Abstract
The temperature and pH dependent solubility of poly(γ-propargyl L-glutamate) (PPLG) functionalized through copper catalyzed 1,3 cycloaddition reaction between an alkyne and azide can be tuned with precision over a broad range of conditions by varying the ratio of substitution of short oligo ethylene glycol and diisopropylamine side groups.
While multi-stimuli responsive polymers have been widely researched, medical applications of polymers that respond quickly and reversibly to changes in both environmental pH and temperature has been especially investigated.1,2 Efforts to fabricate multiplexed tunable polymer systems have built on single stimuli responsive design strategies in seeking to adjust a polymer's macromolecular properties by colocalizing different types of responsive elements in a random,3 block,4 or graft5 copolymer.
While poly(N-isopropylacrylamide) (PNIPAm) is the most established thermoresponsive polymer, polymers based on short oligo(ethylene glycol) side chains have developed as an alternate non-fouling biocompatible thermoresponsive platform.6 Han and colleagues established that the solubility and cloud point of comb-like methacrylate polymers can be modified by controlling oligo(ethyleneglycol) side chain length.7 The polymer's observed cloud point could be systematically varied from 28 °C to 90 °C by adjusting the ratio of a 2-(2-methyloxyethyloxy)ethyl methacrylate to poly(ethylene glycol) methyl ether methacrylate in a random copolymer.8 More recent efforts have expanded methacrylate thermoresponsive platforms by the incorporation of additional functional groups. Strategies have focused on introducing small fractions of comonomers with reactive side chains that can be grafted postpolymerization, including alkynes 9,10 and allyl pendant functionalities.11 Hydroxyl terminated oligo(ethylene glycol) methacrylate monomers have been incorporated, with 2-hydroxyethyl methacrylate, both as a functional handle and as a modifier to tune the copolymer's lower critical solution temperature (LCST) properties.12 Short ester oligo(ethylene glycol) side chains have also been used to confer thermoresponsiveness to polypeptides with defined α-helical secondary structure.13,14
We have recently introduced the N-carboxy anhydride (NCA) polymerization of poly(γ-propargyl L-glutamate) (PPLG) as a particularly attractive platform for exploring multiplexed functionality, as it allows highly efficient postpolymerization grafting through copper catalyzed “click reaction” to an α-helical polypeptide backbone.15 The PPLG backbone has since been demonstrated as the basis for readily functionalized, biocompatible, biodegradable polypeptides with either thermal16 and pH17 responsiveness. Herein we demonstrate that these two properties can be combined through the utility of PPLG grafted with a combination of short oligo ethylene glycol side chains that yield a thermoresponsive highly tunable polypeptide and tertiary amine groups that confer pH sensitivity in a biologically relevant range. The potential for facile, multiplexed conjugation of responsive functional groups is demonstrated through the generation of a family of polymers which can be adjusted to undergo solubilisation at very specific and relevant pH and temperature.
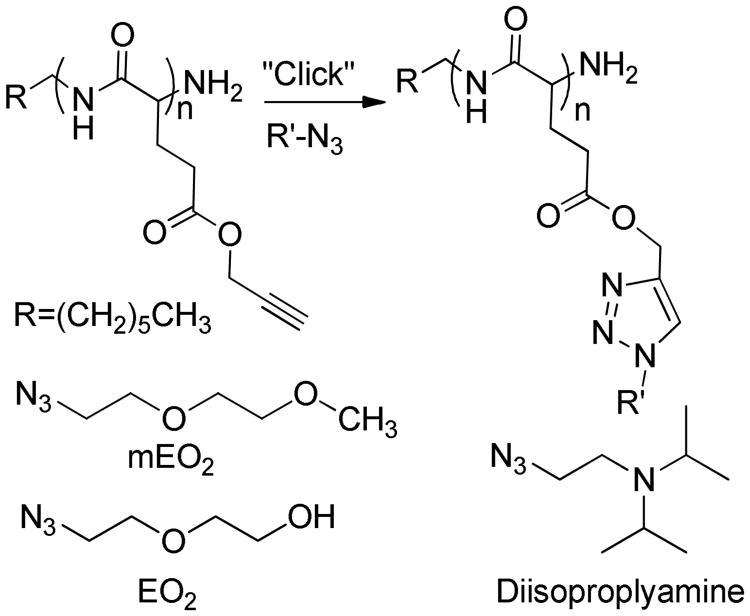
The strategy employed in this study is illustrated in Scheme 1. A series of PPLG backbones is fully substituted with varied ratios of 2-(2-azidoethoxy)ethanol (EG2) and 1-azido-2-(2-methoxyethoxy) ethane (mEG2) to tailor polymer thermoresponsiveness. The highly robust click chemistry offers the potential to multiplex optimized thermal and pH sensitivity with orthogonal functionality including those that confer bioactivity.18-20 This communication establishes PPLG grafted with both mEO2 and N-(2-azidoethyl)-N-isopropylpropan-2-amine (diisopropylamine) as one example of a pH and temperature responsive polymer platform. More broadly, it establishes the systematic, robust, and facile grafting of PPLG with multiple side groups conferring complementary aggregated functionality. While this communication focuses on controlling the responsiveness of aqueous PPLG solutions, the flexibility of mono or bi-functional amine-initiated NCA polymerization of the polymer backbone suggests the facile deployment of the modular functionalized polypeptides in such applications as block copolymers,15 surface coatings, drug delivery systems or responsive hydrogels.2
Scheme 1.
Post polymerization poly(propargyl-L-glutamate) backbones are fully substituted with thermo and pH responsive side groups.
Four PPLG backbones were synthesized by NCA polymerization as recently reported.15 These backbones ranging from 44 to 97, as calculated by NMR (Table 1). Varied ratios of EO2 and mEO2 grafting groups were introduced to tune the polymer's temperature responsiveness. Ratios of EO2 and mEO2 grafting groups, calculated through 1H NMR (Fig. S2a, ESI†), closely match that of the feed ratios (Table 1). By visual inspection, all polymers were soluble at room temperature and only the polymer fully substituted with EO2 did not exhibit an LCST, and become turbid when heated in deionized water.
Table 1. Summary of PPLG backbones and substitutions.
| Polymer | DP by NMR | Mn a | PDI a | Feed Ratio Substitution of EO2 | NMR-based Substitution of EO2 |
|---|---|---|---|---|---|
| PPLG - 44 | 44 | 9500 | 1.13 | 0, 20, 40% | 0, 21, 38% |
| PPLG - 64b | 64 | 16300 | 1.33 | 0, 20, 40% | 0, 16, 39% |
| PPLG - 77 | 77 | 20300 | 1.23 | 0, 20, 40% | 0, 19, 40% |
| PPLG - 97 | 97 | 28100 | 1.14 | 0, 25, 50, 100% | 0, 24, 51, 100% |
DMF GPC with 0.1% lithium bromide with PMMA standards.
Additionally substituted with various ratios diisopropylamine.
1H-NMR-observed percent substitution of EO2 in backbone with remaining fully substituted with mEO2.
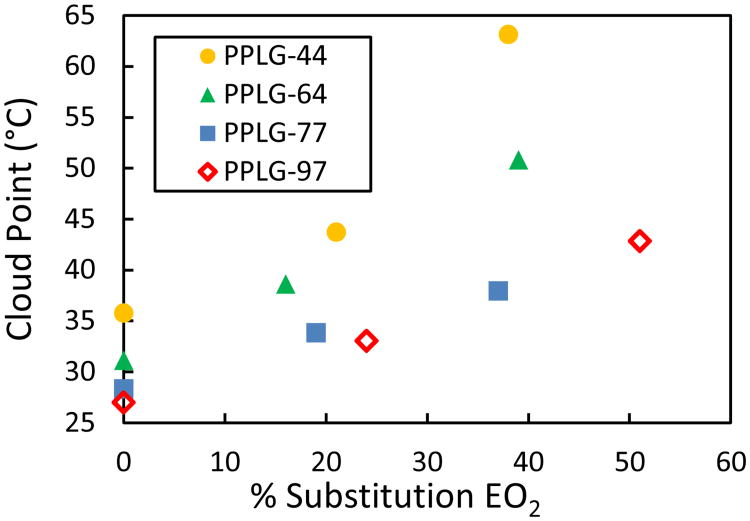
The effects of the backbone degree of polymerization and the ratio of methoxy and hydroxyl terminated grafted side chains on the temperature responsiveness of the PPLG-based platform was characterized by temperature controlled turbidity measurements of the 13 polymers outlined in Table 1. All polymers were characterized at a constant 3 mg/ml in deionized water, as their thermal responsiveness is concentration dependent (Fig. S3, ESI†).16,17 Fig. 1 shows observed cloud points, with reported temperatures corresponding to that of solutions having 50% of the range of transmittance.
Figure 1.
Relationship between cloud point (measured at 3 mg/ml in deionised water) and percent EO2 substituted onto PPLG backbones functionalized with mEO2.
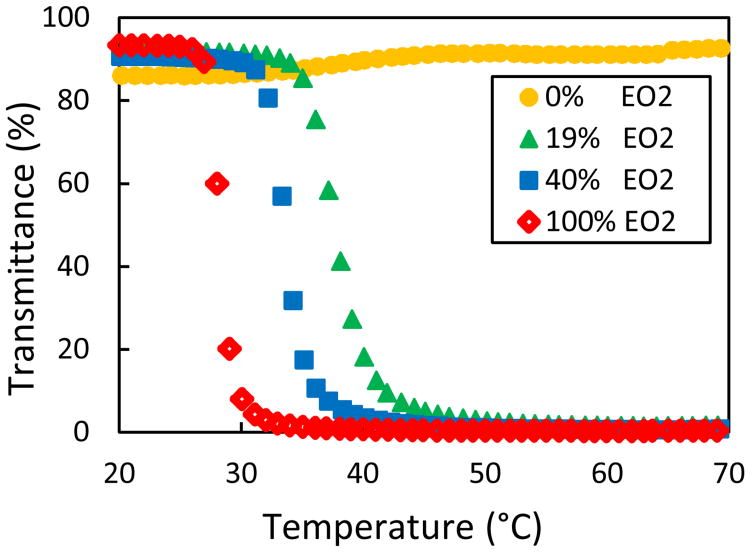
When comparing polymers of similar grafting ratios, all polymers maintain the expected trend of decreasing solubility with increasing molecular weight (Fig. S4, ESI†).16,17 For each backbone length, increasing the fraction of more hydrophilic EO2 side chains predictably increases the cloud point of the fully functionalized polymer (Fig. 1 and Fig. 2). Even the longest tested polymer, PPLG-97, functionalized fully with EO2 remained soluble at all tested temperatures further suggests that the mEO2 functionality specifically confers observed thermoresponsiveness (Fig. 2). The observed thermoresponsive behaviour is reversible under experimental conditions, with the PPLG-64 100% mEO2 showing consistent measured turbidity values across 4 heating and cooling cycles (Fig. S5, ESI†). Further, all sample heating and cooling transmittance curves showed minimal hysteresis, with cloud points varying by less than 3 °C (Fig. S6a, ESI†).The robust dependence of cloud point on grafting group composition across all four backbone lengths demonstrates the ability to systematically tune a given backbone's temperature responsiveness at biologically relevant temperatures by varying only the ratio of hydroxyl and methoxy terminated short PEG grafting groups. This approach modulates temperature sensitivity while preserving much of the polymer's overall structural and chemical identity.
Figure 2.
Influence of temperature on the light transmittance (500 nm, heating 1 °C min−1) of PPLG-97 substituted with 100% EO2 and PPLG-77 substituted with EO2 at 19%, 40%, and 100% and remainder with mEO2 at 3 mg/ml in deionised water.
All cloud points reported in Fig. 1 were generated from heating curves; for most systems, the transmittance decreases from 95% to 5% of the initial value took place over a range of less than 8 °C, with some transitions as sharp as 2°C. The sharpness of these transitions is unusual for a synthetic polymer, which typically would exhibit broader transitions due to a spread of molecular weights and side chain functionalization efficiencies; we attribute these sharper transitions to narrow molecular weights and well-defined grafting efficiencies. The exceptions included three polymers, PPLG-97 50%, PPLG-44 20%, and PPLG-44 40%, which exhibited less defined transitions spanning around 15° C. The less discreet temperature responsiveness of polymers functionalized with mixed grafting groups supports the anticipated result of the stochastic functionalization of the PPLG backbones. When all else is constant, grafting is expected to yield a stochastic, binomial distribution, and one would dictate that short polymers and those with equal substitution of the grafting groups would generate a population with the highest variance and most gradual temperature response (Fig. S6, ESI†).
For application in biological systems, the effects of salt must also be considered. Salts are broadly understood to lower the cloud point of polymers by disrupting the surrounding hydration structure in a process known as “salting out.” As an initial characterization, the temperature response of the PPLG-64 polymers at 3 mg/ml in PBS was compared to that in deionized water. Predictably, all three polymers demonstrate cloud points in physiological buffer that are lower than those in pure water, though the magnitude of this decrease seems dependent on the individual polymer's composition, ranging from 3 °C to 9 °C (Table S1, ESI†).
A dual responsive system with temperature and pH responsiveness was investigated. PPLG was grafted with mEO2 and diisopropylamine, a group conferring PPLG the ability to undergo a solubility phase transition with decreasing degree of amine ionization.17 Four grafted PPLG-64 polymers were synthesized including polymers with feed ratios of 100% diisopropylamine, 50% diisopropylamine and 50% EO2, 50% diisopropylamine and 50% mEO2, and 10% diisopropylamine and 90% mEO2, with calculated grafted values closely matching feed ratios (Fig. S2b, ESI†).
All polymers grafted with diisopropylamine were shown to buffer between pH 6.1 and pH 7.2. PPLG grafted with only diisopropylamine buffered slightly lower than polymers having partial diisopropylamine grafting, while polymers grafted with only mEO2 showed no buffering above pH 4 (Fig. S8, ESI†). 100% diisopropylamine grafted PPLG was insoluble above pH 6.3 while polymers with only partial diisopropylamine grafting precipitated around pH 6.7.
Temperature controlled turbidity measurements of PPLG-64 grafted with 100% diisopropylamine demonstrated robust thermal responsiveness with linear dependence on solution pH between pH 5.0 and pH 6.2, having cloud points ranging from 66 °C to 38 °C (Fig. S7, ESI† and Fig. 5). This temperature responsiveness is not surprising considering the polymer's structural similarities to other known amine containing temperature responsive polymers.10
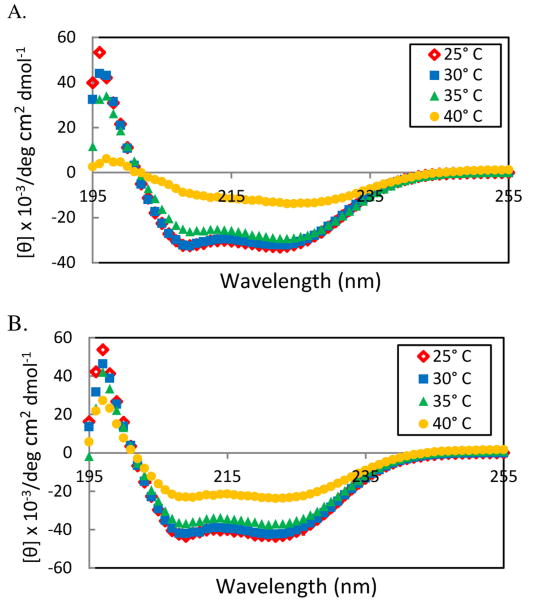
Figure 5.
Circular dichroism spectrum of PPLG-64 measured at 1 mg/ml in 100mM NaCl, 75mM phosphate buffer pH 6.2 at indicated temperatures. A)50% mEO2 and 50% diisoproplyamine) B)90% mEO2 and 10% diisoproplyamine.
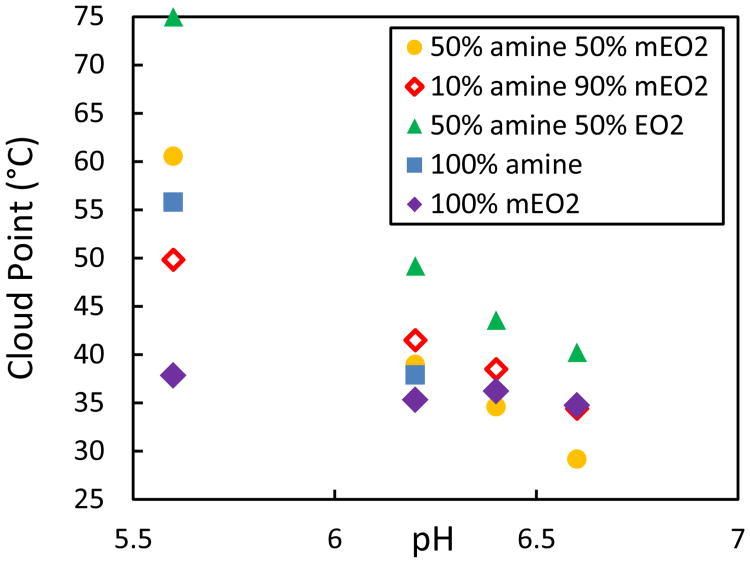
Cloud point measurements of diisopropylamine grafted PPLG polymers between pH 5.6 and pH 6.6 confirm additive properties of dual grafting (Fig. 5). In solutions having pH values below the amine's buffering region, grafted diisopropylamine increased PPLG's solubility compared to PPLG grafted with only mEO2. Specifically, at pH 5.6, PPLG graft 100% mEO2 had the lowest tested cloud point and PPLG graft 50% diisopropylamine and 50% mEO2 was more soluble than PPLG graft 10% diisopropylamine and 90% mEO2 (Fig. 5). However, with increased solution pH, grafted tertiary amines become less protonated and more hydrophobic, leading to a reverse order of cloud points at pH 6.6, where polymers grafted with diisopropylamine exhibit lower cloud points than polymers grafted with only mEO2. Finally, PPLG graft 50% amine and 50% EO2 demonstrated both thermal responsiveness and pH sensitivity but had the highest tested cloud points, confirming both grafted diisopropylamine's contribution to thermal sensitivity and the additive bulk properties of dual PPLG functionalization.
The effect of grafting multiple groups on the secondary structure of the grafted PPLG was explored using circular dichroism (CD), specifically by monitoring the relative absorbances at λ = 208 and 222 nm, characteristic minima of α-helical secondary structures. In both distilled water and buffered salt conditions, CD spectra of polymers functionalized only with combinations of mEO2 and EO2 consistently maintained minima at 208 nm and 222 nm when heated though their cloud points (Fig. S9, ESI†). Decrease in the heated solution's signal intensity is attributed to observed precipitation, and α-helical structure is only confirmed for the soluble fraction. At 25° C in all tested buffers ranging from pH 5.6 to pH 6.6, spectra of PPLG graft 100% and 50% diisopropylamine were also characteristically α-helical. However, as these polymers were heated to their cloud point, their CD absorbance at 208 nm increased relative to 222 nm suggesting a shift from a pure α-helical to a partially random coil secondary structure (Fig. 3A and Fig. S10, ESI†). At the same pH conditions, PPLG graft 10% diisopropylamine and 90% mEO2 maintained minimum at 208 and 222 nm as the temperature of the polymer solutions were raised at least 7° C past their cloud point, (Fig. 3B) suggesting that carefully selected dual grafting designs can also direct polymer secondary structure.
For applications in drug delivery and nanomedicine, these functionalized PPLG's dual pH and temperature sensitivity can be optimized over a broad and biologically relevant range of values to amplify or tailor polymer responsiveness to feed different biological microenvironments simply by adjusting the ratio of mEO2 to diisopropylamine grafting groups or by introducing additional grafting groups. EO2 might be substituted to lower the polypeptide's observed cloud point while grafting on alternative amines such as diethylamine can tune the polymer's buffering capacity and related pH responsiveness.17
In conclusion, we have demonstrated the synthesis of a library of reversibly thermo- responsive α-helical polypeptides with readily tunable temperature dependent solubility at biologically relevant conditions. In both distilled water and PBS, a given polypeptide's cloud point is increased by increasing the substitution of short hydroxyl terminated grafted PEG oligomers on PPLG backbones substituted with short methoxy terminated PEG side chains. Furthermore, by incorporating the diisopropylamine side group, we demonstrated a dual responsive system where the solubility and secondary structure can be tuned. The highly efficient 1,3 cycloaddition grafting chemistry allows the potential to incorporate a wide variety of functional groups including those with demonstrated thermo and pH responsiveness.
Experimental Methods
1H NMR spectra were recorded on Bruker 400 MHz FT-NMR spectrometers. Gel permeation chromatography (GPC) measurements were carried out using a Waters Breeze 1525 HPLC system equipped with two Polypore columns operated at 75 °C, series 2414 refractive index detector, series 1525 binary HPLC pump, and 717 plus autosampler. DMF with 0.01 M LiBr was the eluent for analysis, and samples were dissolved at 4–6 mg/mL in DMF. Data collection and processing was performed using Waters'; Breeze chromatography software version 3.30. The average molecular weight of the sample was calibrated against narrow molecular weight poly(methyl methacrylate) (PMMA) standards.
Temperature responsive solubility was measured by a Cary 500i UV-Vis-NIR Dual-Beam Spectrophotometer automated Multi-Cuvette Sampling Accessory with Temperature Controller. The polymer solutions in cuvettes with a 10mm pathlength at concentrations of 0.1 to 3 mg/ml were equilibrated for at least 5 minutes, heated from 20 °C to 70 °C at a rate of 1 °C/min, held at 70 °C for 3 minutes, and returned to 20 °C. Reported temperatures correspond to the block temperature. A measured by a temperature probe, after ∼2 degrees of a heating or cooling cycle, the block temperature consistently led the solution temperature by 1.6 °C ±0.2 °C. The reported cloud point was defined as the temperature corresponding to 50% of the range of transmittance.
Circular dichroism spectroscopy of polymer solutions was performed using an Aviv model 202 CD spectrometer. Samples were prepared at a concentration of 0.5–1.1 mg mL −1 in Milli-Q water. Measurements were sampled every nm with a 3–5 s average time over the range of 195–260 nm (bandwidth ¼ 1.0 nm) at temperatures ±0.1 °C the reported temperature ranging from 25 °C to 50 °C. Measurements were taken using a cell with a 1 mm path length.
Titration of diisopropylamine mEO2 grafted PPLG was performed on 3 ml solution at 2.5 mM amine solution in 125 mM NaCl adjusted to pH 3. The solution was titrated with 5 μL aliquots of 0.1 M NaOH, and the pH was measuring after each addition. Titration of PPLG graft 11% diisopropylamine was performed at 0.25 mM amine with 0.01 M NaOH.
Poly(γ-propargyl l-glutamate) (PPLG). PPLG was synthesized as previously reported.15,17
mEO2 synthesized closely following the previously reported synthesis of mEO2.21 In brief, 2.75 g (42 mmol) of sodium azide was added to an aqueous solution of 2 g (10.6 mmol) 1-bromo-2-(2methoxyethoxy) ethane. The solution was heated at 75 °C for 15 hours, then treated with 5% NaOH solution and extracted 4 times with 10 mL of diethylether. The combined organic layer was dried over Na2SO4 and concentrated to give a clear viscous liquid. Typical yield is 46%. 1H NMR (CDCl3, δppm) 3.37 (s, 3H), 3.38 (t, 2H, J = 5.24 Hz), 3.54 (m, 2H), 3.63 (m, 4H). 13C NMR (CDCl3, δppm) 72.14, 70.82, 70.22, 59.32, 50.83. HRMS (ESI-TOF) calcd for C5H11O2N3Na+: 168.0743 ([M+Na]), found 168.0750.
Azide-functionalized PPLG. A typical procedure includes grafting onto the PPLG backbone azide terminated side chain at the target ratio feed ratio of alkyne/azide/CuBr/N,N,N′,N′,N″-pentamethyldiethylenetriamine (PMDETA) equal to 1/1.2/0.1/0.1. PPLG (0.025 g, 0.150 mmol alkyne repeat units), azide (0.165 mmol), and PMDETA (3.1 μL, 0.015 mmol) were all dissolved in DMF (3 mL). Azide terminated side chains used in this study include mEO2, EO2 and diisopropylamine. The CuBr catalyst (0.002 g, 0.015 mmol) was added to the degassed solution, and the reaction solution was stirred at room temperature. After more than 2 hours, the reaction solution was precipitated in 40 mL cold diethylether, dissolved in 10 mL distilled water, and incubated for 30 min with 5 mg Dowex M4195 sulfate copper chelating resin. The beads were removed by filtration and the polymer solution was dialyzed against water acidified by HCl (pH <4) for 24 hours and against distilled water for 12 hours. The polymer structure and degree of substitution was verified by 1H-NMR. Typical yield is 75%.
Supplementary Material
Figure 4.
Relationship between cloud point of grafted PPLG backbones and solution pH measured at 1 mg/ml in 100mM NaCl, 75mM phosphate buffer.
Acknowledgments
Funding Sources: This work was supported by NIH R01 EB010246-03, NIH U54-CA112967 and the NIH Biomechanics Training Grant. Use of equipment at the Institute for Soldier Nanotechnologies, the Department of Chemistry Instrumentation Facility, the Center for Materials Science and Engineering Shared Experimental Facilities, and the Biophysical Instrumentation Facility (NSF-0070319 and NIH GM68762) is greatly acknowledged.
Abbreviations
- LCST
lower critical solution temperature
- NCA
N-carboxy anhydride
- PPLG
poly(γ-propargyl L-glutamate)
- EO2
2-(2-azidoethoxy)ethanol
- mEO2
1-azido-2-(2-methoxyethoxy) ethane
- diisopropylamine
N-(2-azidoethyl)-N-isopropylpropan-2-amine
- NMR
nuclear magnetic resonance
- PMMA
poly(methyl methyacrylate)
- CD
circular dichoism
- PEG
poly(ethylene glycol)
- PBS
phosphate buffered saline
Footnotes
Author Contributions: The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
Associated Content Supporting Information. Backbone GPC traces, grafted PPLG NMRs, effect of polymer concentration on thermal responsiveness, hysteresis of heating and cooling polymer solutions, influence of cycling temperature on thermal responsiveness, temperature dependent circular dichroism spectra, Comparison of cloud points measured in distilled water and PBS, and titration of diisopropylamine grafted PPLG. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Schmaljohann D. Advanced Drug Delivery Reviews. 2006;58:1655–1670. doi: 10.1016/j.addr.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 2.Tsvetanov CB, Dimitrov I, Trzebicka B, Muller AHE, Dworak A. Progress in Polymer Science. 2007;32:1275–1343. [Google Scholar]
- 3.Matyjaszewski K, Yamamoto S, Pietrasik J. Macromolecules. 2008;41:7013–7020. [Google Scholar]
- 4.Muller AHE, Schilli CM, Zhang MF, Rizzardo E, Thang SH, Chong YK, Edwards K, Karlsson G. Macromolecules. 2004;37:7861–7866. [Google Scholar]
- 5.Hourdet D, Durand A. Polymer. 1999;40:4941–4951. [Google Scholar]
- 6.Lutz JF. Journal of Polymer Science Part a-Polymer Chemistry. 2008;46:3459–3470. [Google Scholar]
- 7.Han S, Hagiwara M, Ishizone T. Macromolecules. 2003;36:8312–8319. [Google Scholar]
- 8.Lutz JF, Hoth A. Macromolecules. 2006;39:893–896. [Google Scholar]
- 9.Leung MKM, Such GK, Johnston APR, Biswas DP, Zhu ZY, Yan Y, Lutz JF, Caruso F. Small. 2011;7:1075–1085. doi: 10.1002/smll.201002258. [DOI] [PubMed] [Google Scholar]
- 10.Jung SH, Song HY, Lee Y, Jeong HM, Lee HI. Macromolecules. 2011;44:1628–1634. [Google Scholar]
- 11.París R, Liras M, Quijada-Garrido I. Macromolecular Chemistry and Physics. 2011;212:1859–1868. [Google Scholar]
- 12.Laloyaux X, Fautre E, Blin T, Purohit V, Leprince J, Jouenne T, Jonas AM, Glinel K. Advanced Materials. 2010;22:5024–5028. doi: 10.1002/adma.201002538. [DOI] [PubMed] [Google Scholar]
- 13.Yu M, Nowak AP, Deming TJ, Pochan DJ. Journal of the American Chemical Society. 1999;121:12210–12211. [Google Scholar]
- 14.Chen CY, Wang ZH, Li ZB. Biomacromolecules. 2011;12:2859–2863. doi: 10.1021/bm200849m. [DOI] [PubMed] [Google Scholar]
- 15.Engler AC, Lee HI, Hammond PT. Angewandte Chemie-International Edition. 2009;48:9334–9338. doi: 10.1002/anie.200904070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng Y, He C, Xiao C, Ding J, Zhuang X, Chen X. Polymer Chemistry. 2011;49:2665–2676. [Google Scholar]
- 17.Engler AC, Bonner DK, Buss HG, Cheung EY, Hammond PT. Soft Matter. 2011;7:5627–5637. [Google Scholar]
- 18.Parrish B, Breitenkamp RB, Emrick T. Journal of the American Chemical Society. 2005;127:7404–7410. doi: 10.1021/ja050310n. [DOI] [PubMed] [Google Scholar]
- 19.Xiao C, Zhao C, He P, Tang Z, Chen X, Jing X. Macromolecular Rapid Communications. 2010;31:991–997. doi: 10.1002/marc.200900821. [DOI] [PubMed] [Google Scholar]
- 20.Zhang DH, Tang HY. Polymer Chemistry. 2011;2:1542–1551. [Google Scholar]
- 21.Sinha J, Sahoo R, Kumar A. Macromolecules. 2009;42:2015–2022. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.