Abstract
Objective
Deficits in social recognition and learning of social cues are major symptoms of neurodegenerative disorders such as Alzheimer's disease (AD). Here, we studied the role of β1-noradrenergic signaling in cognitive function to determine whether it could be used as a potential therapeutic target for AD.
Methods
Using pharmacological, biochemical, and behavioral tools, we assessed social recognition and the β1-adrenergic receptor (ADR) and its downstream protein kinase A (PKA)/phospho-cAMP response element-binding protein (pCREB) signaling cascade in the medial amygdala (MeA) in Thy1-hAPPLond/Swe+(APP) mouse model of AD.
Results
Our results demonstrated that APP mice display a significant social recognition deficit which is dependent on the β1-adrenergic system. Moreover, betaxolol, a selective β1-ADR antagonist, impaired social but not object/odor learning in C57Bl/6 mice. Our results identifies activation of the PKA/pCREB downstream of β1-ADR in MeA as responsible signaling cascade for learning of social cues in MeA. Finally, we found that xamoterol, a selective β1-ADR partial agonist, rescued the social recognition deficit of APP mice by increasing nuclear pCREB.
Interpretation
Our data indicate that activation of β1-ADR in MeA is essential for learning of social cues, and that an impairment of this cascade in AD may contribute to pathogenesis and cognitive deficits. Therefore, selective activation of β1-ADR may be used as a therapeutic approach to rescue memory deficits in AD. Further safety and translational studies will be needed to ensure the safety of this approach.
Introduction
The inability to recognize faces (social recognition) is a defined endophenotype of several neurological disorders including Alzheimer's disease (AD).1,2 Yet, the underlying causes of this symptom remain elusive. Several studies have highlighted the role of oxytocin and vasopressin in social recognition3,4 and show that low levels of oxytocin could be responsible for deficits in face recognition in autistic patients.5 However, in AD patients, postmortem studies failed to show differences in central vasopressin and oxytocin concentration6 except in the hippocampus where levels were higher than in normal patients.7 This indicates that variations in oxytocin levels do not underlie the deficit in social recognition in AD and points to our incomplete understanding of the neural circuitries responsible for social recognition.
A well-defined pathological hallmark of AD is the degeneration of the neurons in the locus coeruleus, the main source of noradrenaline (NA) in the brain.8,9 This degeneration is associated with a subsequent reduction in NA and reduced activation of the adrenergic receptors. Adrenergic receptors mediate distinctive actions of NA via various intracellular signaling pathways and play important roles in learning and memory processes.10 Recently the contribution of the β-adrenergic system specifically the β1-adrenergic receptor (β1-ADR) in cognitive functions has received interest. For instance, NA action on β-ADR modulates inhibitory synaptic function.11 Other studies have shown that the β1-selective antagonist betaxolol produces spatial navigation retrieval deficit in wild-type (WT) mice and rats, while the retrieval deficit observed in mice with NA deficiency can be rescued by the β1-partial agonist xamoterol.12 Similarly, the cognitive deficits of the Ts65Dn mouse model of Down syndrome, with a similar age-dependent loss of NA-containing neurons,13 can be rescued with xamoterol.14,15 These results indicate that the NAergic neurotransmission mediated by the β1-ADR may play a predominant role in the cognitive deficits observed in neurological disorders characterized by NAergic degeneration.
In this study, we aimed to characterize the role of β1-ADR in social recognition and memory in AD and as a potential therapeutic target. Polymorphisms in the gene coding for the β1-ADR produce AD susceptibility by changing the cell responsiveness to adrenergic stimulation16 pointing to a predominant role of this noradrenergic receptor in AD. Furthermore, the importance of NAergic neurotransmission for social memory has been demonstrated in mouse model of locus coeruleus lesion and in transgenic mice lacking the dopamine beta-hydroxylase.17,18 We thus investigated the social recognition/memory in the Thy1-APPLond/Swe (APP) mouse model of AD using pharmacological and molecular tools and showed the social recognition deficit observed in APP mice is associated with abnormalities in β1-ADR signaling in the medial amygdala (MeA). Pharmacological activation of the β1-ADR and restoring the protein kinase A (PKA)/phospho-cAMP response element-binding protein (pCREB) levels in APP mice rescue the social memory deficit detected in this model of AD. Our results demonstrate for the first time that the β1-ADR and CREB phosphorylation in the MeA is an essential signaling cascade for social learning under normal brain function.
Materials and Methods
Animals
Eight to 10-weeks-old adult C57Bl/6 mice (Jackson Laboratory, Bar Harbor, ME, #000664), 8–10-week-old β2-ADR (knockout) KO mice (kindly provided by Dr. Rona G., Giffard, Department of Anesthesia, Stanford University School of Medicine), 8–10-week-old β1-ADR KO mice (kindly provided by Dr. Daniel Bernstein, Division of Pediatric Cardiology, Stanford University School of Medicine) and their age-matched controls (FVB/NJ mice from Jackson Laboratory, #001800), and Thy1-hAPPLond/Swe+ and their age-matched WT littermate mice, model of AD19 were used. This particular mouse line was chosen because it was shown to have an accelerated pathology with a rapid appearance of mature β-amyloid plaques in the frontal cortex as early as 3 months of age and in the hippocampus, thalamus, and olfactory region in 5–7 months of age.19 In addition, our previous research demonstrated severe cognitive impairments (including social memory deficits) as early as 6 months of age.20 Experiments were performed in accordance with protocols approved by the Institutional Animal Care and Use Committee of Stanford University and were performed based on the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Behavioral testing
Social recognition was tested in two different tasks: the three-chamber social test developed by Nadler et al.21 and the in-home cage social discrimination task developed by Macbeth et al.22 (Data S1). To differentiate between social recognition and recognition of nonsocial odors/objects, nonsocial odor discrimination tests (olfactory habituation/dishabituation and olfactory recognition test), and an object recognition test were used (Data S1).
Drug treatment
The β1-ADR partial agonist, xamoterol, the β1-ADR antagonist, betaxolol and the PKA inhibitor, PKI 14-22 amide myristoylated (Tocris bioscience, Minneapolis, MN) were used. Xamoterol was injected subcutaneously (3 mg/kg). Betaxolol was injected subcutaneously (1 mg/kg) or in the MeA (30 nmole/side). PKI 14-22 amide was dissolved in 30% acetonitrile and injected in the MeA (2.75 nmole/side) (Data S1).
Molecular analyses
Immunohistochemistry was used to assess c-Fos. The quantitative analysis of stained cells was done in different brain regions according to the Mouse Brain Atlas.23 Cell counting was achieved using the unbiased-stereologic method. The total number of positive cells was quantified with the optical fractionator method24 using StereoInvestigator software (MBF Bioscience, Williston, VT). The counting criteria were determined so as to obtain a mean coefficient of error25 ≤0.10. Analysis of pCREB was performed by western blot (Data S1).
Statistics
Data were analyzed using the software Prism 5.01 (GraphPad Software Inc., La Jolla, CA). For behavioral and pharmacological testing, a minimum of five animals per group were used. For immunohistochemistry and western blot four to six animals per group were used.
Results
Social recognition is impaired in a mouse model of AD
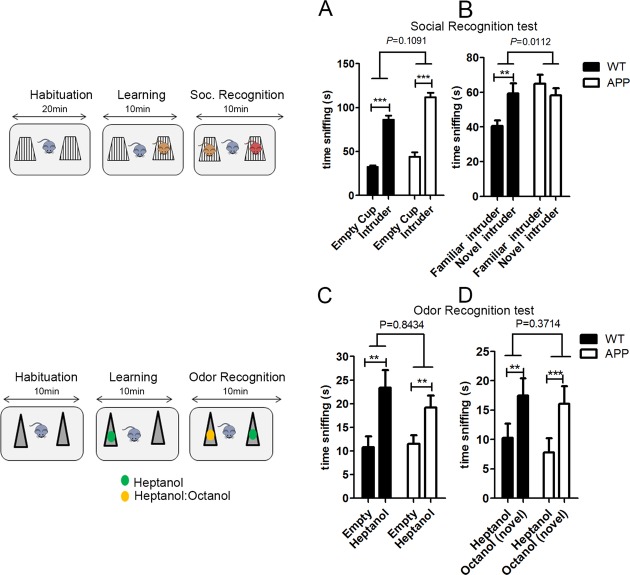
In rodents, social recognition can be measured by an increased amount of time investigating a never-met conspecific as compared to a previously met one. We first investigated the ability of the APP mice to recognize a previously met conspecific. While they showed a preference for an unfamiliar intruder over an empty cup (Fig.1A), they failed to demonstrate a preference for a new intruder over a familiar one, indicative of social recognition deficit (Fig.1B). This deficit was not linked to a deficit in olfactory abilities or in discriminating between nonsocial odors (Fig.1C and D). APP mice display a general hyperactivity and spend more time sniffing social objects during the learning phase and the social recognition phase of the test compared to the WT mice (P < 0.001 and P = 0.046, respectively). This hyperactivity phenotype was previously reported in other behavioral assays, such as the open field test.20 We show for the first time that this APP mouse model of AD recreates one important symptom characteristic of AD, the selective inability to recognize a previously met individual while displaying intact odor and object discrimination.
Figure 1.
APP mouse model of Alzheimer's disease are characterized by impaired social recognition. On the left, schematic representations of the experiments are shown. (A) Both wild-type (WT; n = 11) and APP (n = 9) mice explored a cup containing an unfamiliar C57Bl/6 intruder mouse over an empty cup, showing normal sociability (two-way ANOVA: interaction P = 0.1091, F(1,36) = 2.699; object P < 0.0001, F(1,36) = 203.9; genotype P < 0.0001, F(1,36) = 19.43. Post hoc paired one-tailed t-test corrected for multiple comparisons: WT P < 0.001; APP P < 0.001); (B) however, while WT showed a preference for a novel C56Bl/6 intruder over a familiar one, APP mice did not display this preference, indicative of social recognition deficits (two-way ANOVA: interaction P = 0.0112, F(1,36) = 7.141; object P = 0.2149, F(1,36) = 1.594; genotype P = 0.0200, F(1,36) = 5.929. Post hoc paired one-tailed t-test corrected for multiple comparisons: WT P = 0.0066; APP P = 0.278); (C) Both WT (n = 7) and APP (n = 8) mice sniffed a tube containing an heptanol solution over an empty tube (two-way ANOVA: interaction P = 0.8434, F(1,26) =0.0398; object P = 0.0088, F(1,26) = 8.014; genotype P = 0.4825, F(1,26) = 0.5076. Post hoc paired one-tailed t-test corrected for multiple comparisons: WT P = 0.0024; APP P = 0.0052); (D) Similarly, both WT and APP mice showed a preference for a tube containing a novel alcohol odor (heptanol:octanol solution) over the familiar one (heptanol alone) indicative that both genotypes can remember a familiar nonsocial odor. (Two-way ANOVA: interaction P = 0.3714, F(1,26) = 0.8272; object P = 0.0008, F(1,26) = 14.54; genotype P = 0.5262, F(1,26) = 0.4128. Post hoc paired one-tailed t-test corrected for multiple comparisons: WT P = 0.0062; APP P = 0.001). Data are presented as mean ± SEM.
β1-ADR and its downstream signaling is essential for learning of social cues
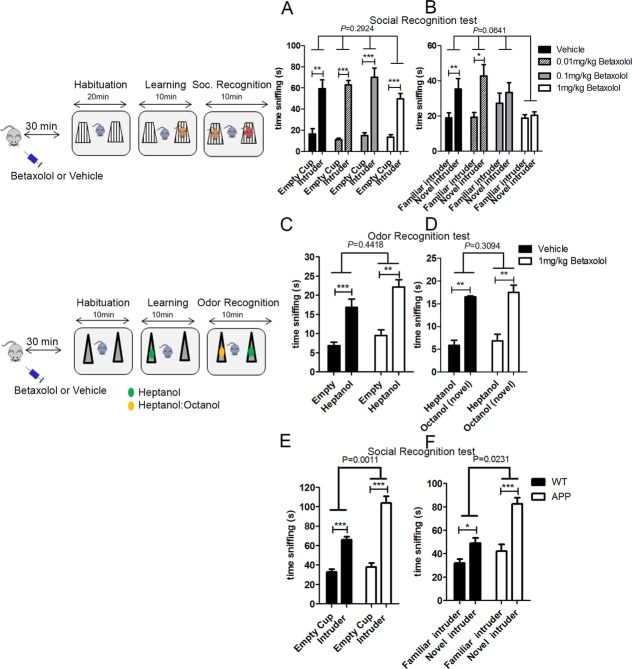
Because expression of the β1-ADR has been suggested to play a critical role in AD pathology,16 we then investigated the potential contribution of the β1-ADR in social recognition to determine whether the abnormalities in the β1-noradrenergic neurotransmission in APP mice could be responsible for their social memory deficit. We assessed the ability of C57Bl/6 mice to recognize a previously met conspecific after an acute subcutaneous injection of various doses of a selective β1-ADR antagonist, betaxolol (0.01, 0.1, and 1 mg/kg). Although betaxolol did not affect the preference for an unfamiliar intruder over an empty cup, 0.1 and 1 mg/kg of betaxolol resulted in an inability of mice to distinguish between a new and a familiar conspecific without affecting the total time spent exploring the two conspecifics (Fig.2A and B). This finding was reproduced in a different behavioral paradigm that also assesses social recognition, the three-chamber test (Fig. S1A). We then demonstrated that blockade of β1-ADR affects the recognition of social cues without affecting general olfactory abilities and discrimination (Fig.2C and D). These results were confirmed in an odor habituation/dishabituation paradigm (Fig. S1B). Finally, we observed that the ability to remember a familiar object was not different in the mice injected with betaxolol or vehicle when conducted with an identical experimental design and apparatus (Fig. S1C).
Figure 2.
Activation of the β1-ADR is necessary for social recognition. In the left, representative diagram of the experimental design followed to assess social recognition (based on22). Blockage of the β1-ADR with various doses of betaxolol ranging from 0.01 to 1 mg/kg (n = 8 per group) did not affect social learning but impaired social recognition. (A) All mice injected betaxolol or vehicle preferred a cup containing an unfamiliar C57Bl/6 mouse over an empty cup (two-way ANOVA: interaction P = 0.2924, F(3,54) = 1.274; object P < 0.0001, F(1,54) = 154.0; treatment P = 0.2459, F(3,54) = 1.424. Post hoc paired one-tailed t-test corrected for multiple comparisons: vehicle P = 0.0016; 0.01 mg/kg P = 0.0004; 0.1 mg/kg P = 0.0008; 1 mg/kg P = 0.0004). (B) However, mice injected with 0.1 or 1 mg/kg of betaxolol did not show a preference for a novel C56Bl/6 intruder mouse over a familiar one (not significant [ns] by paired t-test), indicative of impaired social recognition, while mice injected with vehicle or 0.01 mg/kg of betaxolol showed this preference (two-way ANOVA: interaction P = 0.0641, F(3,54) = 2.565; object P = 0.0004, F(1,54) = 14.29; treatment P = 0.0467, F(3,54) = 2.834. Post hoc paired one-tailed t-test corrected for multiple comparisons: vehicle P = 0.006; 0.01 mg/kg P = 0.0168; 0.1 mg/kg P = 0.5348; 1 mg/kg P = 0.3484); Betaxolol did not impair nonsocial odor recognition: (C) in a test of nonsocial odor recognition, betaxolol did not affect the preference of mice for a tube containing an heptanol solution over an empty tube (two-way ANOVA: interaction P = 0.4418, F(1,24) = 0.6117; object P < 0.0001, F(1,24) = 46.80; treatment P = 0.0254, F(1,24) = 5.680. Post hoc paired one-tailed t-test corrected for multiple comparisons: vehicle P = 0.0004; betaxolol P = 0.0016) and (D) did not affect the ability of mice to discriminate between a familiar odor (heptanol) versus a new by similar odor (solution of heptanol:octanol) (two-way ANOVA: interaction P = 0.3094, F(1,24) = 1.079; object P < 0.0001, F(1,24) = 33.22; treatment P = 0.1070, F(1,24) = 2.804. Post hoc paired one-tailed t-test corrected for multiple comparisons: vehicle P = 0.0018; betaxalol P = 0.0014). (E) Systemic injection of xamoterol (3 mg/kg) prior to social recognition testing did not affect the preference of WT (n = 10) and APP (n = 10) mice for an unfamiliar intruder over an empty cup (two-way ANOVA: interaction P = 0.0011, F(1,36) = 12.67; object P < 0.0001, F(1,36) = 118.3; treatment P < 0.0001, F(1,36) = 22.19. Post hoc paired one-tailed t-test corrected for multiple comparisons: WT P < 0.001; APP P < 0.001); But (F) rescued the social recognition deficit observed previously in APP mice (see Fig.1B): similar to their WT control littermates injected with xamoterol (n = 10), xamoterol injected APP mice (n = 10) showed preference for a novel intruder over a familiar one (two-way ANOVA: interaction P = 0.0231, F(1,36) = 5.636; object P < 0.0001, F(1,36) = 35.09; treatment P < 0.0001, F(1,36) = 20.71. Post hoc paired one-tailed t-test corrected for multiple comparisons: WT P = 0.0198; APP P < 0.001).
These results suggest that the β1-ADR activation is necessary for social learning and recognition in mice. We thus propose that abnormalities in β1-ADR signaling in the APP mice are responsible for their deficit in social recognition, and therefore restoring the function of the β1-ADR in APP mice would rescue the social recognition deficit. Indeed, injection of the β1-ADR partial agonist, xamoterol in APP mice prior to social learning rescued the deficit otherwise observed (Fig.2E and F).
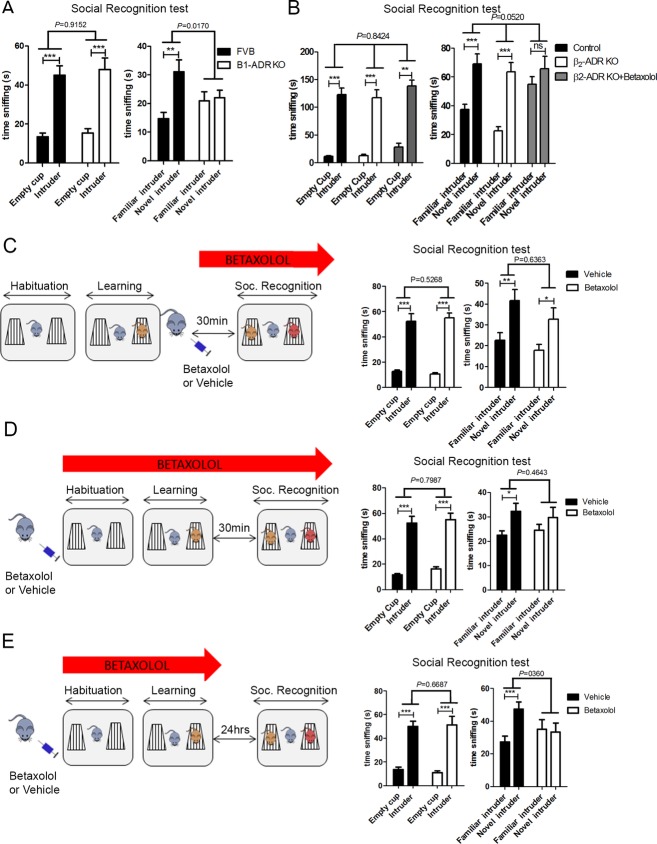
Our pharmacological data indicate that the β1-ADR is necessary for social recognition. As drug selectivity for a specific receptor is often relative, we aimed to test social recognition abilities in both β1-ADR KO and β2-ADR KO mice and their FVB controls to confirm: (1) that betaxolol targets specifically the β1-ADR, and (2) the importance of the β1-ADR (vs. β2-ADR) in social recognition. All mice showed a preference for a cup with an unfamiliar intruder over an empty cup. Whilst β1-ADR KO mice have impaired social recognition (Fig.3A), β2-ADR KO mice perform the task correctly (Fig.3B). In addition, betaxolol injection in β2-ADR KO mice prior to social learning resulted in impaired social recognition (Fig.3B). This suggests that the social recognition deficit induced by betaxolol is mediated by the β1-ADR- not β2-ADR- and that the β1-ADR is essential for social recognition.
Figure 3.
Activation of β1-ADR is necessary for the acquisition of social cues. (A) β1-ADR KO mice (n = 17) tested in the three-chamber assay show a preference for a cup containing an unfamiliar intruder over an empty cup, similar to control mice (FVB mice n = 16) (two-way ANOVA: interaction P = 0.9152, F(1,62) = 0.01143; object P =< 0.0001, F(1,62) = 59.97; treatment P = 0.5622, F(1,62) = 0.3395. Post hoc paired one-tailed t-test corrected for multiple comparisons: FVB P < 0.001; β1-ADR KO P < 0.001). However, β1-ADR KO mice failed to recognize a novel intruder over a familiar one, while their WT controls made this discrimination (two-way ANOVA: interaction P = 0.0170, F(1,62)=6.022; object P = 0.0063, F(1,62) = 7.990; treatment P = 0.6380, F(1,62) = 0.2235. Post hoc paired one-tailed t-test corrected for multiple comparisons: FVB P = 0.0016; betaxolol P = 0.7512). (B) β2-ADR KO mice (n = 8) show a preference for a cup containing an unfamiliar intruder over an empty cup, similar to control FVB mice (n = 8) and β2-ADR KO mice treated with 3 mg/kg of betaxolol (n = 8). Both FVB control and β2-ADR KO mice show a preference for a novel intruder over a familiar one indicative of normal social recognition abilities even in mice lacking the β2-ADR (two-way ANOVA: interaction P = 0.8424, F(3,52) = 0.2761; Object P < 0.0001, F(1,52) = 241.7; treatment P = 0.1272, F(3,52) = 1.988. Post hoc paired one-tailed t-test corrected for multiple comparisons: control P < 0.001; β2-ADR KO P < 0.001; β2-ADR KO + betaxolol P = 0.0015); in addition, betaxolol effectively impaired social recognition abilities in β2-ADR KO mice suggesting that betaxolol's effects on social recognition are not modulated by β2-ADR (two-way ANOVA: interaction P = 0.0520, F(2,38) = 3.199; object P < 0.0001, F(1,38) = 33.43; treatment P = 0.0215, F(2,38) = 4.253. Post hoc paired one-tailed t-test corrected for multiple comparisons: FVB P = 0.0006; β2-ADR KO P = 0.0006; β2-ADR KO + betaxolol P = 0.153). (C) Systemic injection of betaxolol (1 mg/kg) 30 min prior to the retrieval phase of the test did not impair sociability (interaction P = 0.5268, F(1,28) = 0.4107; object P =< 0.0001, F(1,28) = 136.1; treatment P = 0.9198, F(1,28) = 0.01033. Post hoc paired one-tailed t-test corrected for multiple comparisons: vehicle P = 0.0004; betaxolol: P < 0.0002). Testing and social recognition: both vehicle-injected and betaxolol-injected (n = 8 per group) mice preferred a new intruder over a familiar one (two-way ANOVA: interaction: P = 0.6363, F(1,28) = 0.2286; object P = 0.0006, F(1,28) = 14.86; treatment P = 0.1306, F(1,28) = 2.426. Post hoc paired one-tailed t-test corrected for multiple comparisons: vehicle P = 0.0024; betaxolol P = 0.0136). (D) Systemic injection of betaxolol (1 mg/kg) 20 min prior to the learning phase of the test did not impair sociability (two-way ANOVA: interaction P = 0.7987, F(1,28) = 0.06631; object P =< 0.0001, F(1,28) = 110.8; treatment P = 0.3364 F(1,28) = 0.9567. Post hoc paired one-tailed t-test corrected for multiple comparisons: vehicle P < 0.001, betaxolol P < 0.001) but impaired short-term social recognition: betaxolol-injected mice (n = 8) did not prefer a novel intruder over a familiar one, indicative of social recognition deficit, whilst vehicle-injected mice (n = 8) showed this preference (interaction P = 0.4643, F(1,28)=0.5505; object P = 0.0193, F(1,28) = 6.164; treatment P = 0.9264, F(1,28) = 0.008683. Post hoc paired one-tailed t-test corrected for multiple comparisons: vehicle P = 0.0118; betaxolol P = 0.0648). (E) Systemic injection of betaxolol (1 mg/kg) 20 min prior to the learning phase of the test did not impair sociability (two-way ANOVA: interaction: P = 0.6687, F(1,28) = 0.1871; object P =< 0.0001, F(1,28) = 74.64; treatment P = 0.8891, F(1,28) = 0.01980. Post hoc paired one-tailed t-test corrected for multiple comparisons: vehicle P < 0.001, betaxolol P = 0.0004) but impaired long-term social memory: 24 h after the learning phase, betaxolol-injected mice (n = 8) did not preferred the novel C57Bl/6 intruder over the familiar one, indicative of social memory deficit, while vehicle-injected mice (n = 8) showed this preference (two-way ANOVA: interaction P = 0.0360, F(1,28) = 4.855; object P = 0.0762, F(1,28) = 0.0762; treatment P = 0.5298, F(1,28) = 0.4048. Post hoc paired one-tailed t-test corrected for multiple comparisons: vehicle P = 0.0006, betaxolol P = 0.389). Data are presented as mean ± SEM.
We next evaluated if the activation of the β1-ADR is important for the acquisition of social cues (social learning) or during the retrieval of this information. We injected betaxolol either 20 min before the learning phase of the test or 30 min before the retrieval phase of the test. Inhibition of β1-ADR during the learning phase led to a social recognition deficit (Fig.3D), while inhibition of β1-ADR during the retrieval phase of the test did not impair social recognition (Fig.3C). We then confirmed the importance of the β1-ADR for long-term consolidation of social cues by injecting Betaxolol before the learning phase and 24 h prior to the social recognition test. Mice injected with betaxolol prior to the learning phase and tested 24-h later showed impaired social recognition, while vehicle-injected mice had intact social recognition (Fig.3E). Collectively, these data demonstrate the importance of β1-ADR for the learning of social cues, which is necessary for social recognition.
The β1-ADR in the MeA is essential for social recognition
Brain regions involved in social recognition have already been defined.26,27 Among them are the lateral septum, prefrontal cortex, and MeA. We first confirmed the importance of the MeA for social recognition. We used c-Fos as a marker of neuronal activity and compared its expression in a group of C57Bl/6 mice after social learning with a group of mice after both social learning and social recognition. Unbiased stereological counting revealed higher level of c-Fos expression after social recognition in the MeA (Fig. S2A) but not in the basolateral amygdala (Fig. S2B) confirming the importance of the MeA for the processing of social cues.
We then observed that after injection of betaxolol, c-Fos expression in MeA was significantly reduced after social recognition compared to vehicle-injected group (Fig.4A). This reduction was specific to regions activated during social recognition such as the MeA as we did not observe such an effect in the paraventricular nucleus of the thalamus, which is poor in β1-ADR and not preferentially activated by social recognition (Fig. S2C).
Figure 4.
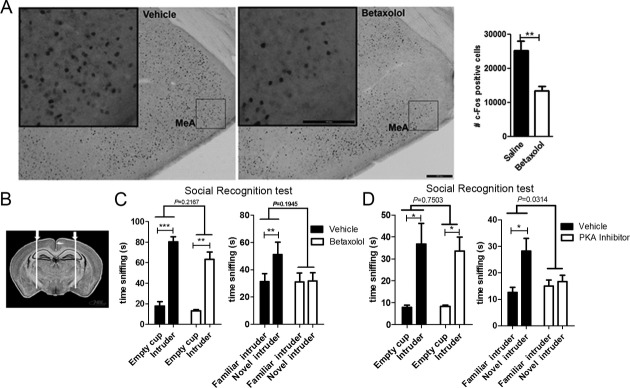
Activation of β1-ADR in medial amygdala (MeA) is necessary for social recognition. (A) Injection of betaxolol (1 mg/kg) prior to testing in a social recognition task decreased the number of c-Fos positive cells in the MeA (vehicle-injected mice n = 4; betaxolol-injected mice n = 4; P = 0.0076 by t-test). Scale bars, 50 μm. (B) Schematic representation of coronal section shows the injection in the MeA. (C) Mice were injected in the MeA with either vehicle (n = 5) or with 30nmole of betaxolol (n = 5). Betaxolol did not affect the social learning (two-way ANOVA: interaction P = 0.2167, F(1,16) = 1.654; object P < 0.0001, F(1,16) = 137.1; treatment P = 0.0361, F(1,16) = 5.236. Post hoc paired one-tailed t-test corrected for multiple comparisons: vehicle P = 0.0008; betaxolol = 0.0030) but impairs social recognition (two-way ANOVA: interaction P = 0.1945, F(1,16) = 1.833; object P = 0.1659, F(1,16) = 2.108; treatment P = 0.1805, F(1,24) = 1.961. Post hoc paired one-tailed t-test corrected for multiple comparisons: vehicle: P = 0.010; betaxolol P = 0.7636). (D) Mice were injected in the MeA with either vehicle (n = 4) or a PKA inhibitor (2.75 nmole – n = 5). PKA inhibition did not affect the preference of mice for an unfamiliar conspecific over an empty cup (two-way ANOVA: interaction P = 0.7503, F(1,14) = 0.1053; object P = 0.0002, F(1,14) = 24.12; treatment P = 0.8032, F(1,14) = 0.06453. Post hoc paired one-tailed t-test corrected for multiple comparisons: vehicle P = 0.0472; PKA inhibitor P = 0.0204) but impaired the ability of mice to recognize between a novel and a familiar conspecific (two-way ANOVA: interaction P = 0.0314, F(1,14) = 5.719; object P = 0.0108, F(1,14) = 8.634; treatment P = 0.1377, F(1,14) = 2.479. Post hoc paired one-tailed t-test corrected for multiple comparisons: vehicle P = 0.0312; PKA inhibitor P = 0.0958.). Data are presented as mean ± SEM.
To further confirm the role of the β1-ADR in the MeA for social recognition, betaxolol was directly injected in the MeA (Fig.4B) of C57Bl/6 mice prior to social learning. Mice treated with betaxolol in the MeA were not able to recognize a familiar conspecific (Fig.4C). Our results demonstrated that the MeA is an important structure for social recognition, and that activation of β1-ADR in the MeA is necessary for social learning and recognition.
The β1-ADR is a G-protein coupled receptor associated with the Gs heterotrimeric G-protein. As such, one of the signaling pathways downstream of the β1-ADR involves activation of the cAMP/PKA cascade resulting in phosphorylation of CREB. We thus analyzed whether the blockade of the pathway downstream of β1-ADR by PKI 14-22 a selective PKA inhibitor impairs social learning and recognition. PKI 14-22 was in the MeA of C57Bl/6 mice prior to social learning to determine whether blockade of the PKA/CREB/pCREB cascade affects social recognition. Inhibition of PKA impairs the ability of mice to recognize a familiar conspecific (Fig.4D) without affecting the preference for an unfamiliar intruder over an empty cup during social learning. These results show that PKA activity is necessary for social learning and recognition. Together our results show that inhibition of β1-ADR signaling pathway in MeA by betaxolol or a PKA inhibitor results in social recognition deficits.
β1-ADR modulates CREB phosphorylation
Because one of the well-known downstream target of PKA is CREB phosphorylation, we aimed at evaluating the possible role of pCREB in social learning; we performed a series of experiments: we first observed that in C57Bl/6 mice learning of social cues induced a strong increase in pCREB in the MeA which picked 5 min after the onset of social learning and returned to baseline after 10 min (Fig.5A). We then tested whether pharmacological modulation of β1-ADR affects CREB phosphorylation in the MeA in the context of social learning. We showed that blockade of the β1-ADR by an acute systemic injection of 1 mg/kg of betaxolol before social learning inhibits the induction of pCREB after social learning (Fig.5B) while activation of the β1-ADR with 3 mg/kg of the partial agonist xamoterol increases pCREB (Fig.5C). With the present data, we show that activation of the PKA/pCREB cascade downstream of the β1-ADR mediates social learning and recognition.
Figure 5.
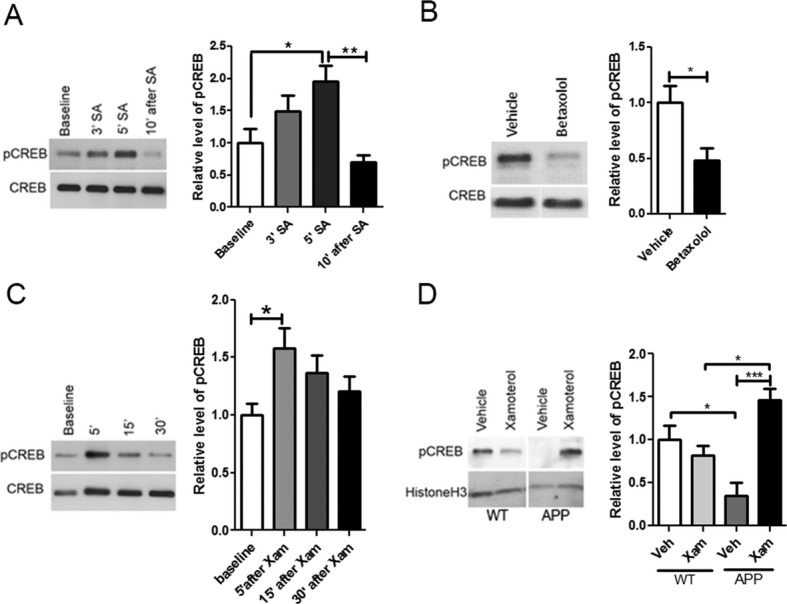
β1-ADR modulates CREB phosphorylation. (A) Western blot analysis of pCREB performed on MeA extracts of C57Bl/6 mice before social acquisition (SA) (baseline) (n = 6), directly after 3 (n = 6) or 5 min (n = 5) of social acquisition and 10 min after a 5 min social acquisition (n = 6). pCREB expression was significantly increased after 5 min of social learning when compared to baseline values and decreased 10 min after social learning was completed. The relative optical density was normalized to CREB. (One-way ANOVA P = 0.0033, F(3,19) = 6.498. Post hoc Bonferroni's multiple comparison test, baseline vs. 5′ SA P < 0.05, 5′ SA vs. 10′ after SA P < 0.01) (B) Western blot analysis of pCREB performed on MeA extracts of C57Bl/6 mice injected systemically 60 min before social learning with betaxolol (1 mg/kg) or vehicle (n = 5 per group) and euthanized after 5 min of social interaction. pCREB expression was significantly decreased in the betaxolol-injected (P = 0.0113 by t-test). (C) Western blot analysis of pCREB performed on MeA extracts of C57Bl/6 mice injected systemically with the β1-ADR partial agonist xamoterol (3 mg/kg) 5 min (n = 6), 15 min (n = 6) and 30 min (n = 5) before euthanasia. pCREB expression was increased 5 min after injection when compared to baseline (n = 5). The relative optical density is normalized to CREB (One-way ANOVA P = 0.0675, F(3,18) = 2.831. Post hoc Bonferroni's multiple comparison test, baseline vs. 5′ after Xam P < 0.05). (D) Western blot analysis of the nuclear fraction of medial amygdala of APP mice and the WT controls injected with xamoterol (n = 4 APP, n = 5 WT) or vehicle (n = 4 APP, n = 4 WT) prior to social learning showed that the level of nuclear pCREB was lower in APP mice (one-way ANOVA P = 0.0011, F(3,13) = 10.08. Post hoc Bonferroni's multiple comparison test, WT Veh vs. APP veh P < 0.05, WT Xam vs. APP Xam P < 0.05, APP Veh vs. APP Xam P < 0.001). The relative optical density is normalized to histone H3. Data are presented as mean ± SEM.
To assess whether the rescuing effects of xamoterol on social memory in APP mice is associated with modifications in the level of pCREB, we first measured the level of pCREB in the MeA of APP mice and their WT littermates and found a higher level of pCREB in MeA of APP mice (Fig. S3). It was reported that in AD several phosphorylated proteins and transcription factors are preferentially located in the cytoplasm (vs. the nucleus)28–30 and are thus inefficient in inducing expression of genes important for learning and memory. We thus determined whether the level of pCREB in the nucleus of APP mice (effective pCREB) was affected by xamoterol. We observed that despite the high levels of pCREB in whole tissue lysates of MeA in APP mice, the levels of pCREB in nuclear fraction of the tissue was lower in APP mice compared to control mice and xamoterol increased pCREB level in the nuclear fraction of the MeA in APP mice (Fig.5D). This indicates that activation of the β1-ADR in a mouse model of AD using the partial agonist xamoterol leads to an increased level of nuclear pCREB in the MeA and an improvement in learning of the social cues.
Discussion
Many neurological disorders, such as AD, are characterized by impairment in social memory and discrimination. The causes of these symptoms are not yet well-defined and as a consequence, no treatment currently exists to rescue these deficits. We have now identified the β1-ADR and it downstream PKA/pCREB signaling cascade as key modulators of social learning and recognition. Interestingly, we have shown pathological changes in this cascade in a model of AD. We have shown that the level of nuclear pCREB in the MeA of APP mice is significantly reduced when compared to WT mice. Moreover, we have shown that APP mice present severe social learning and recognition deficits, that relies mainly on integrity of the MeA. Finally, we have shown that a selective partial agonist of the β1-ADR can restore this function by increasing the level of nuclear pCREB in the MeA, highlighting a possible approach for improving or restoring social function in AD.
We have reported that APP mice display social recognition deficits, despite demonstrating normal recognition for nonsocial olfactory cues. Even if at the level of the olfactory bulb, NA has been shown to be important for the processing of neutral and social olfactory information31 by promoting selective attention to olfactory stimuli,32,33 our results indicate that two independent pathways downstream of the olfactory bulb regulate memory for social and nonsocial cues. For the first time, we show that the blockade of the β1-ADR prior to learning social or neutral olfactory cues leads to impaired social recognition without affecting nonsocial olfactory recognition. In addition, our results indicate that the β1-ADR pathway is necessary for the learning of social cues but not for their recall.
The MeA is known to be involved in the processing of sensory information necessary for the regulation of social and sexual behaviors in both humans and nonhuman mammals.34 Many previous rodent studies have shown the central role of the MeA for social recognition.26,27 Several studies have demonstrated pathological changes occur in amygdala at the early stages of AD and accumulation of neuritic plaques and neurofibrillary tangles is observed in amygdala35,36. The early impairment of amygdala structures, whose central role in the recognition and memorization of emotions has been demonstrated, has been associated with deficits in terms of social cognition in AD.37 Furthermore, positron emission tomography studies in AD patients during face memory task have pointed toward dysfunction of the amygdala.38 Our results demonstrate the importance of β1-ADR noradrenergic neurotransmission in the MeA for social recognition, and suggest that abnormality in this signaling cascade in this brain region in APP mice could contribute to the disease related social recognition deficit.
Our data indicate that the β1-ADR in MeA regulates social learning by activation of the PKA/CREB phosphorylation-signaling cascade. Indeed, pharmacological inhibition of PKA in the MeA prior to social learning impairs social recognition. We also demonstrate that blockade of β1-ADR with a selective antagonist which induces social recognition deficit results in a severe decrease in CREB phosphorylation in the MeA. These results specify a direct link between the PKA/pCREB cascade and social learning in mice. Generally it is assumed that pCREB is necessary for long-term memory, rather than short-term memory, because of its known effect on gene expression and protein synthesis that are required for long-term memory.39,40 However, Suzuki et al.41 recently demonstrated that an upregulation of CREB activity leading to higher expression of brain-derived neurotrophic factor enhances social and nonsocial short-term memory in mice. Here, we are suggesting that a similar phenomenon is occurring as a consequence of activation of the β1-ADR. Social learning induces the release of NA, which activates β1-ADR in the MeA, leading to activation of the cAMP/PKA/pCREB signaling cascade. We have shown that activation of β1-ADR and its downstream signaling is necessary for the learning of social cues.
While a treatment with the β1-ADR partial agonist xamoterol rescues the social recognition deficit observed in APP mice, it did not affect their total level of pCREB but did show significantly increased levels of nuclear pCREB. Therefore, we suggest that social recognition deficit observed in APP mice is explained rather by diminished levels of nuclear pCREB. It has been shown in cellular system that high levels of amyloid-beta causes hyperphosphorylation of CREB and a lack of nuclear translocation.42 Therefore, we suggest that activation of the β1-ADR with xamoterol affects the nuclear level of pCREB in the MeA of APP mice and activates mechanisms allowing the processing of social cues necessary for further social recognition.
Finally, our findings indicate that the β1-ADR can be used as a potential therapeutic target to improve the social memory and possibly other cognitive deficits in AD. Our conclusions are based on the APP mouse model of AD which has been shown to be highly successful for AD treatment compound testing.43 However, due to significant involvement of the noradrenergic system in cardiovascular function, further safety and translational studies will be needed to ensure the safety and efficacy of this approach. Ultimately developing a molecule with minimal systemic activity and high central nervous system (CNS) penetration may provide us with means to enhance the positive CNS effects of adrenergic system while minimizing the cardiovascular effects. While this novel cognitive enhancement strategy remains extremely attractive, further investigation of the β1-ADR as modulator of the neuroinflammation is also warranted.
Acknowledgments
We thank Leo Kim and Nay Saw for excellent technical assistance. We also thank R. G. Giffard and D. Bernstein for providing us with β2-ADR KO and β1-ADR KO mice, respectively. This study was partially supported by National Institute of Neurological Disorders and Stroke P30 center core grant (NS069375-01A1).
Conflict of Interest
None declared.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Data S1.Supplementary methods.
Figure S1. Blocking the β1-ADR with betaxolol (1 mg/kg) impaired social recognition independently of nonsocial odor recognition and object recognition. (A) In the three-chamber social recognition test, systemic injection of betaxolol prior the learning phase of the test did not alter the preference of mice for an unfamiliar intruder in a cup over an empty cup (vehicle-injected mice n = 10, betaxolol-injected mice n = 10) (two-way ANOVA interaction: P = 0.2801, F(1,36) = 1.203; object P < 0.0001, F(1,36) = 33.05; treatment P = 0.6501, F(1,36) = 0.2093. Post hoc paired one-tailed t-test corrected for multiple comparisons: vehicle P = 0.0004; betaxolol P = 0.0014. However, betaxolol impairs social recognition: betaxolol-injected mice did not show a preference for a new intruder over a familiar one while vehicle-injected mice show this preference (two-way ANOVA interaction: P = 0.0906, F(1,36) = 3.024; object P = 0.0293, F(1,36) = 5.155; treatment P = 0.6749, F(1,36) = 0.1789. Post hoc paired one-tailed t-test correlated for multiple comparisons: vehicle P = 0.0082, betaxolol P = 0.7128). (B) Betaxolol did not impair nonsocial odor recognition; in a test of nonsocial odor habituation, dishabituation, and recognition, both vehicle-injected (n = 10) and betaxolol-injected (n = 10) mice habituated to nonsocial odors (water and vanilla) over the course of three successive exposures and dishabituated when presented to a new odor (two-way RM ANOVA: interaction: P = 0.9623, F(6,108) = 0.2401; treatment P = 0.5234, F(1,108) = 0.4236; object P < 0.0001, F(6,108) = 21.42; Post hoc paired one-tailed t-test correlated for multiple comparisons: Water habituation: vehicle ***P < 0.001; betaxolol τττP < 0.001; Water dishabituation: vehicle **P = 0.0024; betaxolol τττP < 0.001; Vanilla habituation: vehicle ***P < 0.001; betaxolol ττP = 0.006). All mice were also able to discriminate between a previously presented odor (vanilla) and a new odor (mint) as indicated by higher sniffing time when presented with the mint odor versus sniffing time when presented with the vanilla odor for a third time (vehicle **P = 0.0048; betaxolol ττP = 0.0036) (C) The ability of mice to recognize a familiar object over a new one was not affected by betaxolol. Both vehicle-injected (n = 10) and betaxolol-injected (n = 10) mice preferred a new object over a familiar one in an object recognition test (two-way ANOVA interaction: P = 0.7487, F(1,36) = 0.1042; object P < 0.0001 F(1,36) = 28.12; treatment P = 0.2604, F(1,36) = 0.2604; Post hoc paired one-tailed t-test correlated for multiple comparisons: vehicle P < 0.001; betaxolol P < 0.001). Data are presented as mean ± SEM.
Figure S2. (A) c-Fos expression is induced in the MeA 90 min after social recognition. Quantification of c-Fos positive cells was performed using a nonbiased stereological methods in control mice (n = 4) and mice exposed to a social recognition task (n = 4) (P = 0.0303 by t-test). (B) c-Fos expression was not induced in the basolateral amygdala (BLA) after social recognition. Quantification of c-Fos positive cells was performed using a nonbiased stereological methods in control mice (n = 4) and mice exposed to a social recognition task (n = 4) (P = 0.4857 by Mann–Whitney test). (C) Injection of betaxolol prior to testing in a social recognition task did not affect the number of c-Fos positive cells in the thalamus (PV), brain region not involved in social memory and poor in β1-ADR (vehicle-injected mice n = 4; betaxolol-injected mice n = 4; P = 0.8571 by Mann–Whitney test). Data are presented as mean ± SEM. Scale bars, 100 µm (magnified box) and 200 µm.
Figure S3. Western blot analysis of pCREB performed on whole tissue homogenates from medial amygdala of control mice and APP mice (n = 4 per group). pCREB level was significantly higher in APP mice (P = 0.0286 by Mann–Whitney test). The relative optical density is normalized to CREB. Data are presented as mean ± SEM.
References
- Werheid K, Clare L. Are faces special in Alzheimer's disease? Cognitive conceptualisation, neural correlates, and diagnostic relevance of impaired memory for faces and names. Cortex. 2007;43:898–906. doi: 10.1016/s0010-9452(08)70689-0. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Kaszniak AW, Bacon LD, et al. Facial recognition memory in dementia. Cortex. 1982;18:329–336. doi: 10.1016/s0010-9452(82)80031-2. [DOI] [PubMed] [Google Scholar]
- Bielsky IF, Hu SB, Szegda KL, et al. Profound impairment in social recognition and reduction in anxiety-like behavior in vasopressin V1a receptor knockout mice. Neuropsychopharmacology. 2004;29:483–493. doi: 10.1038/sj.npp.1300360. [DOI] [PubMed] [Google Scholar]
- Young LJ. The neurobiology of social recognition, approach, and avoidance. Biol Psychiatry. 2002;51:18–26. doi: 10.1016/s0006-3223(01)01268-9. [DOI] [PubMed] [Google Scholar]
- Higashida H, Yokoyama S, Huang JJ, et al. Social memory, amnesia, and autism: brain oxytocin secretion is regulated by NAD+ metabolites and single nucleotide polymorphisms of CD38. Neurochem Int. 2012;61:828–838. doi: 10.1016/j.neuint.2012.01.030. [DOI] [PubMed] [Google Scholar]
- Meynen G, Unmehopa UA, Hofman MA, et al. Hypothalamic vasopressin and oxytocin mRNA expression in relation to depressive state in Alzheimer's disease: a difference with major depressive disorder. J Neuroendocrinol. 2009;21:722–729. doi: 10.1111/j.1365-2826.2009.01890.x. [DOI] [PubMed] [Google Scholar]
- Mazurek MF, Beal MF, Bird ED, Martin JB. Oxytocin in Alzheimer's disease: postmortem brain levels. Neurology. 1987;37:1001–1003. doi: 10.1212/wnl.37.6.1001. [DOI] [PubMed] [Google Scholar]
- Bondareff W, Mountjoy CQ, Roth M, et al. Neuronal degeneration in locus ceruleus and cortical correlates of Alzheimer disease. Alzheimer Dis Assoc Disord. 1987;1:256–262. doi: 10.1097/00002093-198701040-00005. [DOI] [PubMed] [Google Scholar]
- Tomlinson BE, Irving D, Blessed G. Cell loss in the locus coeruleus in senile dementia of Alzheimer type. J Neurol Sci. 1981;49:419–428. doi: 10.1016/0022-510x(81)90031-9. [DOI] [PubMed] [Google Scholar]
- Gibbs ME, Summers RJ. Role of adrenoceptor subtypes in memory consolidation. Prog Neurobiol. 2002;67:345–391. doi: 10.1016/s0301-0082(02)00023-0. [DOI] [PubMed] [Google Scholar]
- Madison DV, Nicoll RA. Norepinephrine decreases synaptic inhibition in the rat hippocampus. Brain Res. 1988;442:131–138. doi: 10.1016/0006-8993(88)91440-0. [DOI] [PubMed] [Google Scholar]
- Murchison CF, Zhang XY, Zhang WP, et al. A distinct role for norepinephrine in memory retrieval. Cell. 2004;117:131–143. doi: 10.1016/s0092-8674(04)00259-4. [DOI] [PubMed] [Google Scholar]
- Kleschevnikov AM, Belichenko PV, Salehi A, Wu C. Discoveries in Down syndrome: moving basic science to clinical care. Prog Brain Res. 2012;197:199–221. doi: 10.1016/B978-0-444-54299-1.00010-8. [DOI] [PubMed] [Google Scholar]
- Faizi M, Bader PL, Tun C, et al. Comprehensive behavioral phenotyping of Ts65Dn mouse model of Down syndrome: activation of beta1-adrenergic receptor by xamoterol as a potential cognitive enhancer. Neurobiol Dis. 2011;43:397–413. doi: 10.1016/j.nbd.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehi A, Faizi M, Colas D, et al. Restoration of norepinephrine-modulated contextual memory in a mouse model of Down syndrome. Sci Transl Med. 2009;1:7ra17. doi: 10.1126/scitranslmed.3000258. [DOI] [PubMed] [Google Scholar]
- Bullido MJ, Ramos MC, Ruiz-Gomez A, et al. Polymorphism in genes involved in adrenergic signaling associated with Alzheimer's. Neurobiol Aging. 2004;25:853–859. doi: 10.1016/j.neurobiolaging.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Marino MD, Bourdelat-Parks BN, Cameron Liles L, Weinshenker D. Genetic reduction of noradrenergic function alters social memory and reduces aggression in mice. Behav Brain Res. 2005;161:197–203. doi: 10.1016/j.bbr.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Heneka MT, Ramanathan M, Jacobs AH, et al. Locus ceruleus degeneration promotes Alzheimer pathogenesis in amyloid precursor protein 23 transgenic mice. J Neurosci. 2006;26:1343–1354. doi: 10.1523/JNEUROSCI.4236-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockenstein E, Mallory M, Mante M, et al. Early formation of mature amyloid-beta protein deposits in a mutant APP transgenic model depends on levels of Abeta(1-42) J Neurosci Res. 2001;66:573–582. doi: 10.1002/jnr.1247. [DOI] [PubMed] [Google Scholar]
- Faizi M, Bader PL, Saw N, et al. Thy1-hAPP(Lond/Swe+) mouse model of Alzheimer's disease displays broad behavioral deficits in sensorimotor, cognitive and social function. Brain Behav. 2012;2:142–154. doi: 10.1002/brb3.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadler JJ, Moy SS, Dold G, et al. Automated apparatus for quantitation of social approach behaviors in mice. Genes Brain Behav. 2004;3:303–314. doi: 10.1111/j.1601-183X.2004.00071.x. [DOI] [PubMed] [Google Scholar]
- Macbeth AH, Edds JS, Young WS., III Housing conditions and stimulus females: a robust social discrimination task for studying male rodent social recognition. Nat Protoc. 2009;4:1574–1581. doi: 10.1038/nprot.2009.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. 2nd ed. Amsterdam, Boston: Elsevier Academic Press; 2004. Compact. [Google Scholar]
- West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ, Jensen EB, Kieu K, Nielsen J. The efficiency of systematic sampling in stereology–reconsidered. J Microsc. 1999;193:199–211. doi: 10.1046/j.1365-2818.1999.00457.x. [DOI] [PubMed] [Google Scholar]
- Borelli KG, Blanchard DC, Javier LK, et al. Neural correlates of scent marking behavior in C57BL/6J mice: detection and recognition of a social stimulus. Neuroscience. 2009;162:914–923. doi: 10.1016/j.neuroscience.2009.05.047. [DOI] [PubMed] [Google Scholar]
- Ferguson JN, Aldag JM, Insel TR, Young LJ. Oxytocin in the medial amygdala is essential for social recognition in the mouse. J Neurosci. 2001;21:8278–8285. doi: 10.1523/JNEUROSCI.21-20-08278.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu CT, Plowey ED, Wang Y, et al. Location, location, location: altered transcription factor trafficking in neurodegeneration. J Neuropathol Exp Neurol. 2007;66:873–883. doi: 10.1097/nen.0b013e318156a3d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung SH. Aberrant phosphorylation in the pathogenesis of Alzheimer's disease. BMB Rep. 2009;42:467–474. doi: 10.5483/bmbrep.2009.42.8.467. [DOI] [PubMed] [Google Scholar]
- Ke YD, Dramiga J, Schutz U, et al. Tau-mediated nuclear depletion and cytoplasmic accumulation of SFPQ in Alzheimer's and Pick's disease. PLoS One. 2012;7:e35678. doi: 10.1371/journal.pone.0035678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan P, Kaba H, Keverne EB. Olfactory recognition: a simple memory system. Science. 1990;250:1223–1226. doi: 10.1126/science.2147078. [DOI] [PubMed] [Google Scholar]
- Dluzen DE, Muraoka S, Landgraf R. Olfactory bulb norepinephrine depletion abolishes vasopressin and oxytocin preservation of social recognition responses in rats. Neurosci Lett. 1998;254:161–164. doi: 10.1016/s0304-3940(98)00691-0. [DOI] [PubMed] [Google Scholar]
- Sara SJ, Vankov A, Herve A. Locus coeruleus-evoked responses in behaving rats: a clue to the role of noradrenaline in memory. Brain Res Bull. 1994;35:457–465. doi: 10.1016/0361-9230(94)90159-7. [DOI] [PubMed] [Google Scholar]
- Rasia-Filho AA, Londero RG, Achaval M. Functional activities of the amygdala: an overview. J Psychiatry Neurosci. 2000;25:14–23. [PMC free article] [PubMed] [Google Scholar]
- Kromer Vogt LJ, Hyman BT, Van Hoesen GW, Damasio AR. Pathological alterations in the amygdala in Alzheimer's disease. Neuroscience. 1990;37:377–385. doi: 10.1016/0306-4522(90)90408-v. [DOI] [PubMed] [Google Scholar]
- Unger JW, Lapham LW, McNeill TH, et al. The amygdala in Alzheimer's disease: neuropathology and Alz 50 immunoreactivity. Neurobiol Aging. 1991;12:389–399. doi: 10.1016/0197-4580(91)90063-p. [DOI] [PubMed] [Google Scholar]
- Morrone I, Besche-Richard C, Mahmoudi R, Novella JL. Identification and memorisation of emotions in Alzheimer's disease: a critical review of litterature. Geriatr Psychol Neuropsychiatr Vieil. 2012;10:307–314. doi: 10.1684/pnv.2012.0357. [DOI] [PubMed] [Google Scholar]
- Grady CL, Furey ML, Pietrini P, et al. Altered brain functional connectivity and impaired short-term memory in Alzheimer's disease. Brain. 2001;124:739–756. doi: 10.1093/brain/124.4.739. [DOI] [PubMed] [Google Scholar]
- De Luca A, Giuditta A. Role of a transcription factor (CREB) in memory processes. Riv Biol. 1997;90:371–384. [PubMed] [Google Scholar]
- Izquierdo LA, Barros DM, Vianna MR, et al. Molecular pharmacological dissection of short- and long-term memory. Cell Mol Neurobiol. 2002;22:269–287. doi: 10.1023/A:1020715800956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Fukushima H, Mukawa T, et al. Upregulation of CREB-mediated transcription enhances both short- and long-term memory. J Neurosci. 2011;31:8786–8802. doi: 10.1523/JNEUROSCI.3257-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvanitis DN, Ducatenzeiler A, Ou JN, et al. High intracellular concentrations of amyloid-beta block nuclear translocation of phosphorylated CREB. J Neurochem. 2007;103:216–228. doi: 10.1111/j.1471-4159.2007.04704.x. [DOI] [PubMed] [Google Scholar]
- Windisch M, Flunkert S, Havas D, Hutter-Paier B. Commentary to the recently published review “Drug pipeline in neurodegeneration based on transgenic mice models of Alzheimer's disease” by Li, Evrahimi and Schluesener Ageing Res. Rev. 2013;12(1):116-40. Ageing Res Rev. 2013;12:852–854. doi: 10.1016/j.arr.2013.06.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1.Supplementary methods.
Figure S1. Blocking the β1-ADR with betaxolol (1 mg/kg) impaired social recognition independently of nonsocial odor recognition and object recognition. (A) In the three-chamber social recognition test, systemic injection of betaxolol prior the learning phase of the test did not alter the preference of mice for an unfamiliar intruder in a cup over an empty cup (vehicle-injected mice n = 10, betaxolol-injected mice n = 10) (two-way ANOVA interaction: P = 0.2801, F(1,36) = 1.203; object P < 0.0001, F(1,36) = 33.05; treatment P = 0.6501, F(1,36) = 0.2093. Post hoc paired one-tailed t-test corrected for multiple comparisons: vehicle P = 0.0004; betaxolol P = 0.0014. However, betaxolol impairs social recognition: betaxolol-injected mice did not show a preference for a new intruder over a familiar one while vehicle-injected mice show this preference (two-way ANOVA interaction: P = 0.0906, F(1,36) = 3.024; object P = 0.0293, F(1,36) = 5.155; treatment P = 0.6749, F(1,36) = 0.1789. Post hoc paired one-tailed t-test correlated for multiple comparisons: vehicle P = 0.0082, betaxolol P = 0.7128). (B) Betaxolol did not impair nonsocial odor recognition; in a test of nonsocial odor habituation, dishabituation, and recognition, both vehicle-injected (n = 10) and betaxolol-injected (n = 10) mice habituated to nonsocial odors (water and vanilla) over the course of three successive exposures and dishabituated when presented to a new odor (two-way RM ANOVA: interaction: P = 0.9623, F(6,108) = 0.2401; treatment P = 0.5234, F(1,108) = 0.4236; object P < 0.0001, F(6,108) = 21.42; Post hoc paired one-tailed t-test correlated for multiple comparisons: Water habituation: vehicle ***P < 0.001; betaxolol τττP < 0.001; Water dishabituation: vehicle **P = 0.0024; betaxolol τττP < 0.001; Vanilla habituation: vehicle ***P < 0.001; betaxolol ττP = 0.006). All mice were also able to discriminate between a previously presented odor (vanilla) and a new odor (mint) as indicated by higher sniffing time when presented with the mint odor versus sniffing time when presented with the vanilla odor for a third time (vehicle **P = 0.0048; betaxolol ττP = 0.0036) (C) The ability of mice to recognize a familiar object over a new one was not affected by betaxolol. Both vehicle-injected (n = 10) and betaxolol-injected (n = 10) mice preferred a new object over a familiar one in an object recognition test (two-way ANOVA interaction: P = 0.7487, F(1,36) = 0.1042; object P < 0.0001 F(1,36) = 28.12; treatment P = 0.2604, F(1,36) = 0.2604; Post hoc paired one-tailed t-test correlated for multiple comparisons: vehicle P < 0.001; betaxolol P < 0.001). Data are presented as mean ± SEM.
Figure S2. (A) c-Fos expression is induced in the MeA 90 min after social recognition. Quantification of c-Fos positive cells was performed using a nonbiased stereological methods in control mice (n = 4) and mice exposed to a social recognition task (n = 4) (P = 0.0303 by t-test). (B) c-Fos expression was not induced in the basolateral amygdala (BLA) after social recognition. Quantification of c-Fos positive cells was performed using a nonbiased stereological methods in control mice (n = 4) and mice exposed to a social recognition task (n = 4) (P = 0.4857 by Mann–Whitney test). (C) Injection of betaxolol prior to testing in a social recognition task did not affect the number of c-Fos positive cells in the thalamus (PV), brain region not involved in social memory and poor in β1-ADR (vehicle-injected mice n = 4; betaxolol-injected mice n = 4; P = 0.8571 by Mann–Whitney test). Data are presented as mean ± SEM. Scale bars, 100 µm (magnified box) and 200 µm.
Figure S3. Western blot analysis of pCREB performed on whole tissue homogenates from medial amygdala of control mice and APP mice (n = 4 per group). pCREB level was significantly higher in APP mice (P = 0.0286 by Mann–Whitney test). The relative optical density is normalized to CREB. Data are presented as mean ± SEM.