Abstract
Recently in Developmental Cell, Zhao et al. (2013) reported a mechanism for the directed turnover of the mouse Piwi protein MIWI during sperm maturation. This study implicates the anaphase-promoting complex as a mediator of MIWI ubiquitination and expands the avenues for regulating small RNA processes.
Spermatogenesis begins with spermatogonia that self-renew through mitosis, whereas daughter cells enter meiosis as spermatocytes. After completing meiosis and becoming round spermatids, sperm mature by replacing histones with protamines, forming the flagella tail, and extruding most of its cytoplasm (O'Don-nell et al., 2011). In mice, male germ cells rely on Piwi proteins—MIWI, MIWI2, and MILI—that bind to Piwi-interacting RNAs (piRNAs) to mediate transposon silencing, RNA regulation, and genome stabilization (Siomi et al., 2011). These Piwi proteins are expressed at different stages during spermatogenesis. MIWI2 is expressed transiently in the spermatogonia of embryonic testes, whereas MIWI is expressed only from spermatocytes to late spermatids, and MILI is expressed throughout most stages of spermatogenesis.
Although the significance of the temporal expression of Piwi proteins is not well understood, MIWI, MILI, and piRNAs are clearly depleted in mature sperm cells (Siomi et al., 2011), perhaps as a result of the spermiation process of indiscriminate cytoplasm exclusion (O'Donnell et al., 2011). Alternatively, a directed protein turnover mechanism, such as the ubiquitin-proteasome system, could be operating earlier in spermatogenesis to degrade the MIWI-piRNA complex. The precedence for this mechanism is the RNF8-mediated ubiquitination of histones, which leads to massive nucleosome removal (Lu et al., 2010).
Currently in Developmental Cell, Zhao et al. (2013) provide evidence suggesting that the MIWI-piRNA complex is a target of ubiquitination by the E3 ubiquitin ligase anaphase-promoting complex/cyclosome (APC/C) that leads to subsequent proteasome-mediated degradation. The APC/C is best known for regulating key cell-cycle transitions, such as controlling sister chromatid separation (Peters, 2006). In addition to cell-cycle-regulatory targets, the APC/C can also direct the degradation of substrates in nonmitotic cells. The Zhao et al. (2013) study shows that MIWI interacts specifically with the APC10 subunit of the APC/C and that this interaction is dependent upon the addition of piRNAs.
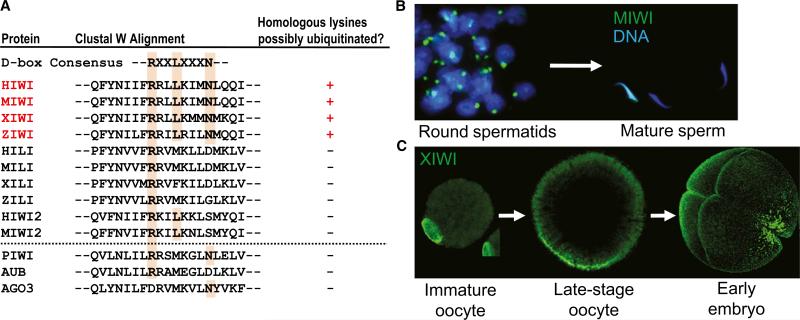
This study suggests that, when MIWI binds piRNAs, a “destruction” box (D-box), and perhaps other motifs (e.g., the KEN box), for APC/C recognition (Peters, 2006) is revealed, triggering APC/C binding and the ubiquitination of specific lysines in MIWI. The D-box is conserved among other vertebrate orthologs of MIWI, although other Piwi homologs such as MILI, MIWI2, and Drosophila Piwi proteins lack this motif (Figure 1). Ubiquitination assays with in vitro-expressed APC/C and MIWI suggested that piRNA loading was necessary for MIWI ubiquitination. Using a heterologous system of HEK293T cells overexpressing MIWI, the authors showed polyubiquitination of MIWI only when adult mouse testes piRNAs or a single synthetic piRNA was cotransfected. Surprisingly, significant piRNA loading occurred in these transformed cells, even though they lacked endogenous piRNAs, MIWI, and most likely other factors required for piRNA maturation. The authors showed that MIWI ubiquitination depended on cotransfection of piRNAs. Disruption of piRNA loading by the mutation of key residues in the overexpressed MIWI also reduced its ubiquitination. Polyubiquitination and modest decreases in the MIWI protein level in the in vitro system hint at a 26S proteasome-dependent degradation mechanism, although the molecular basis of specific APC/C recognition of the MIWI and piRNA complex remains unclear.
Figure 1. Piwi Protein Turnover and Perdurance in Vertebrate Gametes.
(A) Alignment of the vertebrate (top) and Drosophila (bottom) Piwi protein amino acid sequence portions that highlights the region containing a possible destruction box (D-box) consensus. Only MIWI and its orthologs have both the D-box consensus and the combination of lysines that might be substrates for ubiquitination. (B) MIWI is highly concentrated in the chromatoid body of mouse round spermatids but is depleted in mature sperm. (C) XIWI is expressed throughout oogenesis and is retained in late-stage Xenopus oocytes and the early embryo.
Looking in a physiological context, Zhao et al. (2013) provide evidence that MIWI interacts with the APC/C and that it is ubiquitinated in late spermatids of adult mouse testes. The authors use lentiviruses expressing either small hairpin RNAs to knock down APC10 or to express epitope-tagged MIWI in spermatids in order to assess the in vivo effects of APC disruption. Amazingly, APC10 depletion or point mutations in a MIWI transgene that affect piRNA loading increased the stability of MIWI. The next challenge will be to determine whether this effect is direct in vivo, as implied by the in vitro and heterologous system experiments, because reducing APC/C levels may disrupt many other cellular processes.
Although MIWI and piRNAs levels are greatly depleted from mature sperm, MIWI does not appear to be ubiquitinated in earlier stages of spermatogenesis, such as in spermatocytes and round spermatids, when the levels of piRNAs and Cdc-20- and Cdh-1-activated APC/C are high. How is piRNA-induced MIWI degradation prevented during these earlier stages? One explanation for this could be an inhibitor that occludes APC/C from acting on MIWI and piRNAs. Although Zhao et al. (2013) do not identify a specific inhibitor, their data suggest a spatial segregation of the APC/C from MIWI in spermatocytes and round spermatids. Since MIWI is concentrated in the chromatoid body (Siomi et al., 2010), this study opens the question as to what bars the APC/C entry into the chromatoid body until the late spermatid stages? Additionally, could the MILI-piRNA complex also be the subject of APC/C regulation?
What may be the physiological necessity to target the MIWI-piRNA complex for active degradation? First, this mechanism might be part of a checkpoint for completion of proper transposon silencing before late spermatozoa maturation. Second, it might be necessary to prevent paternal piRNAs from being transmitted to the embryo as this may impair some zygotic processes (e.g., imprinting). Although the Zhao et al. (2013) study suggests a requirement for MIWI degradation for final sperm maturation, other studies have described transmission of other small RNAs—paternal microRNAs (miRNAs)—from sperm to zygote (Liu et al., 2012). Perhaps the mechanism in sperm that targets MIWI degradation allows miRNAs to remain intact for epigenetic transmission.
This is in contrast with female gameto-genesis, where Piwi proteins and piRNAs are clearly maternally transmitted to the embryo in Drosophila and Caenorhabditis and are key mechanisms for progeny reproductive health (Siomi et al., 2011). MIWI persists in late-stage mouse oocytes and is restricted to the cytoplasm (Ding et al., 2012). Perhaps as in sperm, the APC/C and 26S proteasome are sequestered from MIWI by residing in the oocyte nucleus, where they are poised to mediate cyclin B1 decay and arrest the oocyte before meiosis II entry at ovulation. Alternatively, APC/C activity could be suppressed in oocytes and un-fertilized eggs by the inhibitors Emi1 and Emi2 (also known as Xerp1), respectively (Peters, 2006). This could explain why MIWI and its other vertebrate orthologs, ZIWI and XIWI, remain stable in late-stage oocytes and are maternally deposited into the embryo (Figure 1).
Data are accumulating on posttranslational modifications that affect the stability and activity of Argonaute-family proteins such as Piwi and Argonaute (Ago). For example, Ago2 can be stabilized by prolyl 4-hydroxylation (Qi et al., 2008) or subjected to ubiquitination by Lin41 (Rybak et al., 2009). The methylation of arginines in Piwi proteins also seems to be important for stabilizing Drosophila Piwi and fostering protein interactions (Siomi et al., 2010). Adding to this list could be a potential mechanism for MIWI turnover through APC/C-directed ubiquitination and proteasome degradation, an intriguing concept that awaits additional experimentation to address its impact on Piwi protein function and its effects on spermatogenesis.
REFERENCES
- Ding X, Guan H, Li H. Theriogenology. 2012 doi: 10.1016/j.theriogenology.2012.11.013. [DOI] [PubMed] [Google Scholar]
- Liu WM, Pang RT, Chiu PC, Wong BP, Lao K, Lee KF, Yeung WS. Proc. Natl. Acad. Sci. USA. 2012;109:490–494. doi: 10.1073/pnas.1110368109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu LY, Wu J, Ye L, Gavrilina GB, Saunders TL, Yu X. Dev. Cell. 2010;18:371–384. doi: 10.1016/j.devcel.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell L, Nicholls PK, O'Bryan MK, McLachlan RI, Stanton PG. Spermatogenesis. 2011;1:14–35. doi: 10.4161/spmg.1.1.14525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters JM. Nat. Rev. Mol. Cell Biol. 2006;7:644–656. doi: 10.1038/nrm1988. [DOI] [PubMed] [Google Scholar]
- Qi HH, Ongusaha PP, Myllyharju J, Cheng D, Pakkanen O, Shi Y, Lee SW, Peng J, Shi Y. Nature. 2008;455:421–424. doi: 10.1038/nature07186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybak A, Fuchs H, Hadian K, Smirnova L, Wulczyn EA, Michel G, Nitsch R, Krappmann D, Wulczyn FG. Nat. Cell Biol. 2009;11:1411–1420. doi: 10.1038/ncb1987. [DOI] [PubMed] [Google Scholar]
- Siomi MC, Mannen T, Siomi H. Genes Dev. 2010;24:636–646. doi: 10.1101/gad.1899210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siomi MC, Sato K, Pezic D, Aravin AA. Nat. Rev. Mol. Cell Biol. 2011;12:246–258. doi: 10.1038/nrm3089. [DOI] [PubMed] [Google Scholar]
- Zhao S, Gou L-T, Zhang M, Zu L-D, Hua M-M, Hua Y, Shi H-J, Li Y, Li J, Li D, et al. Dev. Cell. 2013;24:13–25. doi: 10.1016/j.devcel.2012.12.006. [DOI] [PubMed] [Google Scholar]