investigation is warranted to see if there are unique characteristics of HSV-2 in Zambia that make it less neurotropic.
Seizures frequently occurred in our patients, with similar incidence regardless of whether there was a detectable pathogen in the CSF. Without comprehensive neuroimaging data, it is difficult to identify the etiology of seizures in cases devoid of specific pathogen(s). HIV infection of the CNS itself may cause seizures. Hyponatremia is a common etiology in this population secondary to CNS and pulmonary disease [23]. Seizures are also likely to be underreported, as patients in Zambia have a limited ability to identify seizures that are not convulsive. There is also significant stigma associated with seizures in Zambia [24], although antiepileptic drugs are available, most commonly in the form of carbamazepine and phenobarbitone [25].
Mortality rate was extremely high in our study population. More than one-third of patients died during their hospitalization. This rate of mortality is higher than reported in resource-rich settings at the beginning of the AIDS epidemic [26, 27]. Our study was not designed for long-term follow-up. It is possible that death occurred in the post-hospitalization period due to loss of follow-up, poor compliance with treatment, and poor access to emergency health services. As expected, mortality was higher in patients with lower scores on GCS. Men presented with a shorter time since the initial HIV diagnosis, had lower CD4+ T-cell counts, and had higher mortality rates. These results suggest that men present at later stages of their HIV disease than do women. This is consistent with multiple studies across Africa regarding the enrollment characteristics and treatment outcomes of patients presenting to cART clinics [28–30].
Our study highlights several factors that could improve management and outcome in this patient population. First, early diagnosis of HIV infection, especially in men, could prevent immunosuppression and infectious complications. Second, widespread availability of CrAg testing in CSF would save lives. The recently developed point-of-care CrAg lateral flow immunochromatographic assay has the potential to address this issue through greater ease of use and cheaper cost than the more widespread CrAg latex agglutination test. Third, implementation of innovative technologies utilizing PCR to detect viral pathogens amenable to treatment (EBV, tuberculosis, CMV, VZV, HSV) may improve the survival of these patients. This could potentially be done through utilization of the GeneXpert real-time PCR platform, which is currently undergoing scale-up in resource-limited settings to improve tuberculosis diagnostics [31]. The current cost of a GeneXpert cartridge for PCR testing of a single tissue sample for tuberculosis is US$9.98 [32]. A comparable cartridge-based system for testing of CSF pathogens could easily be developed. In Zambia, there is a national program that provides antituberculosis treatment free of charge, but antiviral treatment such as intravenous acyclovir can only be purchased from private pharmacies at a cost of US$17 per 250-mg dose. A typical course for viral encephalitis would cost approximately US$663 and is out of reach for most patients.
Our study has several limitations. The true degree of infections and coinfections was likely underestimated. The actual rate of tuberculosis in this population is unknown. Tuberculosis culture is not routinely performed on CSF in Zambia, as it can take up to 6 weeks to become positive. The diagnosis of tuberculosis meningitis is problematic regardless of setting because of the limited sensitivity of CSF PCR testing, estimated to be approximately 50% [33]. Numerous patients were suspected of having probable or possible tuberculosis meningitis despite negative CSF tuberculosis PCR testing. As a result, the true number of patients presenting with seizure who had tuberculosis meningitis is also underestimated. Cryptococcal meningitis was also likely underdiagnosed as not all patients received adequate testing.
The average yearly salary in Zambia is equivalent to US$1350 [34]. CrAg testing is covered by the public health system but is not always available. In this context, out-of-pocket expenses for CrAg testing are significant, as less than half of patients for whom it was clinically indicated had this test performed on their CSF. Likewise head CT and MRI costs are incurred directly by the patients, and each exam corresponds to approximately 13%–20% of yearly salary. There is a tiered payment system in place where patients pay according to their level of income. However, many patients are not able to afford these supplemental payments. Finally, neurocysticercosis and neurosyphilis may also affect HIV-infected Zambian patients presenting to UTH, but due to limited resources, not all patients received an adequate evaluation for these 2 pathogens even when clinically indicated.
Our study shows that numerous pathogens can be found in the CSF of HIV-infected patients presenting with neurological symptoms in Zambia, and the mortality of this population is very high. Systematic review of the literature showed no similar comprehensive molecular diagnostic study of the CNS in sub-Saharan Africa. There have been targeted studies utilizing PCR for the diagnosis of herpesviruses in aseptic meningitis and bacterial coinfection in HIV-infected patients in Malawi [11, 35]. Diagnostic testing for a broad array of CSF pathogens may be required in resource-limited settings to achieve better outcomes and avoid unnecessary treatments.
Notes
Acknowledgments. The authors thank the UTH microbiology lab for technical assistance.
Financial support. This work was supported by the National Institutes of Health (grant numbers P30A1060354 and R24TW007988 to O. K. S. and R01NS047029, NS074995, and K24NS060950 to I. J. K.).
Potential conflicts of interest. I. J. K. has served on scientific advisory boards for Hoffman La Roche, GlaxoSmithKline, Merck Serono, and Genentech; has received consulting fees from Bristol-Myers Squibb, Ono Pharmaceuticals, Merck Serono, Hoffman La Roche, GlaxoSmithKline, Perseid Therapeutics, Vertex Pharmaceutical, and Johnson & Johnson; is an editorial board member for the Journal of NeuroVirology; and receives royalties from UpToDate for topics on the management of HIV and CNS mass lesions and on PML. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Portegies P, Berger JR. HIV/AIDS and the nervous system. In: Aminoff MJ, Boller F, Swaab DF, editors. Handbook of clinical neurology. Vol. 85. Edinburgh: Elsevier; 2007. pp. 1–2. [Google Scholar]
- 2.United Nations Joint Programme on HIV/AIDS. UNAIDS report on the global AIDS epidemic 2010. Available at: http://www.unaids.org/documents/20101123_globalreport_em.pdf. Accessed 31 January 2014.
- 3.United Nations Joint Programme on HIV/AIDS. Zambia. Available at: http://www.unaids.org/en/regionscountries/countries/zambia/ Accessed 31 January 2014.
- 4.Ryschkewitsch C, Jensen P, Hou J, Fahle G, Fischer S, Major EO. Comparison of PCR-southern hybridization and quantitative real-time PCR for the detection of JC and BK viral nucleotide sequences in urine and cerebrospinal fluid. J Virol Methods. 2004;121:217–21. doi: 10.1016/j.jviromet.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 5.Broccolo F, Scarpellini P, Locatelli G, et al. Rapid diagnosis of mycobacterial infections and quantitation of Mycobacterium tuberculosis load by two real-time calibrated PCR assays. J Clin Microbiol. 2003;41:4565–72. doi: 10.1128/JCM.41.10.4565-4572.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mesquita RT, Ziegler AP, Hiramoto RM, Vidal JE, Pereira-Chioccola VL. Real-time quantitative PCR in cerebral toxoplasmosis diagnosis of Brazilian human immunodeficiency virus-infected patients. J Med Microbiol. 2010;59(pt 6):641–7. doi: 10.1099/jmm.0.016261-0. [DOI] [PubMed] [Google Scholar]
- 7.Weidmann M, Meyer-Konig U, Hufert FT. Rapid detection of herpes simplex virus and varicella-zoster virus infections by real-time PCR. J Clin Microbiol. 2003;41:1565–8. doi: 10.1128/JCM.41.4.1565-1568.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Modi M, Mochan A, Modi G. Management of HIV-associated focal brain lesions in developing countries. QJM. 2004;97:413–21. doi: 10.1093/qjmed/hch080. [DOI] [PubMed] [Google Scholar]
- 9.Martelius T, Lappalainen M, Palomaki M, Anttila VJ. Clinical characteristics of patients with Epstein Barr virus in cerebrospinal fluid. BMC Infect Dis. 2011;11:281. doi: 10.1186/1471-2334-11-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weinberg A, Bloch KC, Li S, Tang YW, Palmer M, Tyler KL. Dual infections of the central nervous system with Epstein-Barr virus. J Infect Dis. 2005;191:234–7. doi: 10.1086/426402. [DOI] [PubMed] [Google Scholar]
- 11.Kelly MJ, Benjamin LA, Cartwright K, et al. Epstein-Barr virus coinfection in cerebrospinal fluid is associated with increased mortality in Malawian adults with bacterial meningitis. J Infect Dis. 2012;205:106–10. doi: 10.1093/infdis/jir707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kimura H, Ito Y, Suzuki R, Nishiyama Y. Measuring Epstein-Barr virus (EBV) load: the significance and application for each EBV-associated disease. Rev Med Virol. 2008;18:305–19. doi: 10.1002/rmv.582. [DOI] [PubMed] [Google Scholar]
- 13.Tunkel AR, Glaser CA, Bloch KC, et al. The management of encephalitis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2008;47:303–27. doi: 10.1086/589747. [DOI] [PubMed] [Google Scholar]
- 14.Dellemijn PL, Brandenburg A, Niesters HG, van den Bent MJ, Rothbarth PH, Vlasveld LT. Successful treatment with ganciclovir of presumed Epstein-Barr meningo-encephalitis following bone marrow transplant. Bone Marrow Transplant. 1995;16:311–2. [PubMed] [Google Scholar]
- 15.Mwaba P, Mwansa J, Chintu C, et al. Clinical presentation, natural history, and cumulative death rates of 230 adults with primary cryptococcal meningitis in Zambian AIDS patients treated under local conditions. Postgrad Med J. 2001;77:769–73. doi: 10.1136/pmj.77.914.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shankar SK, Satishchandra P, Mahadevan A, et al. Low prevalence of progressive multifocal leukoencephalopathy in India and Africa: is there a biological explanation? J Neurovirol. 2003;9(suppl 1):59–67. doi: 10.1080/13550280390195397. [DOI] [PubMed] [Google Scholar]
- 17.Gheuens S, Wuthrich C, Koralnik IJ. Progressive multifocal leukoencephalopathy: why gray and white matter. Annu Rev Pathol. 2013;8:189–215. doi: 10.1146/annurev-pathol-020712-164018. [DOI] [PubMed] [Google Scholar]
- 18.Cingolani A, De Luca A, Ammassari A, et al. PCR detection of Toxoplasma gondii DNA in CSF for the differential diagnosis of AIDS-related focal brain lesions. J Med Microbiol. 1996;45:472–6. doi: 10.1099/00222615-45-6-472. [DOI] [PubMed] [Google Scholar]
- 19.Dupon M, Cazenave J, Pellegrin JL, et al. Detection of Toxoplasma gondii by PCR and tissue culture in cerebrospinal fluid and blood of human immunodeficiency virus-seropositive patients. J Clin Microbiol. 1995;33:2421–6. doi: 10.1128/jcm.33.9.2421-2426.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manzardo C, Del Mar Ortega M, Sued O, Garcia F, Moreno A, Miro JM. Central nervous system opportunistic infections in developed countries in the highly active antiretroviral therapy era. J Neurovirol. 2005;11(suppl 3):72–82. doi: 10.1080/13550280500513846. [DOI] [PubMed] [Google Scholar]
- 21.Zumla A, Savva D, Wheeler RB, et al. Toxoplasma serology in Zambian and Ugandan patients infected with the human immunodeficiency virus. Trans R Soc Trop Med Hyg. 1991;85:227–9. doi: 10.1016/0035-9203(91)90034-v. [DOI] [PubMed] [Google Scholar]
- 22.Weiss HA, Buve A, Robinson NJ, et al. The epidemiology of HSV-2 infection and its association with HIV infection in four urban African populations. AIDS. 2001;15(suppl 4):S97–108. doi: 10.1097/00002030-200108004-00011. [DOI] [PubMed] [Google Scholar]
- 23.Siddiqi O, Birbeck GL. Safe treatment of seizures in the setting of HIV/AIDS. Curr Treat Options Neurol. 2013;15:529–43. doi: 10.1007/s11940-013-0237-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Atadzhanov M, Haworth A, Chomba EN, Mbewe EK, Birbeck GL. Epilepsy-associated stigma in Zambia: what factors predict greater felt stigma in a highly stigmatized population? Epilepsy Behav. 2010;19:414–8. doi: 10.1016/j.yebeh.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chomba EN, Haworth A, Mbewe E, et al. The current availability of antiepileptic drugs in Zambia: implications for the ILAE/WHO “out of the shadows” campaign. Am J Trop Med Hyg. 2010;83:571–4. doi: 10.4269/ajtmh.2010.10-0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fortgang IS, Moore RD. Hospital admissions of HIV-infected patients from 1988 to 1992 in Maryland. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;8:365–72. [PubMed] [Google Scholar]
- 27.Turner BJ, Ball JK. Variations in inpatient mortality for AIDS in a national sample of hospitals. J Acquir Immune Defic Syndr. 1992;5:978–87. [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention. Differences between HIV-Infected men and women in antiretroviral therapy outcomes—six African countries, 2004–2012. MMWR Morb Mortal Wkly Rep. 2013;62:945–52. [PMC free article] [PubMed] [Google Scholar]
- 29.Mosha F, Muchunguzi V, Matee M, et al. Gender differences in HIV disease progression and treatment outcomes among HIV patients one year after starting antiretroviral treatment (ART) in Dar es Salaam, Tanzania. BMC Public Health. 2013;13:38. doi: 10.1186/1471-2458-13-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cornell M, Schomaker M, Garone DB, et al. Gender differences in survival among adult patients starting antiretroviral therapy in South Africa: a multicentre cohort study. PLoS Med. 2012;9:e1001304. doi: 10.1371/journal.pmed.1001304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.World Health Organization. WHO monitoring of Xpert MTB/RIF roll-out. Available at: http://www.who.int/tb/laboratory/mtbrifrollout/en/index.html. Accessed 28 January 2014.
- 32.World Health Organization. Public-private partnership announces immediate 40 percent cost reduction for rapid TB test. Available at: http://www.who.int/tb/features_archive/GeneXpert_press_release_final.pdf. Accessed 3 February 2014.
- 33.Garg RK. Tuberculosis of the central nervous system. Postgrad Med J. 1999;75:133–40. doi: 10.1136/pgmj.75.881.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bank TW. Zambia. Available at: http://data.worldbank.org/country/zambia. Accessed 17 September 2014.
- 35.Benjamin LA, Kelly M, Cohen D, et al. Detection of herpes viruses in the cerebrospinal fluid of adults with suspected viral meningitis in Malawi. Infection. 2013;41:27–31. doi: 10.1007/s15010-012-0292-z. [DOI] [PMC free article] [PubMed] [Google Scholar]