Abstract
Background
Additional drugs are needed for the treatment of cytomegalovirus (CMV) infection. Artesunate is an antimalarial drug that has activity against CMV in vitro and in a rodent model. Only a small number of case reports are available describing the clinical effects of artesunate on CMV infection, and these yielded inconsistent results.
Objective
To evaluate the effect of artesunate on CMV infection, using blood samples collected from children who participated in malaria treatment trials.
Study design
Quantitative CMV DNA PCR was performed on dried blood spots collected from 494 Ugandan children, who were randomized either to artesunate plus amodiaquine or sulfadoxine-pyrimethamine plus amodiaquine for acute malaria infection. Poisson regression was used to compare treatment regimens with respect to the change in the frequency and quantity of CMV detected that occurred before and after treatment.
Results
CMV was detected in 11.4% of children immediately prior to treatment and 10.7% 3 days later (p=0.70). The average quantity of CMV was 0.30 log10 copies per million cells higher on day 3 than at treatment initiation (95% CI 0.01 to 0.58, p=0.041). There was no measurable difference in either the frequency or quantity of CMV detected in blood between children randomized to the two treatment arms.
Conclusions
A standard 3-day artesunate-containing antimalarial regimen had no detectable effect on CMV viremia in children with malaria. Longer treatment courses and/or higher doses of artesunate than those routinely used for malaria may be required for effective treatment of CMV infection.
Background
Cytomegalovirus (CMV) is an important cause of disease in congenitally-infected or immunocompromised patients.1 Available drugs with proven efficacy for the treatment of CMV infection all directly or indirectly target the viral polymerase.2 Ganciclovir and its pro-drug valganciclovir are highly effective but frequently cause bone marrow suppression, and ganciclovir-resistant CMV can emerge with prolonged use. Cidofovir and foscarnet are second-line drugs to treat CMV infection, but they are only available in intravenous formulations, are highly nephrotoxic, and can select for drug resistance. Therefore, alternative agents with activity against CMV are needed.
Recently, the antimalarial drug artesunate was shown to have activity against CMV in vitro and in a rodent model.3-6 Artesunate is widely used in combination malaria therapies, is very well tolerated, is orally bioavailable, and can be produced relatively inexpensively.7, 8 However, data on the clinical use of artesunate for CMV infection is limited to 8 patients, in whom efficacy varied widely.9-11 To gain insight into potential effects of artesunate on CMV infection in a larger cohort, we utilized banked samples from two randomized malaria treatment trials in Uganda.1, 12
Study design
DBS were obtained from two Phase 3 randomized controlled trials of antimalarial treatments performed in Uganda.13, 14 Children aged 0.5 - 13 years were randomized to receive combination regimens for uncomplicated malaria infection, including artesunate (4 mg/kg/day for three days) plus amodiaquine (10 mg/kg on the first 2 days and then 5 mg/kg on the third day), or sulfadoxine-pyrimethamine (sulfadoxine 25 mg/kg and pyrimethamine 1.25 mg/kg as a single dose on the first day) plus amodiaquine (at the same dosing scheme). Notably, neither sulfadoxine-pyrimethamine nor amodiaquine has known antiviral activity. A dried whole blood spot (DBS) on filter paper was collected from each child immediately prior to initiation of study treatment on the day malaria infection was diagnosed (day 0), and one day following the completion of therapy (day 3). Given the short half-life of artesunate (39 – 95 minutes),7 an antiviral effect was not anticipated to arise later after discontinuation of therapy.
DNA was extracted from 6 mm DBS punches using QIAGEN BioSprint 96 DNA Blood kits and quantitative TaqMan PCR assays for CMV and beta-globin (as a positive control and to normalize CMV results to the quantity of cells) were performed using established methods.15, 16 The lower limit of detection was approximately 3 copies of CMV per reaction. Multiple negative controls were included in each PCR run. Laboratory investigators and staff were blinded to treatment assignment prior to the completion of testing.
Non-linear mixed models with a log (or Poisson) link were used to determine whether the frequency of CMV detection differed between days 0 and 3. An interaction term was used to determine whether any observed change differed by treatment arm. Poisson regression was also used to examine detection rates by age, and the potential interaction between age and treatment effect. The quantity of virus detected was compared using positive samples only, using linear mixed models. All of these models account for repeated measures on the same subjects and effectively adjust for baseline CMV detection when comparing the responses between treatment arms.
Results
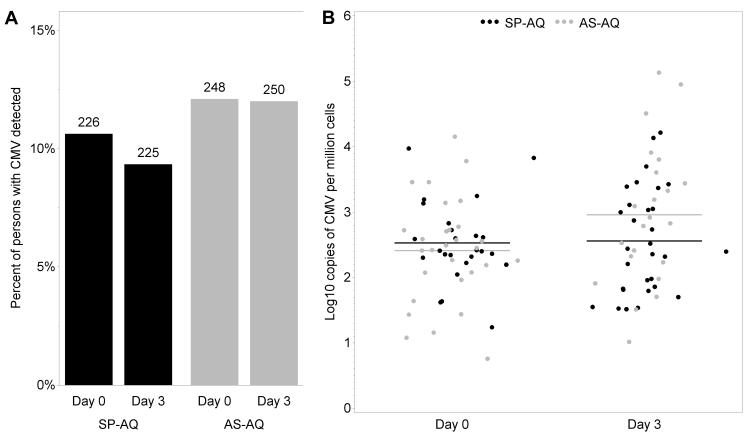
DBS samples were available from 494 children, 261 of whom were randomized to artesunate plus amodiaquine and 233 to sulfadoxine-pyrimethamine plus amodiaquine. Age (median 5 years), parasite density and other participant characteristics were comparable between the two treatment arms.13, 14 A total of 988 specimens were tested, of which 955 (96.7%) had interpretable PCR results. Of these samples, 105 (11.1%) had CMV detected, with a median quantity of 2.5 log10 CMV genomes per million cells (range 0.8 to 5.1). CMV viremia was detected in a similar proportion of all children on each day (11.4% on day 0 and 10.7% on day 3; p=0.70). The proportion of children with viremia detected on day 0 was 26% in those <2 years old compared to 4% for those 11-13 years old, and decreased, on a relative scale, by 10% for each year of age (RR=0.90, 95% CI 0.82 to 0.99, p=0.035). The average quantity of CMV was 0.30 log10 copies per million cells higher on day 3 versus day 0 (95% CI 0.01 to 0.58, p=0.041). No effect of treatment arm was observed in the change of either the rate (p=0.27) or quantity (p=0.69) of CMV viremia that was detected between day 0 and day 3 (Figure 1). There was no interaction between age and treatment arm (p=0.18). Thus, there was no measurable antiviral activity of artesunate against CMV when given in combination with amodiaquine as treatment for malaria.
Figure 1. A standard artesunate-containing antimalarial regimen does not affect CMV viremia in children being treated for malaria.
CMV PCR was performed on dried blood spots collected at the initiation of (day 0) and following treatment (day 3) with sulfadoxinepyrimethamine plus amodiaquine (SP-AQ) or artesunate plus amodiaquine (AS-AQ). There was no association between treatment arm and either (A) the proportion of children with viremia (the number of participants with results is shown above each bar) or (B) the quantity of CMV detected (lines indicate mean values).
Discussion
The lack of effect seen with an artesunate-containing antimalarial regimen in this study contrasts with a case report of a pediatric transplant patient treated for asymptomatic multi-drug-resistant CMV infection.9 Oral artesunate (100 mg/day; weight-based dosing was not provided) was given on two separate occasions, and each time there was approximately a 2 log10 reduction of CMV copies/mL of whole blood after 7 days of treatment.9 In a pilot trial, 6 adult transplant recipients with CMV viremia were each given a loading dose of oral artesunate of 200 mg twice on the first day of treatment, followed by 100 mg/day on subsequent days.11 Of the 6 subjects, 2 had an apparent virologic response, with a reduction in viremia within 3 days of artesunate treatment; the remaining 4 patients either lacked a clear trend or had increasing CMV copy numbers, and artesunate was discontinued after 7 days. The only other relevant published report of which we are aware is of an adult patient treated for ganciclovir-resistant CMV colitis following renal transplantation, in which there was no clinical or virologic response to 20 days of intravenous artesunate at doses up to 240 mg/day, but a rapid response upon initiation of foscarnet.10
Strengths of this study include the relatively large number of subjects who were treated in a randomized fashion, and evaluation of an artesunate regimen that has proven to be safe and well tolerated in millions of patients.8 The main limitation of the study is that only a 3-day antimalarial regimen of artesunate combined with amodiaquine was studied. Although CMV log10 copies/mL in whole blood or plasma decrease with first-order kinetics during (val)ganciclovir treatment in transplant patients, viral load reductions after 3 days of therapy are typically small (<0.5 log10 copies/mL) and relatively variable.11, 17-19 The use of DBS could have also limited our ability to detect an early effect of artesunate on CMV infection. However, CMV PCR results from DBS and plasma samples are highly correlated in transplant patients, including during ganciclovir treatment.16 Finally, the effects of artesunate on CMV infection might differ between children with malaria and other populations, such as transplant patients. Our results do not preclude the possibility that artesunate may have a role in the treatment of CMV infection, but they strongly suggest that longer courses and/or higher doses than those used for malaria treatment are required to observe an effect on CMV viremia. In summary, a standard artesunate-containing antimalarial regimen had no detectable effect on the frequency or quantity of CMV viremia in African children.
Acknowledgments
Funding: University of Washington Royalty Research Fund Award, the National Institutes of Health (U01 AI052142; K23 AI49212; K23 AI43301), and the Fogarty International Center (TW00007; TW01506).
Abbreviations
- CMV
cytomegalovirus
- DBS
dried blood spot
Footnotes
Conflict of interests: None declared.
Ethical approval: All aspects of this study were approved by institutional review boards at the University of Washington, the University of California, San Francisco, Makerere University, Kampala, Uganda, and the Uganda National Council for Science and Technology, and informed consent was obtained from all participants.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mocarski ES, Shenk T, Pass RF. Cytomegaovirus. In: Knipe DM, Howley PM, Griffin DE, Lamb R, Martin M, Roizman B, et al., editors. Field’s Virology. 5th ed Lippincott Williams & Wilkins; Philadelphia: 2007. [Google Scholar]
- 2.Biron KK. Antiviral drugs for cytomegalovirus diseases. Antiviral Res. 2006;71:154–63. doi: 10.1016/j.antiviral.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 3.Kaptein SJ, Efferth T, Leis M, Rechter S, Auerochs S, Kalmer M, et al. The anti-malaria drug artesunate inhibits replication of cytomegalovirus in vitro and in vivo. Antiviral Res. 2006;69:60–9. doi: 10.1016/j.antiviral.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 4.Efferth T, Marschall M, Wang X, Huong SM, Hauber I, Olbrich A, et al. Antiviral activity of artesunate towards wild-type, recombinant, and ganciclovir-resistant human cytomegaloviruses. J Mol Med. 2002;80:233–42. doi: 10.1007/s00109-001-0300-8. [DOI] [PubMed] [Google Scholar]
- 5.Chou S, Marousek G, Auerochs S, Stamminger T, Milbradt J, Marschall M. The unique antiviral activity of artesunate is broadly effective against human cytomegaloviruses including therapy-resistant mutants. Antiviral Res. 2011;92:364–8. doi: 10.1016/j.antiviral.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 6.Schnepf N, Corvo J, Pors MJ, Mazeron MC. Antiviral activity of ganciclovir and artesunate towards human cytomegalovirus in astrocytoma cells. Antiviral Res. 2011;89:186–8. doi: 10.1016/j.antiviral.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 7.Efferth T, Romero MR, Wolf DG, Stamminger T, Marin JJ, Marschall M. The antiviral activities of artemisinin and artesunate. Clin Infect Dis. 2008;47:804–11. doi: 10.1086/591195. [DOI] [PubMed] [Google Scholar]
- 8.Zwang J, Dorsey G, Djimde A, Karema C, Martensson A, Ndiaye JL, et al. Clinical tolerability of artesunate-amodiaquine versus comparator treatments for uncomplicated falciparum malaria: an individual-patient analysis of eight randomized controlled trials in sub-Saharan Africa. Malar J. 2012;11:260. doi: 10.1186/1475-2875-11-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shapira MY, Resnick IB, Chou S, Neumann AU, Lurain NS, Stamminger T, et al. Artesunate as a potent antiviral agent in a patient with late drug-resistant cytomegalovirus infection after hematopoietic stem cell transplantation. Clin Infect Dis. 2008;46:1455–7. doi: 10.1086/587106. [DOI] [PubMed] [Google Scholar]
- 10.Lau PK, Woods ML, Ratanjee SK, John GT. Artesunate is ineffective in controlling valganciclovir-resistant cytomegalovirus infection. Clin Infect Dis. 2011;52:279. doi: 10.1093/cid/ciq050. [DOI] [PubMed] [Google Scholar]
- 11.Wolf DG, Shimoni A, Resnick IB, Stamminger T, Neumann AU, Chou S, et al. Human cytomegalovirus kinetics following institution of artesunate after hematopoietic stem cell transplantation. Antiviral Res. 2011;90:183–6. doi: 10.1016/j.antiviral.2011.03.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walter S, Atkinson C, Sharland M, Rice P, Raglan E, Emery VC, et al. Congenital cytomegalovirus: association between dried blood spot viral load and hearing loss. Arch Dis Child Fetal Neonatal Ed. 2008;93:F280–5. doi: 10.1136/adc.2007.119230. [DOI] [PubMed] [Google Scholar]
- 13.Staedke SG, Mpimbaza A, Kamya MR, Nzarubara BK, Dorsey G, Rosenthal PJ. Combination treatments for uncomplicated falciparum malaria in Kampala, Uganda: randomised clinical trial. Lancet. 2004;364:1950–7. doi: 10.1016/S0140-6736(04)17478-3. [DOI] [PubMed] [Google Scholar]
- 14.Dorsey G, Staedke S, Clark TD, Njama-Meya D, Nzarubara B, Maiteki-Sebuguzi C, et al. Combination therapy for uncomplicated falciparum malaria in Ugandan children: a randomized trial. Jama. 2007;297:2210–9. doi: 10.1001/jama.297.20.2210. [DOI] [PubMed] [Google Scholar]
- 15.Boeckh M, Huang M, Ferrenberg J, Stevens-Ayers T, Stensland L, Nichols WG, et al. Optimization of quantitative detection of cytomegalovirus DNA in plasma by real-time PCR. J Clin Microbiol. 2004;42:1142–8. doi: 10.1128/JCM.42.3.1142-1148.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Limaye AP, Hayes TK, Huang ML, Magaret A, Boeckh M, Jerome KR. Quantitation of Cytomegalovirus (CMV) DNA Load In Dried Blood Spots (DBS) Correlates Well with Plasma Viral Load. J Clin Microbiol. 2013 doi: 10.1128/JCM.00316-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Asberg A, Humar A, Rollag H, Jardine AG, Mouas H, Pescovitz MD, et al. Oral valganciclovir is noninferior to intravenous ganciclovir for the treatment of cytomegalovirus disease in solid organ transplant recipients. Am J Transplant. 2007;7:2106–13. doi: 10.1111/j.1600-6143.2007.01910.x. [DOI] [PubMed] [Google Scholar]
- 18.Emery VC, Cope AV, Bowen EF, Gor D, Griffiths PD. The dynamics of human cytomegalovirus replication in vivo. J Exp Med. 1999;190:177–82. doi: 10.1084/jem.190.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mattes FM, Hainsworth EG, Hassan-Walker AF, Burroughs AK, Sweny P, Griffiths PD, et al. Kinetics of cytomegalovirus load decrease in solid-organ transplant recipients after preemptive therapy with valganciclovir. J Infect Dis. 2005;191:89–92. doi: 10.1086/425905. [DOI] [PubMed] [Google Scholar]