INTRODUCTION
Gastroesophageal reflux disease (GERD) is a complex problem in the pediatric population and has received significant attention in the literature. Extraesophageal reflux disease, commonly called laryngopharyngeal reflux disease (LPRD), continues to be an entity with more questions than answers. Although the role of LPRD has been implicated in various pediatric diseases, it has been inadequately studied in others. LPRD is believed to contribute to failure to thrive, laryngomalacia, recurrent respiratory papillomatosis (RRP), chronic cough, hoarseness, esophagitis, and aspiration among other pathologies.
Although the exact prevalence is unknown, it is estimated that nearly 1 in 5 children likely suffers from reflux disease.1 Currently, given that childhood obesity is on the increase, and with the association between obesity and reflux, it is likely that the incidence is increasing. As our understanding of various diseases of the aerodigestive tract increases, the role of reflux as a contributing factor continues to gain attention. Although the definitions have not changed dramatically, knowledge regarding the benefits of treating LPRD as a contributing factor in many afflictions of the upper aerodigestive tract has certainly increased.
LPRD is defined by the reflux of either gastric acid or refluxate (containing pepsin) into the larynx, oropharynx, and/or nasopharynx.2 Although once believed to be an extension of gastroesophageal reflux disease, the differences in symptoms, findings, and treatments has led to the evolution of LPRD as a unique and distinct disease process.3 It is a disease classically diagnosed by symptomatology in the patient. Although confirmation of the disease requires objective findings on various tests, including endoscopy, pH probes, and radiographic studies, a high index of suspicion must be maintained to diagnose the child.
Although LPRD is present in both infants and younger children, it usually presents with a different set of symptoms depending on age (Box 1). Infants typically present with regurgitation, vomiting, dysphagia, anorexia, failure to thrive, apnea, recurrent croup, laryngomalacia, subglottic stenosis, or chronic respiratory issues. School-age children tend to demonstrate chronic cough, dyspnea, dysphonia, persistent sore throat, halitosis, and globus sensation. Older children may also complain of regurgitation, heartburn, vomiting, nausea, or have chronic respiratory issues. The symptoms in these children tend to bridge the gap between those seen in infants and those in teenagers/adults.2 Certain complaints, including dysphagia, vomiting, regurgitation, dyspnea, and globus sensation, are more broad. The role and manifestations of LPRD in specific disease processes requires further attention (Box 2).
Box 1. Various extraesophageal manifestations of GERD.
Infants
Failure to thrive
Wheezing
Stridor
Persistent cough
Apnea
Feeding difficulties
Aspiration
Regurgitation
Recurrent croup
Children
Cough
Hoarseness
Stridor
Sore throat
Asthma
Vomiting
Globus sensation
Wheezing
Aspiration
Recurrent pneumonia
Box 2. Diseases affected by reflux.
Subglottic stenosis
Laryngomalacia
Asthma
Recurrent otitis media
Vocal cord nodules
Vocal cord granuloma
Eosinophilic esophagitis
Allergic rhinitis
Recurrent respiratory papillomatosis
IMPAIRED SWALLOWING AND ASPIRATION
In infants, swallowing is a highly coordinated function requiring the infant to perform and coordinate the actions of suck-swallow-breathe, in that order, to avoid aspiration.4 Performing this sequence requires an intact laryngeal sensation, which explains why children with neurologic deficits tend to have greater feeding difficulties and increased episodes of microaspirations.
Understanding the effects of reflux on the larynx is the key to better treatment of the neonate and infant. The supraglottic mucosa must be able to sense the upcoming food bolus. This sensation leads to appropriate vocal fold closure, while also stimulating the opening of the hypopharynx and upper esophageal sphincter. This highly sensitive mechanism is important at all ages, but more so in newborns as they begin to learn cognitive functioning. Edema from chronic irritation by gastric aspirate causes decreased sensation in these tissues, and thereby increases the risk of aspiration in these patients.5–7
Testing of the laryngeal adductor reflex can be checked by endoscopy combined with a pulse of air to the aryepiglottic folds to simulate a food bolus. This testing begins with a pressure of 2.5 mm Hg and gradually increases in increments of 0.5 mm Hg to 10 mm Hg. The goal is to identify at what pressure the reflex is triggered, with a positive response being a cough or break in respiration. The need for greater than 4.5 mm Hg of pressure to obtain a positive response is suggestive of microaspiration and poor laryngeal adductor reflex in children.
Suskind and colleagues4 showed significant improvement in videofluoroscopic swallow evaluations and pharyngeal impairment scores when infants with swallowing issues were treated for GERD. Aviv and colleagues5 demonstrated that just 3 months of GERD treatment was sufficient time to demonstrate normalization of laryngopharyngeal sensation. This improvement in turn led to improved swallowing function and decreased posterior laryngeal edema. In addition to antireflux medication, thickening of feeds has always been a traditional method of preventing microaspiration. Thickening helps by improving overall laryngopharyngeal sensing of the bolus and thus improves the coordination of swallowing.8,9
Although swallowing is a highly coordinated activity, laryngopharyngeal reflux likely plays a role in dysfunction of the swallow reflex and therefore requires treatment. Currently, there are no universally accepted methods for evaluating the role of LPRD in these patients, although fiber-optic endoscopy can provide visual clues of changes in the larynx. Empirical trials of a proton pump inhibitor and thickening of feeds can delineate the role of LPRD and therefore benefit the child in most cases.
LARYNGOMALACIA
In the pediatric population, laryngomalacia is one of the most common causes of airway distress.10 It typically presents as inspiratory stridor, coughing, choking, or regurgitation. The lack of inherent strength in the larynx leads to collapse of tissues and subsequent upper airway obstruction. A recent meta-analysis11 showed that 65% of patients with severe laryngomalacia had reflux. Further analysis revealed that those children with moderate to severe laryngomalacia were nearly 10 times more likely to suffer from reflux than those with only mild laryngomalacia. The proposed mechanism is that aerophagia during feedings causes gastric distention leading to vagal reflexes followed by postprandial vomiting and regurgitation.
The increased association between laryngomalacia and reflux has led to the question of whether reflux causes laryngomalacia or is simply present concurrently. Although supraglottic biopsies did show mild intraepithelial infiltrate, pathognomonic for reflux, the gross morphologic changes did not seem to correspond to laryngomalacia.12,13 These findings only seem to confuse the issue regarding the role of reflux in laryngomalacia. On the other hand, 2 studies using 24-hour dual-probe pH manometry showed 100% correlation between laryngomalacia and reflux, where reflux is defined as at least 1 episode of pH less than 4 for at least 4 seconds.14,15 Unfortunately, there is still some uncertainty regarding the role of pharyngeal pH monitoring in these patients. Little and colleagues16 demonstrated that nearly half of patients with extraesophageal reflux were only accurately diagnosed after pharyngeal monitoring and a negative esophageal study. Rabinowitz and colleagues17 stated that
Beyond its value in clinical practice, upper esophageal reflux testing should be employed in research studies that evaluate the impact of GER [gastroesophageal reflux] therapy on ENT [ear, nose, and throat] symptoms.
Although several retrospective studies have reported an improvement in laryngomalacia symptoms (cough, stridor, choking) with antireflux treatment, a prospective trial done by Thompson15 indicates a strong correlation between reflux treatment and decreased laryngomalacia symptoms. Three levels of laryngomalacia severity were categorized, ranging from mild (inconsequential stridor during feeding) to moderate (inspiratory stridor, no failure to thrive, and inconsequential dyspnea, cyanosis, or brief apneas) to severe (inspiratory stridor and life-threatening complications). Nearly 89% of patients in the moderate and severe groups showed improvement in coughing and choking after 7.3 months of GERD therapy. Improvements in regurgitation were reported in nearly 70% of patients.
These findings seem to encourage the treatment of laryngomalacia with LPRD. However, resolution of symptoms may simply be attributed to the natural course of the disease. Thompson18 commented on the natural history of laryngomalacia in infants, noting, “Symptoms worsen at 4–8 months, improve between 8 and 12 months, and usually resolve by 12–18 months of age.” The mean age at diagnosis for patients in the 2007 study was older than 3 months (102.8 days). Therefore, it seems possible that many of the symptom improvements reported in this study reflect the natural history of the disease. No studies have compared the outcome of patients with laryngomalacia treated for LPRD with those who receive no treatment. In summary, although further studies are needed, treatment of laryngomalacia with antireflux therapy may be beneficial. Each patient should be evaluated independently by an otolaryngologist to determine disease severity and decide on therapy.
SUBGLOTTIC STENOSIS
Subglottic stenosis typically presents in the neonate/infant with recurrent crouplike episodes and chronic cough. Often, these patients may not demonstrate any difficulty breathing at rest; however, episodes of dyspnea and stridor can quickly develop, caused by the limited airway circumference in young children, which cannot handle even minimal inflammatory insults. Three major causes of subglottic stenosis are trauma, infection, and LPRD.19
The role of LPRD in causing subglottic stenosis has been studied in canine models, giving it significant credence; however, the effect of acid and pepsin on the human subglottic mucosa has not been as thoroughly studied. The formation of vocal cord granulomas has long been known as a sequela of reflux, and these same histologic changes were noted in early subglottic stenosis lesions.20 Although irritation and mucosal damage begin the stenosis process, the role of reflux in preventing reepithelialization should not be understated.21 Further studies have demonstrated the effect of reflux at the cellular level, including downregulation of epidermal growth factor receptor, which reduces mucosal turnover, increased transforming growth factor β1, which causes fibroblast differentiation, and excessive connective tissue deposition.22,23
Although the correlation between reflux and subglottic mucosal changes has been evaluated, no prospective data are available to better correlate the clinical relationship. Reports of children whose stridor and degree of stenosis decreases with reflux management advocate for LPRD treatment in this patient population.24 Reviews show that nearly two-thirds of children with subglottic stenosis have reflux disease to some extent.24–31 Furthermore, subglottic stenosis correlates with reflux disease with a relative risk of 2.5.26–28,32 In 1 study, nearly one-third of patients with subglottic stenosis who were treated for reflux were able to avoid surgical intervention.24 With these findings and correlations in mind, early evaluation for and treatment of LPRD may lead to prevention of disease progression and should be encouraged in patients with subglottic stenosis.
RRP
RRP is a complex, often prolonged, infection of the upper airway by the human papilloma virus. The complexity of this disease is beyond the scope of this article; however, LPRD treatment provides benefit to these patients. There has been increased anecdotal evidence in the literature of cases where children with mild to moderate LPRD have shown improvement or even resolution of disease with antireflux therapy.33,34 The ciliated respiratory epithelium of the larynx has increased sensitivity when chronically exposed to pepsin and gastric refluxate. This increased sensitivity may contribute to more advanced presentations of the disease or a more frequent need for surgical debridement. Some of the concerning findings in disease progression of RRP, such as laryngeal webs, may be diminished or even prevented if these children receive antireflux therapy.35 Although treatment of RRP with LPRD therapy alone is not recommended, ensuring that these patients are placed on adjuvant anti-reflux therapy may help to minimize the consequences or progression of their disease and in some cases may even lead to resolution.
ASTHMA
Because of concerns about the airway often noted in children with LPRD, asthma and the role of treatment of LPRD to improve asthma has been postulated. It has been estimated that gastroesophageal reflux may be present in 40% to 80% of children with asthma.36 There is a growing belief that rhinitis and asthma are often present together, and that rhinitis can cause laryngeal changes that may mimic LPRD changes. Therefore, when evaluating asthmatics for LPRD with laryngoscopy, strict criteria should be used, such as limiting positive findings to vocal cord nodules and granulomas.37 Among asthmatic adults and children, LPRD changes in the larynx have been identified on laryngoscopy in nearly 70% of cases.37,38 Thus, any patient with suspected LPRD in addition to asthma and/or allergic rhinitis should undergo pH probe testing to confirm the diagnosis.
The use of β-agonists has also been studied as a possible trigger for reflux by reducing the tone of the lower esophageal sphincter.39 However, a recent study seems to prove that no such correlation exists.37 Another belief was that uncontrolled asthma may worsen LPRD, but it seems that children with asthma, whether controlled or uncontrolled, have similar rates of LPRD.16 From the literature to date, the main concept to note is that these 2 conditions, LPRD and asthma, are often present together, and they both require treatment. The effect of treatment of one on the status of the other unfortunately requires further research.
HOARSENESS
Although laryngopharyngeal reflux is often described as a significant contributor to adult hoarseness, its role in children has not been as well established. Vocal cord nodules are widely considered to be the most common cause of pediatric hoarseness.40 Various studies have begun to question whether LPRD should be given greater consideration as a cause of this problem. Gumpert and colleagues41 counted interarytenoid edema or chronic inflammation as a sign of LPRD and found these findings in 90% of children with hoarseness. Although this was a retrospective study, it does consider the prevalence of at least some LPRD changes in children with hoarseness.
A possible explanation as to why LPRD can often be underdiagnosed is that findings on endoscopy may mimic vocal cord nodules. There is a subtle difference between nodules seen at the junction between the anterior one-third and posterior two-thirds of the vocal folds and the pseudonodules of LPRD noted as edema in the anterior one-third of the vocal folds.42 Although direct laryngoscopy under general anesthesia may easily highlight these differences, it is significantly more challenging to note these subtle changes on flexible fiber-optic laryngoscopy in an awake child. This difficulty may be a key reason why the prevalence of LPRD in children may be underappreciated.
Early recognition of the role of LPRD and appropriate treatment are key to the most effective management of the hoarse patient. In adults, the finding of vocal cord nodules43 is managed with a combination of speech therapy and antireflux medication, typically a proton pump inhibitor. Block and Brodsky42 found similar such benefits in children. Children with only LPRD findings showed some improvement with pharmacotherapy and speech therapy compared with pharmacotherapy alone. Perhaps more remarkable was the improvement seen in children with nodules who were treated with a combination of pharmacotherapy and speech therapy compared with those treated with only 1 modality.
These findings, in addition to the treatment of LPRD and nodules in the adult population, seem to imply that appropriate treatment of a child with hoarseness would be to combine pharmacotherapy with speech therapy.44 Unfortunately, there has been limited objective evidence in children with hoarseness, specifically with respect to pH probe findings and pepsin immunoassays. Although further studies are necessary, it seems prudent that the treatment of children with hoarseness attributed to LPRD and/or nodules should include pharmacotherapy as well as speech therapy.
COUGH
Cough is a typical complaint in children seen by a pediatrician; it is seen in the clinic in nearly 35% of preschool children.45 The cough mechanism itself is a complex process generated in the cerebral cortex. It can be generated spontaneously or occur involuntarily as a protective mechanism. Rapidly adapting receptors as well as nocireceptors are the primary sensory receptors involved in chronic cough.46 Rapidly adapting receptors are typically involved in response to physical stimuli such as smoke, ingested solutions, and pulmonary congestion.47 On the other hand, nocireceptors respond to chemical stimuli (histamines, bradykinins, prostaglandins, and substance P).48
Although nocireceptors tend to maintain their sensitivity, rapidly adapting receptors respond and transform based on frequency of involvement. Children with chronic allergies, postnasal drainage, asthma, or GERD may demonstrate changes in the severity and frequency of their cough over time. Thus, some of these diseases may silently worsen despite no overt symptoms. Recognizing the role of reflux as a cause of a cough is imperative and should be high in the differential diagnosis once infection has been ruled out. Specifically, in the setting of a chronic cough (cough for more than 4 weeks), LPRD, allergy, and asthma should be strongly suspected.49
As described in other sections, irritation of the larynx by reflux is best categorized by the frequency and severity of reflux events. Although it may seem that these acute events should trigger a spell of coughing, this relationship is not so direct. A recent study50 evaluating 20 children with reflux evaluated their coughing spells while being monitored with a pH probe. Findings showed that most coughs, nearly 90%, did not correspond with a reflux event. The chronic irritation of laryngeal tissues from reflux is the predominant culprit, rather than acute events, thereby stressing the importance of long-term treatment to reverse the process.
In the evaluation of chronic cough, it is always possible for 2 pathologies to be present simultaneously. In 1 study,25 children with chronic cough underwent esophageal biopsies for evaluation of GERD. Of those children with positive biopsies, 75% also had a history of asthma. In a unique study51 involving multiple disciplines, several specialists evaluated 40 children with cough for longer than 8 weeks. They found that reflux and asthma were present in nearly half the patients and another quarter of the patients had multiple causes.
Thus, the evaluation of chronic cough should be extensive with the idea that multiple factors may be playing a role. LPRD can often be considered as a possible primary cause. Furthermore, the possibility of a second contributory cause such as LPRD, allergies, or asthma should always be evaluated in patients who are unresponsive to therapy (Fig. 1).
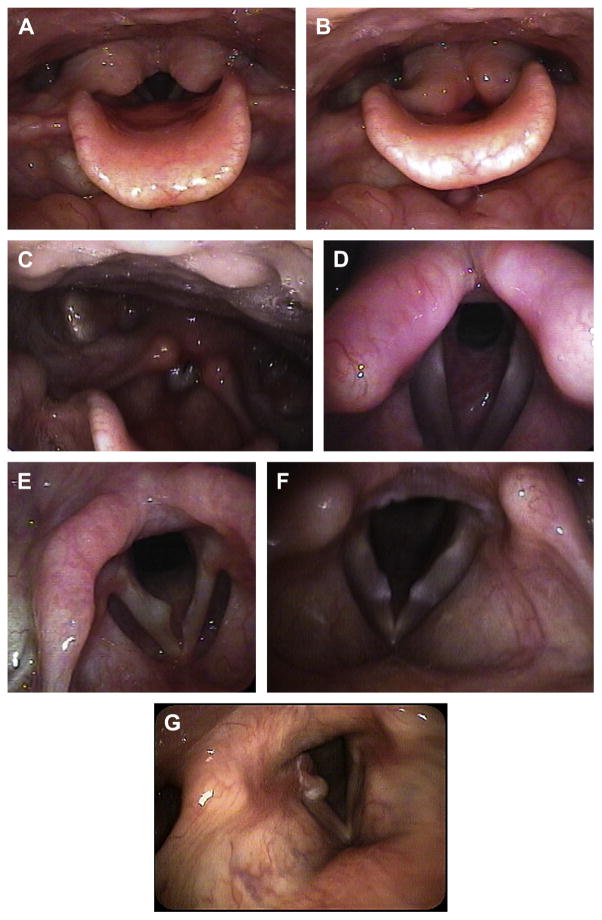
Fig. 1.
(A) Laryngomalacia with vocal cords open. (B) Laryngomalacia with collapse and obstruction of airway in same patient. (C) Posterior pharyngeal wall cobblestoning. (D) Subglottic stenosis. (E) Right true vocal fold cyst. (F) Bilateral true vocal fold nodules. (G) Early formation of right true vocal fold granuloma (Noted posteriorly).
DIAGNOSIS
Because of its subtlety, LPRD may be difficult to recognize in patients with the chronic effects of this disease. To the otolaryngologist, many physical examination findings may suggest LPRD; however, most of these findings can only be seen when viewing the larynx (Box 3).52 Without the ability to view the larynx, the index of suspicion must be even higher for the pediatrician. A child with any of the conditions discussed as well as unresponsive or unexplained difficulty with feeding or the airway should suggest the possibility of reflux as a contributing factor and prompt a referral to a specialist.
Box 3. Reflux findings seen on laryngoscopy.
Lingual tonsil swelling
Postglottic edema
Postglottic erythema
Arytenoid edema
Arytenoid erythema
Laryngeal ventricle obliteration
True vocal fold edema
Laryngomalacia
Tracheomalacia
Vocal fold nodules
Subglottic stenosis
Hypopharyngeal cobblestoning
Narrowed trachea
Increased secretions
Diagnosis of reflux begins with a thorough history paying special attention to feeding and airway symptoms. With regard to feeding, it is crucial to know if a child has regurgitation or emesis and the timing of these events (ie, how long after meals?). Deciding if the child has adequate weight gain that correlates with oral intake is also important. Previous airway issues should be identified as well, because diseases such as subglottic stenosis, laryngomalacia, and RRP may have already been diagnosed. Knowledge of choking incidents, chronic cough, and recurrent crouplike episodes may suggest an underlying anatomic airway issue or even microaspiration. If there is a sufficient index of suspicion, the practitioner should enlist the help of specialists and obtain objective data for the evaluation of LPRD.
Reflux disease has been evaluated and diagnosed using a variety of objective tests, including histopathology from esophageal biopsies, barium esophograms, and double and/or single pH probes. The 24-hour pH probe study is considered the current gold standard for the diagnosis of LPRD and can be helpful in evaluating the severity of disease.
TREATMENT
Although diagnostic measures can be used to determine if a child has LPRD, the decision to proceed with work-up and treatment is key. Empirical therapy with either proton pump inhibitors or histamine (H2) blockers is often the preferred initial approach in children with presumed GERD. The primary care practitioner must decide when to initiate diagnostic work-up of LPRD/GERD.
With regard to extraesophageal symptoms and laryngopharyngeal involvement of reflux, there are certain diseases that can be readily diagnosed by simple endoscopy, such as subglottic stenosis, laryngomalacia, laryngeal edema, RRP, vocal cord granulomas, or vocal cord nodules. The diagnosis of 1 of these conditions should prompt a course of medical therapy and a period of observation for symptomatic improvement. In the case of more complex issues (swallowing difficulty, aspiration, cough) additional diagnostic testing before medical therapy is often warranted. Treatment options for LPRD include lifestyle modifications, medical therapy, and/or surgical therapy.1,53
Lifestyle modification for reflux typically centers on 3 actions: altering food composition, adjusting the diet to eliminate known triggers of reflux, and adjusting postural positioning. As children grow older, they can begin to follow similar guidelines as for adults. As stated earlier, thickening of feeds improves laryngeal sensation and overall swallowing function. Frequent but smaller meals can also help to reduce reflux. Sleep positioning may provide further benefit to infants by encouraging sleep in the lateral position as well as elevating the head of the bed if possible.3 Avoidance of known triggers of LPRD is often a key dietary modification that improves symptoms in older children. Much like GERD, avoidance of certain foods (ie, juices and spicy foods, chocolates, and mints) and not eating meals just before sleep can help to decrease reflux.2 These changes alone (elevating the head of the bed, milk thickening, and fasting before bedtime) may even lead to complete resolution of LPRD.54,55
If lifestyle changes are insufficient in resolving symptoms of LPRD, medical therapy is the next avenue to explore. As with adults, the recommended first-line therapy is proton pump inhibitors (PPIs). They bind irreversibly to active proton pumps and provide greatest efficacy when taken just before a meal.55 In adults, a 30-minute gap between consumption of medication and commencing a meal is typically recommended. Among the various PPIs, esomeprazole has been shown to provide the greatest benefit.56 In children, similar studies have not been performed; however, in neonates and infants, lansoprazole and omeprazole are the only PPIs approved by the US Food and Drug Administration.
Histamine-2 receptor antagonists (H2RAs), once a mainstay in treatment, have now become second-line therapy. They have typically been used to either help wean patients off PPIs or to supplement PPI therapy. Unlike PPIs, they are typically taken at night to provide nocturnal suppression of acid as they reduce meal-stimulated gastric acid production by nearly 70%.57 In children, the use of a H2RA is most helpful for weaning off PPIs. Ranitidine is available as a syrup, which may help to increase patient compliance. Although the use of PPIs and H2RAs have been studied in adults, there is no consensus on their role in the treatment of reflux.58 Although further studies are needed, the use of a PPI or H2RA in short courses of therapy can often yield beneficial results. The failure to respond to either a PPI or H2RA in cases of suspected reflux indicates a lack of significant reflux, insufficient dosage, or the need to consider surgical intervention.
Surgical therapy for reflux should be reserved for those children whose symptoms are life threatening or drastically affecting their quality of life despite maximum medical therapy. Although surgery may seem appealing by promising the possibility of eliminating the need for medication, it carries with it the risk of high failure rates, significant morbidity, and even death.59 As some of these situations (coughing, choking, aspiration, or recurrent pneumonias) compromise a patient’s respiratory status, the risks of surgery may greatly outweigh the projected benefits. If all factors have been considered, fundoplication can be entertained as a possibility. Nissen fundoplication is the gold standard procedure performed for the surgical treatment of GERD. This procedure attempts to restore the integrity of the lower esophageal sphincter. When performed on appropriate patients, it has a 90% symptom control rate.60,61 Furthermore, when performed on patients without respiratory issues, the success rate increases even further.62
SUMMARY
Extraesophageal symptoms of GERD have long been recognized and referred to as LPRD. Despite its similarities with GERD, LPRD has been more difficult to diagnose accurately and consistently. This variability has made creating comprehensive treatment guidelines difficult. Much of the current information regarding the role of LPRD has been learned by noting its presence in conjunction with another well-understood disease (laryngomalacia, subglottic stenosis, vocal cord nodules, and so forth). Currently, the treatment of LPRD seems to demonstrate symptomatic benefits as well as improvements in these concomitant diseases. Thus, LPRD should be considered as a chronic disease with a variety of presentations. High clinical suspicion along with consultation with an otolaryngologist, who can evaluate for laryngeal findings, is necessary to accurately diagnose LPRD. Future studies will work to illuminate the role of gastric acid and refluxate on the upper aerodigestive tract. However, until excluded, the role of LPRD should never be underestimated and should always be treated in symptomatic patients.
KEY POINTS.
Extraesophageal symptoms of gastroesophageal reflux disease (GERD) have long been recognized and referred to as laryngopharyngeal reflux disease (LPRD).
Despite its similarities with GERD, LPRD has been more difficult to diagnose accurately and consistently.
This variability has made creating comprehensive treatment guidelines difficult.
Currently, the treatment of LPRD seems to provide symptomatic benefits as well as improvements in these concomitant diseases.
LPRD should be considered as a chronic disease with a variety of presentations.
References
- 1.Vandenplas Y, Sacre-Smith L. Continuous 24-hour esophageal pH monitoring in 285 asymptomatic infants 0 to 15 months old. J Pediatr Gastroenterol Nutr. 1987;6:220–4. doi: 10.1097/00005176-198703000-00010. [DOI] [PubMed] [Google Scholar]
- 2.Stavroulaki P. Diagnostic and management problems of laryngopharyngeal reflux disease in children. Int J Pediatr Otorhinolaryngol. 2006;70:579–90. doi: 10.1016/j.ijporl.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 3.McGuirt WF., Jr Gastroesophageal reflux and the upper airway. Pediatr Clin North Am. 2003;50:487–502. doi: 10.1016/s0031-3955(03)00033-6. [DOI] [PubMed] [Google Scholar]
- 4.Suskind DL, Thompson DM, Gulati M, et al. Improved infant swallowing after gastroesophageal reflux disease treatment: a function of improved laryngeal sensation? Laryngoscope. 2006;116:1397–403. doi: 10.1097/01.mlg.0000225942.33102.9b. [DOI] [PubMed] [Google Scholar]
- 5.Aviv JE, Liu H, Parides M, et al. Laryngopharyngeal sensory deficits in patients with laryngopharyngeal reflux and dysphagia. Ann Otol Rhinol Laryngol. 2000;109:1000–6. doi: 10.1177/000348940010901103. [DOI] [PubMed] [Google Scholar]
- 6.Link DT, Willging JP, Miller CK, et al. Pediatric laryngopharyngeal sensory testing during flexible endoscopic evaluation of swallowing: feasible and correlative. Ann Otol Rhinol Laryngol. 2000;109:899–905. doi: 10.1177/000348940010901002. [DOI] [PubMed] [Google Scholar]
- 7.Thompson DM. Laryngopharyngeal sensory testing and assessment of airway protection in pediatric patients. Am J Med. 2003;115(Suppl 3A):166S–8S. doi: 10.1016/s0002-9343(03)00217-1. [DOI] [PubMed] [Google Scholar]
- 8.Orenstein SR, Magill HL, Brooks P. Thickening of infant feedings for therapy of gastroesophageal reflux. J Pediatr. 1987;110:181–6. doi: 10.1016/s0022-3476(87)80150-6. [DOI] [PubMed] [Google Scholar]
- 9.Henry SM. Discerning differences: gastroesophageal reflux and gastroesophageal reflux disease in infants. Adv Neonatal Care. 2004;4:235–47. doi: 10.1016/j.adnc.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 10.Holinger LD. Etiology of stridor in the neonate, infant, and child. Ann Otol Rhinol Laryngol. 1980;89:397–400. doi: 10.1177/000348948008900502. [DOI] [PubMed] [Google Scholar]
- 11.Hartl TT, Chadha NK. A systematic review of laryngomalacia and acid reflux. Otolaryngol Head Neck Surg. 2012;147(4):619–26. doi: 10.1177/0194599812452833. [DOI] [PubMed] [Google Scholar]
- 12.Chandra RK, Gerber ME, Holinger LD. Histological insight into the pathogenesis of severe laryngomalacia. Int J Pediatr Otorhinolaryngol. 2001;61:31–8. doi: 10.1016/s0165-5876(01)00541-9. [DOI] [PubMed] [Google Scholar]
- 13.Iyer VK, Pearman K, Raafat F. Laryngeal mucosal histology in laryngomalacia: the evidence for gastrooesophageal reflux laryngitis. Int J Pediatr Otorhinolaryngol. 1999;49:225–30. doi: 10.1016/s0165-5876(99)00205-0. [DOI] [PubMed] [Google Scholar]
- 14.Matthews BL, Little JP, Mcguirt WF, Jr, et al. Reflux in infants with laryngomalacia: results of 24-hour double-probe pH monitoring. Otolaryngol Head Neck Surg. 1999;120:860–4. doi: 10.1016/S0194-5998(99)70327-X. [DOI] [PubMed] [Google Scholar]
- 15.Thompson DM. Abnormal sensorimotor integrative function of the larynx in congenital laryngomalacia: a new theory of etiology. Laryngoscope. 2007;117(6 Pt 2 Suppl 114):1–33. doi: 10.1097/MLG.0b013e31804a5750. [DOI] [PubMed] [Google Scholar]
- 16.Little JP, Matthews BL, Glock MS, et al. Extraesophageal pediatric reflux: 24-hour double-probe pH monitoring of 222 children. Ann Otol Rhinol Laryngol Suppl. 1997;169:7. [PubMed] [Google Scholar]
- 17.Rabinowitz SS, Piecuch S, Jibaly R, et al. Optimizing the diagnosis of gastroesophageal reflux in children with otolaryngologic symptoms. Int J Pediatr Otorhinolaryngol. 2003;67:625. doi: 10.1016/s0165-5876(03)00072-7. [DOI] [PubMed] [Google Scholar]
- 18.Thompson DM. Laryngomalacia: factors that influence disease severity and outcomes of management. Curr Opin Otolaryngol Head Neck Surg. 2010;18:564–70. doi: 10.1097/MOO.0b013e3283405e48. [DOI] [PubMed] [Google Scholar]
- 19.Karkos PD, Leong SC, Apostolidou MT, et al. Laryngeal manifestations and pediatric laryngopharyngeal reflux. Am J Otol. 2006;27(3):200–3. doi: 10.1016/j.amjoto.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 20.Delahunty JE, Cherry J. Experimentally produced vocal cord granulomas. Laryngoscope. 1968;78(11):1941–7. doi: 10.1288/00005537-196811000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Little FB, Koufman JA, Kohut RI, et al. Effect of gastric acid on the pathogenesis of subglottic stenosis. Ann Otol Rhinol Laryngol. 1985;94(5):516–9. doi: 10.1177/000348948509400521. [DOI] [PubMed] [Google Scholar]
- 22.Yellon RF, Parameswarran M, Brandom BW. Decreasing morbidity following laryngotracheal reconstruction in children. Int J Pediatr Otorhinolaryngol. 1997;41(2):145–54. doi: 10.1016/s0165-5876(97)00067-0. [DOI] [PubMed] [Google Scholar]
- 23.Jarmuz T, Roser S, Rivera H, et al. Transforming growth factor-beta 1, myofibroblasts, and tissue remodelling in the pathogenesis of tracheal injury: potential role of gastroesophageal reflux. Ann Otol Rhinol Laryngol. 2004;113(6):488–97. doi: 10.1177/000348940411300614. [DOI] [PubMed] [Google Scholar]
- 24.Halstead LA. Gastroesophageal reflux: a critical factor in pediatric subglottic stenosis. Otolaryngol Head Neck Surg. 1999;120:683–8. doi: 10.1053/hn.1999.v120.a91766. [DOI] [PubMed] [Google Scholar]
- 25.Yellon RF, Coticchia J, Dixit S. Esophageal biopsy for the diagnosis of gastroesophageal reflux-associated otolaryngologic problems in children. Am J Med. 2000;108(Suppl 4a):131S–8S. doi: 10.1016/s0002-9343(99)00352-6. [DOI] [PubMed] [Google Scholar]
- 26.Mitzner R, Brodsky L. Multilevel esophageal biopsy in children with airway manifestations of extraesophageal reflux disease. Ann Otol Rhinol Laryngol. 2007;116:571–5. doi: 10.1177/000348940711600803. [DOI] [PubMed] [Google Scholar]
- 27.Halstead LA. Role of gastroesophageal reflux in pediatric upper airway disorders. Otolaryngol Head Neck Surg. 1999;120:208–14. doi: 10.1016/S0194-5998(99)70408-0. [DOI] [PubMed] [Google Scholar]
- 28.Carr MM, Nagy ML, Pizzuto MP, et al. Correlation of findings at direct laryngoscopy and bronchoscopy with gastroesophageal reflux disease in children: a prospective study. Arch Otolaryngol Head Neck Surg. 2001;127:369–74. doi: 10.1001/archotol.127.4.369. [DOI] [PubMed] [Google Scholar]
- 29.Carr MM, Abu-Shamma U, Brodsky LS. Predictive value of laryngeal pseudosulcus for gastroesophageal reflux in pediatric patients. Int J Pediatr Otorhinolaryngol. 2005;69:1109–12. doi: 10.1016/j.ijporl.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 30.Giannoni C, Sulek M, Friedman EM, et al. Gastroesophageal reflux association with laryngomalacia: a prospective study. Int J Pediatr Otorhinolaryngol. 1998;43:11–20. doi: 10.1016/s0165-5876(97)00151-1. [DOI] [PubMed] [Google Scholar]
- 31.Can MM, Nguyen A, Poje C, et al. Correlation of findings on direct laryngoscopy and bronchoscopy with presence of extraesophageal reflux disease. Laryngoscope. 2000;110:1560–2. doi: 10.1097/00005537-200009000-00030. [DOI] [PubMed] [Google Scholar]
- 32.Stroh BC, Faust RA, Rimell FL. Results of esophageal biopsies performed during triple endoscopy in the pediatric patient. Arch Otolaryngol Head Neck Surg. 1998;124:545–9. doi: 10.1001/archotol.124.5.545. [DOI] [PubMed] [Google Scholar]
- 33.McKenna M, Brodsky L. Extraesophageal acid reflux and recurrent respiratory papillomatosis in children. Int J Pediatr Otorhinolaryngol. 2005;69:597–605. doi: 10.1016/j.ijporl.2004.11.021. [DOI] [PubMed] [Google Scholar]
- 34.Borkowski G, Sommer P, Stark T, et al. Recurrent respiratory papillomatosis associated with gastroesophageal reflux disease in children. Eur Arch Otorhinolaryngol. 1999;256(7):370–2. doi: 10.1007/s004050050166. [DOI] [PubMed] [Google Scholar]
- 35.Holland BW, Koufman JA, Postma GN, et al. Laryngopharyngeal reflux and laryngeal web formation in patients with pediatric recurrent respiratory papillomas. Laryngoscope. 2002;112(11):1926–9. doi: 10.1097/00005537-200211000-00003. [DOI] [PubMed] [Google Scholar]
- 36.Thakkar K, Boatright RO, Gilger MA, et al. Gastroesophageal reflux and asthma in children: a systematic review. Pediatrics. 2010;125:925–30. doi: 10.1542/peds.2009-2382. [DOI] [PubMed] [Google Scholar]
- 37.Kilic M, Ozturk F, Kirmemis O, et al. Impact of laryngopharyngeal and gastroesophageal reflux on asthma control in children. Int J Pediatr Otorhinolaryngol. 2013;77(3):341–5. doi: 10.1016/j.ijporl.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 38.Eryuksel E, Dogan M, Golabi P, et al. Treatment of laryngopharyngeal reflux improves asthma symptoms in asthmatics. J Asthma. 2006;43:539–42. doi: 10.1080/02770900600857234. [DOI] [PubMed] [Google Scholar]
- 39.Parsons JP, Mastronarde JG. Gastroesophageal reflux disease and asthma. Curr Opin Pulm Med. 2010;16:60–3. doi: 10.1097/MCP.0b013e328332ca2f. [DOI] [PubMed] [Google Scholar]
- 40.Wohl DL. Nonsurgical management of pediatric vocal fold nodules. Arch Otolaryngol Head Neck Surg. 2005;131(1):68–70. doi: 10.1001/archotol.131.1.68. [DOI] [PubMed] [Google Scholar]
- 41.Gumpert L, Kalach N, Dupont C, et al. Hoarseness and gastroesophageal reflux in children. J Laryngol Otol. 1998;112(1):49–54. doi: 10.1017/s002221510013988x. [DOI] [PubMed] [Google Scholar]
- 42.Block BB, Brodsky L. Hoarseness in children: the role of laryngopharyngeal reflux. Int J Pediatr Otorhinolaryngol. 2007;71:1361–9. doi: 10.1016/j.ijporl.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 43.Kuhn J, Toohill RJ, Ulualp SO, et al. Pharyngeal acid reflux events in patients with vocal cord nodules. Laryngoscope. 1998;108:1146–9. doi: 10.1097/00005537-199808000-00008. [DOI] [PubMed] [Google Scholar]
- 44.Karkos PD, Wilson JA. Empiric treatment of laryngopharyngeal reflux with proton pump inhibitors; a systematic review. Laryngoscope. 2006;116:144–8. doi: 10.1097/01.mlg.0000191463.67692.36. [DOI] [PubMed] [Google Scholar]
- 45.Kogan MD, Pappas G, Yu SM, et al. Over-the-counter medication use among preschool-age children. JAMA. 1994;272:1025–30. [PubMed] [Google Scholar]
- 46.Mazzone SB. Sensory regulation of the cough reflex. Pulm Pharmacol Ther. 2004;17:361–8. doi: 10.1016/j.pupt.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 47.Palmer R, Anon JB, Gallagher P. Pediatric cough: what the otolaryngologist needs to know. Curr Opin Otolaryngol Head Neck Surg. 2011;19:204–9. doi: 10.1097/MOO.0b013e328345aa7c. [DOI] [PubMed] [Google Scholar]
- 48.Millqvist E, Bende M. Role of the upper airways in patients with chronic cough. Curr Opin Allergy Clin Immunol. 2006;6:7–11. doi: 10.1097/01.all.0000199796.64304.ca. [DOI] [PubMed] [Google Scholar]
- 49.Chang AB, Landau LI, van Asperen PP, et al. Cough in children: definitions and clinical evaluation. Med J Aust. 2006;184:398–403. doi: 10.5694/j.1326-5377.2006.tb00290.x. [DOI] [PubMed] [Google Scholar]
- 50.Chang AB, Connor FL, Petsky HL, et al. An objective study of acid reflux and cough in children using an ambulatory pHmetry-cough logger. Arch Dis Child. 2011;96(5):468–72. doi: 10.1136/adc.2009.177733. [DOI] [PubMed] [Google Scholar]
- 51.Khoshoo V, Edell D, Mohnot S, et al. Associated factors in children with chronic cough. Chest. 2009;136:811–5. doi: 10.1378/chest.09-0649. [DOI] [PubMed] [Google Scholar]
- 52.May JG, Shah P, Lemonnier L, et al. Systematic review of endoscopic airway findings in children with gastroesophageal reflux disease. Ann Otol Rhinol Laryngol. 2011;120(2):116–22. doi: 10.1177/000348941112000208. [DOI] [PubMed] [Google Scholar]
- 53.Cezard JP. Managing gastro-oesophageal reflux disease in children. Digestion. 2004;69(Suppl):3–8. doi: 10.1159/000076370. [DOI] [PubMed] [Google Scholar]
- 54.Bach KK, McGuirt WF, Jr, Postma GN. Pediatric laryngopharyngeal reflux. Ear Nose Throat J. 2002;81(9 Suppl 2):27–31. [PubMed] [Google Scholar]
- 55.Meyer TK, Olsen E, Merati A. Contemporary diagnostic and management techniques for extraoesophageal reflux disease. Curr Opin Otolaryngol Head Neck Surg. 2004;12(6):519–24. doi: 10.1097/01.moo.0000144390.95132.9b. [DOI] [PubMed] [Google Scholar]
- 56.Miner P, Jr, Katz PO, Chen Y, et al. Gastric acid control with esomeprazole, lansoprazole, omeprazole, pantoprazole, and rabeprazole: a five-way crossover study. Am J Gastroenterol. 2003;98:2616–20. doi: 10.1111/j.1572-0241.2003.08783.x. [DOI] [PubMed] [Google Scholar]
- 57.Katz PO. Optimizing medical therapy for gastroesophageal reflux disease: state of the art. Rev Gastroenterol Disord. 2003;3:59–69. [PubMed] [Google Scholar]
- 58.Chang A, Lasserson T, Gaffney J, et al. Gastro-oesophageal reflux treatment for prolonged non-specific cough in children and adults. Cochrane Database Syst Rev. 2005;(2):CD004823. doi: 10.1002/14651858.CD004823.pub2. [DOI] [PubMed] [Google Scholar]
- 59.Hassall E. Wrap session: is the Nissen slipping? Can medical treatment replace surgery for severe gastroesophageal reflux disease in children? Am J Gastroenterol. 1995;90(8):1212–20. [PubMed] [Google Scholar]
- 60.Fung KP, Seagram G, Pasieka J, et al. Investigation and outcome of 121 infants and children requiring Nissen fundoplication for management of gastroesophageal reflux. Clin Invest Med. 1990;13:237–46. [PubMed] [Google Scholar]
- 61.Little AG, Ferguson MK, Skinner DB. Reoperation for failed antireflux operations. J Thorac Cardiovasc Surg. 1986;91:511–7. [PubMed] [Google Scholar]
- 62.Pennell RC, Lewis JE, Cradock TV, et al. Management of severe gastroesophageal reflux in children. Arch Surg. 1984;119:553–7. doi: 10.1001/archsurg.1984.01390170049010. [DOI] [PubMed] [Google Scholar]