Abstract
Increasingly, the discovery and characterization of small regulatory RNAs from a variety of organisms have all required deep-sequencing methodologies. However, the crux to successful deep-sequencing analysis depends upon optimal construction of a cDNA library compatible for the high-throughput sequencing platform. Challenges to small RNA library constructions arise when dealing with minute tissue samples because certain structural RNA fragments can dominate and mask the desired characterization of regulatory small RNAs like microRNAs (miRNAs), endogenous small interfering RNAs (endo-siRNAs), and Piwi-interacting RNAs (piRNAs). Here, we describe methods that improve the chances of constructing a successful library from small RNAs isolated from minute tissues such as enriched follicle cells from the Drosophila ovarium. Because the ribosomal RNA (rRNA) fragments are frequently the major contaminants in small RNA preparations from minute amounts of tissue, we demonstrate the utility of antisense oligonucleotide depletion and an acryloylaminophenylboronic acid (APB) polyacrylamide gel system for separating the abundant 2S rRNA in Drosophila from endo-siRNAs and piRNAs. Finally, our methodology generates libraries amenable to multiplex sequencing on the Illumina Hi-Seq platform.
Keywords: Small RNAs, Illumina deep-sequencing, Library construction
1. INTRODUCTION
Amongst the various methods to detect small endogenous RNAs in organism samples, the procedure to convert RNAs into a library of cDNAs followed by sequencing is considered to be the most comprehensive technique because it enables discovery of the molecules’ sequence as well as confirmation of its identity. For example, the seminal discoveries that expanded the catalog of microRNAs beyond lin-4 and let-7 resulted from the sequencing of cDNA libraries from small RNAs purified from nematode, fly and human cells (1–3). With the commercialization and adoption of high-throughput next generation sequencing technologies such as 454, Illumina and ABI SoLID, the detection of molecules based on the number of reads have lent to unparalleled sensitivity and accuracy in small RNA quantitation.
Despite the immense depth of sequencing now achievable by high-throughput systems like the Illumina Hi-Seq 2000 capable of routinely generating >200 million reads per flow cell lane, the success of a small RNA sequencing run depends most on the composition of the library in terms of being free of undesired degradation remnants or naturally abundant short ribosomal RNA fragments. For example, Drosophila and other insects generate an abundant 31-nucleotide (nt) 2S rRNA that migrates very closely to the piRNAs that range from 24–32 nt long (4). In addition, Xenopus eggs also contain a slew of ribosomal RNA fragments that range from 23–35 nt long, which also co-migrate in size with Xenopus piRNAs and miRNAs (5). When there are plenty of tissues and cells from which to generate a cell lysate, this lysate can be subjected to immunoprecipitation (IP) of ARGONAUTE and PIWI proteins, or with cation exchange chromatography (6), both of which can quite effectively deplete undesired contaminating RNAs.
However, when one wishes to profile small RNAs from minute samples, such as a single egg or from a very small population of cells enriched in a particular cell type, the IP and chromatography methods are not practical, and typically just total RNA is isolated. From these total RNA preparations, rRNA fragments persist and will become a major nuisance. For example, when total small RNAs from single Xenopus eggs were profiled, the rRNA fragments reduced the representation of other small regulatory RNAs down to below 20% of the library (5). This issue can be partially mitigated by sequencing libraries on the Illumina Hi-Seq platform versus the Illumina Genome Analyzer (GA) platform because the ~20-fold increase in depth from the former platform may yield enough desired small regulatory RNA reads despite sacrificing the non-useful rRNA contaminants. However, new considerations in the multiplexing of small RNA libraries must be followed for the Illumina Hi-Seq platform because the lower stringency of base-calling versus the GA platform can also reduce the yield of reads passing quality. In the first set of methods detailed in this chapter, we will describe our experiences in isolating a small sample of tissues, an enriched population of follicle cells from the Drosophila ovarium, and our considerations in generating small RNA libraries from these minute samples for the Illumina Hi-Seq platform, which includes a new format of linkers that are amenable for multiplex sequencing of small RNA libraries.
In the second set of methods, we describe the adaptation of a boronate affinity-gel matrix applied to the resolution of small amounts of RNA from Drosophila ovary cells. The boronate gel matrix consists of a denaturing polyacrylamide gel impregnated with acryloylamino-phenylboronic acid (APB), and short RNAs (<~80 nt) with unmodified 2′-3′ cis-diols will exhibit a stronger dynamic affinity to the boronate than endo-siRNAs and piRNAs which are naturally methylated at the 3′ end by Hen-1 on the 2′ OH (7, 8). With standard polyacrylamide electrophoresis, the abundant 2S rRNA and other rRNA fragments can co-migrate or resolve poorly from piRNAs and endo-siRNAs. However, on an APB-gel the rRNA fragments are retarded while bonafide piRNAs and endo-siRNAs migrate faster, thus facilitating further the removal of the contaminating RNAs from the regulatory small RNAs.
As biologists began to interrogate the small RNA profiles of particular niches of cells, from stem cells to specialized neuronal cell types, the need to improve methodologies to generate libraries from minute samples will become more evident. These procedures we have developed will increase the likelihood that properly diverse cDNA libraries can be constructed, and although our reagents are based on lab-made stocks, the antisense oligo-mediated depletion step and the boronate affinity gel matrix can be applied to steps from commercial small RNA library construction protocols.
2. MATERIALS
2.1. Drosophila Culture and Ovary Dissection
Standard fly food in bottles with enough extra yeast added to make a fine layer on the top of the food.
Flynap or carbon dioxide venting flypad for anesthetizing flies.
Stereo dissection microscope, paintbrush for fly pushing, and fine pointed Dumont 50 tweezers
Watch glass dish chilled in a small box of ice
Chilled 1X PBS
2.2. Follicle Cell Enrichment
Programmable thermoshaker (Eppendorf)
Concentrated trypsin stock (Sigma)
Refrigerated microcentrifuge
40 micron mesh filter (BD Scientific)
Microscope slides dipped in 1mg/ml Concanavalin A solution (Sigma)
Formaldehyde in 1X PBS (1% for fixing follicle cells; 3.7% for fixing ovaries)
4′,6-diamidino-2-phenylindole (DAPI, Invitrogen) and Vectasheild (Vector Labs)
Fluorescent microscope with filter for imaging DAPI (digital camera optional).
2.3. Small RNA Purification and antisense oligo depletion of 2S rRNA
TriReagent or TriZol solutions for RNA extraction and precipitation (Molecular Research Center or Invitrogen)
Standard materials for urea polyacrylamide gel electrophoresis
2x RNA Urea-TE loading buffer (8M Urea, 100mM Tris-HCl, pH 8.0, 50 mM EDTA, 0.05% w/v bromphenol blue, 0.05% w/v xylene cyanol FF);
10bp ladder (Invitrogen) and gamma-32P-labeled markers (18mer, and 34mer-to ensure getting small RNA size range)
SyberGreen II RNA stain (Invitrogen)
Phosphorimaging cassette and phosphorimager (i.e. Typhoon Scanner, GE Healthcare)
Siliconized tubes 1.7ml microcentrifuge tubes
0.3M NaCl
Rotisserie rocker
10μM biotinylated DNA oligo antisense to 2S rRNA: TACAACCCTCAACCATATGTAGTCCAAGCATACAACCCTCAACCATATGTAGTCCAA GCAGTCGA-3′ biotin
0.5M EDTA, pH 8.0
20X and 0.5X SSC
0.5M NaCl
Streptavidin magnetic beads (Promega), and small magnetic stand
2.4. Acryloylaminophenylboronic acid (APB) polyacrylamide gel
APB that was originally produced by the protocol of (9) is now available from tRNA Probes Inc (College Station, TX).
Sequagel Urea gel system (National Diagnostics);
50x Tris-Acetate-EDTA (TAE) buffer (2M Tris-Acetate, 50 mM EDTA, pH 8.0);
10% Ammonium Persulfate (APS) and TEMED
Vertical electrophoresis system (i.e. Model V16 from Whatman Inc.);
2x Urea-TE loading buffer (8M Urea, 10mM Tris-HCl pH 8.0, 50 mM EDTA, 0.05% w/v bromphenol blue, 0.05% w/v xylene cyanol FF);
Synthetic RNA Marker oligonucleotides of any sequence at 18, 22, and 32 nt long.
Synthetic piRNAs are synthetic RNAs of 27 nt long such as the following sequences:
iRNA with standard terminal 2′-OH: AGGAAAGUUGUGCACACUUGUAAUCCGAAA
piRNA with terminal 2′-O-Me: UGGGAUUACAAGUGUGCACAACUUUCCUGmC
2.5. Linker-Ligation small RNA library construction from minute samples
Chimeric marker oligonucleotides where upper-case bold represents an RNA base, lowercase italics is a standard DNA base, and “dU” is deoxy-uridine, which can be degraded by uracil-deoxyglycosylase (UDG):
34 nt chimeric marker: CAGUAC ggatcca dUdUdUdU tatgctc AGCGUACGAA
18 nt chimeric marker: C agtac dUdUdU gctag CUAA
3′ Linker adaptor: p-cgtcgtatgccgtcttctgcttgt-/3AmMO/,
where /3AmMO/ is a 3′ amino modifier and is chemical phosphorylated on the 5′ end.
Original 5′ Adaptor: GUUCAGAGUUCUACAGUCCGACGAUC
-
5′ HiSeq adaptors with barcodes underlined, RNA bases in uppercase bold, DNA bases in lowercase italics, and “N” represents random incorporation of all four DNA bases:
Barcode CAA: gttcagagttctacagtccgacgatc NNN CAAAA
Barcode ACC: gttcagagttctacagtccgacgatc NNN ACCAA
Barcode GUU: gttcagagttctacagtccgacgatc NNN GUUAA
Barcode UGG: gttcagagttctacagtccgacgatc NNN UGGAA
Reverse Transcription primer & 5′ PCR primer: caagcagaagacggcata
3′ PCR primer: aatgatacggcgaccaccgacaggttcagagttctacagtccga
Mth RNA ligase, Polynucleotide kinase (PNK), Uracil-DNA glycosylase, Phusion Polymerase and T4 RNA ligase I enzymes (New England Biolabs)
RiboLock RNase inhibitor, 40U/μl (Thermo Scientific)
5x RNA ligase buffer: 250mM HEPES (pH 8.3), 50mM MgCl2, 16.5mM DTT, 50μg/mL BSA, 41.5% glycerol
ATP (10 mM)
Low melting temperature agarose (i.e. Agarose II, Amresco)
Gel extraction kit (Qiagen)
RNA Clean & Concentrator kit (Zymo Research)
SuperScript III Reverse Transcriptase, Quant-iT Pico-green dsDNA assay kit, and Zero-Blunt TOPO PCR cloning kit for sequencing (Invitrogen)
3. METHODS
3.1. Harvesting Ovaries and Enriching for Follicle Cells
About 3 days prior to dissections, flies should be given extra yeast in the food in order to fatten up the ovaries so that they are easily distinguishable and removable from the rest of the abdomen. Typically, ~400 female flies are dissected in one sitting to obtain ~100 μl of tissue. Follicle cell enriching must be performed immediately after one dissection sitting, and practice is required to enable obtaining tissue efficiently. The amount of trypsin to add varies depending on amount of tissue (usually 7.5 μl of a 10X trypsin stock from Sigma per 100 μl of tissue yields a good enriched follicle cell sample).
Anesthetize fattened flies with either flynap or carbon dioxide, and sort for females (larger yet lighter abdomen).
Transfer females to a watch glass containing cold 1X PBS. Use fine tweezers to tease out the ovaries by pulling away from the abdomen and place ovaries in a microcentrifuge tube containing ice cold 1X PBS.
Wash ovaries once in 1mL cold 1X PBS (save a 20 μl drop for DAPI staining).
Add 700ul of cold 1X PBS to the ovaries, then add 77 μl of a 10X trypsin stock. Vortex briefly to mix.
Place tube in a thermoshaker to shake at 1400 rpm (or the maximum rpm) for 20 minutes at 30°C. Shaking must be vigorous (>1200 rpm) to ensure that the majority of the nurse cells and oocytes are properly lysed during enzymatic digestion.
Rest the tube on ice to allow undigested tissue to settle and remove the supernatant of liberated follicle cells to a new tube which is centrifuged at 4°C for 8 minutes at 4200 rpm to pellet the follicle cells.
Repeat Steps 4 to 6 at least two more times to collect enriched follicle cell samples. The remaining mostly consists of fertilized eggs (up to 70%) whose egg shells are resistant to trypsin digestion and can be discarded.
Wash and centrifuge the follicle cell pellet once in ice cold 1X PBS (2 minutes, 1600 x g), and resuspend pellet in 300 μl of cold 1X PBS. Remove 5 μl for DAPI staining verification. Spin down cells, remove PBS, and store the cell pellet at −80°C until ready to start the small RNA library construction.
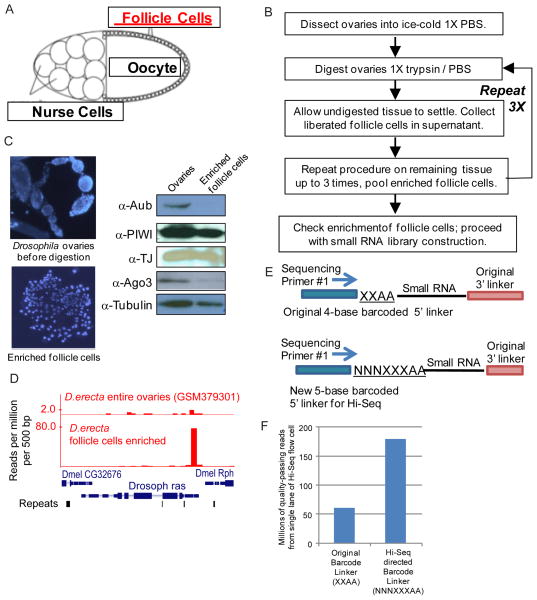
To verify efficacy of follicle cell enrichment, drops of dissected ovaries and follicle cells are placed on a microscope glass slide previously coated with Concanavalin A, which promotes cell adhesion to glass. After 5 minutes at room temp, cells are fixed with formaldehyde in PBS for 5 minutes, then dipped in a 50 mL solution of 10 μg/ml DAPI in 1X PBS in a coplin jar for 5 minutes. Cells and ovaries are then mounted in Vectashield with a cover slip and observed under a fluorescent microscope with a DAPI filter to visualize the clear difference in size and morphology between intact ovaries and follicle cells (Figure 1C).
Figure 1.
Drosophila ovary follicle cell enrichment and small RNA library construction from this minute tissue sample. A) A schematic of a Drosophila egg chamber highlighting the relative small size of the follicle cells. B) Flowchart of the procedure to enrich follicle cells from Drosophila ovaries. C) Validation of follicle cell enrichment procedure with 4′,6-diamidino-2-phenylindole (DAPI) staining in left panels and western blots that show retention of Piwi and Traffic Jam (TJ) proteins concomitant with depletion of Aubergine (Aub) and Argonaute-3 (Ago3) proteins. D) Counts of genic 3′UTR-directed piRNAs from the Raspberry (ras) gene are enriched from the follicle cells compared to sequencing from total ovary RNA. E) Schematic comparing the original 5′ linker barcode format that was suitable for sequencing on the Illumina Genome Analyzer and the new barcode format we developed for the Illumina Hi-Seq platform. F) A small RNA library with the original 4-base barcode suffers from poor read quality discrimination, while the simple addition of a 3-base random element in the new 5-base barcode greatly increases the number of quality-passing reads.
3.2. Small RNA purification and antisense oligo-mediated 2S rRNA depletion
Typically, two rounds of dissections and follicle cell enrichment would yield 10 μg of total RNA. However, we have been able to perform library construction from as little as 1–3 μg of total RNA (5). Total RNA is extracted using TriReagent (also known as Trizol), and the very small pellet is resuspended in 20 μl of water.
Small RNAs are purified by gel purification from a 15% urea denaturing gel by electrophoresis at 30W for 75 minutes or until the darker Bromphenol blue dye is near the bottom of the gel. Total RNA is first denatured in 2X Urea Loading buffer with heating at 95 degrees for 5 minutes, and samples are run with a 10 bp ladder and/or radioactive RNA markers.
If using radioactive RNA markers, expose gel covered in plastic wrap against a phosphor plate for 15 minutes. If only a 10 bp DNA ladder is used, stain the gel with SyberGreen II for 10 minutes. Phosphor plate or gel is directly scanned on the image scanner (phosphorimaging or fluorescent setting). Print a full-scale picture of the gel, and placing this behind the gel, cut out the region of small RNAs from ~20 nt to 30 nt (the 2S rRNA migrates at 31 nt).
Elute the gel samples overnight in 500 μl of 0.3M NaCl. Precipitate the RNA in 2 volumes absolute ethanol. 1 μg of mussel glycogen greatly enhances precipitation of small RNAs, while using siliconized tubes reduces non-specific adherence of RNA to plastic. Resuspend the RNA in 25 μl dH2O.
Antisense oligo-mediated depletion of 2S rRNA begins by pre-binding oligo to the 25 μl RNA with the following: 10 μl Anti-2S rRNA biotin oligo (10 μM), 2 μl 0.5M EDTA, pH 8.0, 10 μl 20X SSC, and 68 μl dH2O. Heat the sample to 70° C for 5 minutes and slowly cool to 37° C.
Meanwhile, prepare 600 μl per sample of MagneSphere streptavidin beads by washing according to manufacturer’s instruction with 0.5X SSC, and add the 100 μl beads suspension to the RNA/oligo mix. Incubate at room temperature for 20 minutes with mild agitation (can be placed on a rotisserie rocker to rotate).
Bind beads to a magnetic stand for 3 minutes, remove the supernatant to a new tube, add 200 μl 0.5M NaCl, 1 μl glycogen, and 1 mL absolute ethanol to supernatant and precipitate overnight at −20° C. Next day pellet RNA and resuspend in 10 μl dH2O for library construction.
3.3. Boronate affinity gel electrophoresis to resolve piRNAs from other small RNAs
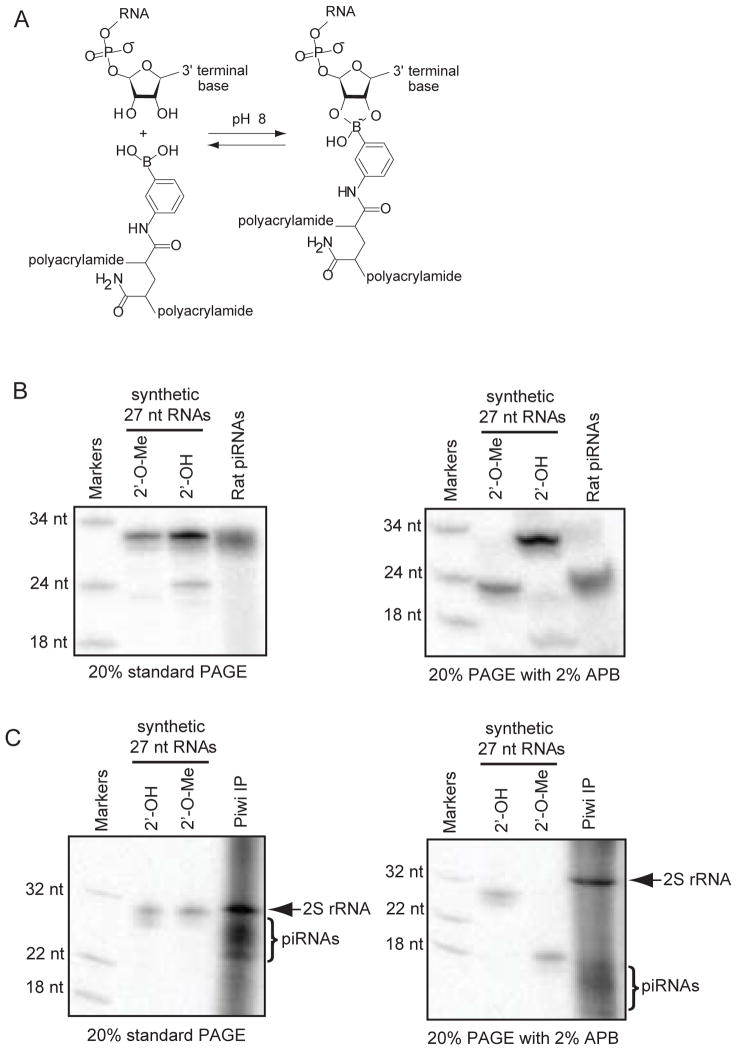
Another way to purify piRNAs from 2S rRNA and other abundant rRNA fragments is to exploit the differential affinity of modified versus unmodified terminal 3′-ends of nucleic acids to special gel matrixes during electrophoresis. For example, an acrylamide gel matrix impregnated with a boronate group can react in a dynamic equilibrium with the 3′-terminal 2′ and 3′ hydroxyl groups of unmodified short RNAs (Fig. 2A), but this reaction does not occur when there is a 2′ O-methyl group. Boronate groups are incorporated by adding acryloylaminophenylboronic acid (APB) to a standard denature urea polyacrylamide gel (9). Short RNAs of similar lengths but with 3′-terminal modifications will not sufficiently resolve simply by size on a standard polyacrylamide gel (Fig. 2B, 2C), however when APB is added to 10% of the acrylamide concentration (e.g. for a 20% polyacrylamide gel we add APB to 2%), the piRNAs with a 3′-terminal 2′-O-methyl group now migrate much faster in the gel and separate very effectively from unmodified rRNAs. Electrophoresis with APB gels must utilize Tris-Acetate (TAE) buffer instead of the typical Tris-Borate (TBE) buffer used for polyacrylamide gels because excess borate ions perturb complex formation between the boronate and the RNA.
Figure 2.
Boronate affinity gel electrophoresis of small regulatory RNAs. A) Scheme describing the structure of acryloylaminophenylboronic acid (APB) linked to polyacrylamide, and the reversible, dynamic reaction between boronate and the 3′ end of unmodified RNAs such as rRNA fragments. Small regulatory RNAs such as endogenous siRNAs and Piwi-interacting RNAs are methylated on the 3′-terminal 2′OH, which prevents them from reacting with boronate. B) 5′ radiolabeled synthetic RNAs with a 3′-terminal 2′-OH or 2′-O-Me electrophoresed against radiolabeled rat piRNAs on a standard and APB polyacrylamide gel. The methylated synthetic RNA and rat piRNAs migrate much faster than their typical size when compared to the unmodified RNAs C) The mobility of Drosophila piRNAs are increased in the APB gel, allowing for better resolution of the piRNAs from the contaminating and highly abundant 2S rRNA that co-precipitate non-specifically in a Piwi immunoprecipitation (IP). The Drosophila piRNAs from OSS cells were derived from a Piwi antibody IP performed with protein A/G magnetic beads followed by RNA extraction with TRI Reagent RT.
Prepare a 20% APB-polyacrylamide mix for a 30 ml, 0.8mm thick gel with 24 ml of Sequagel Concentrate, 3 ml of Sequagel Diluent, 1.5 ml of a 50X TAE stock, 600mg of APB power, and 1 ml of dH2O. Warm the mix to 60°C till APB powder dissolves completely and then cool back to room temperature.
Add 120 μl of 10% APS and 12 μl TEMED, mix and pour into the glass plates and spacers, and let the gel polymerize for at least 1 hour at room temperature.
In our control experiments which are optional for actual library construction, we electrophoresed the same RNA samples on a standard Urea-TAE gel versus an APB gel to demonstrate the different mobility behaviors of piRNAs relative to synthetic RNA markers. Natural RNAs were dephosphorylate with phosphatase, extracted with phenol and chloroform, and then ethanol precipitated. Natural and synthetic RNAs were then labeled with 32P-γ-ATP and PNK and denatured in Urea Loading Dye for 5 min at 95 °C prior to gel loading. Note in our demonstration that on a standard polyacrylamide gel, residual 2S rRNAs persisting in a Piwi immunoprecipitation do not resolve well from piRNAs, however on an APB gel, piRNAs can now be resolved several millimeters away from 2S rRNA (Fig. 2C).
When electrophoresing an RNA sample on the APB gel, we recommend resolving no more than 2 μg of RNA for a 1 cm wide, 0.8 mm thick gel to avoid overloading the capacity of the APB. Since modified RNAs run very differently compared to unmodified RNAs of the same size, we recommend that a standard synthetic RNA and a 2′-O-methylated RNA oligo of similar size to piRNAs be radiolabeled and run alongside your organismal RNA sample to guide where to cut out the gel region of interest from which to elute the RNA in 0.5M NaCl for downstream small RNA library construction.
3.4. Generating small RNA libraries from minute RNA quantities for multiplexing on the Illumina HiSeq platform
Several modifications of our original small RNA library construction protocol (1) have been described to generate libraries for deep-sequencing on the Illumina Genome Analyzer (4, 5, 10–18). We further extend the list of modifications with a protocol geared towards preparations for the Illumina HiSeq sequencer and starting with a small quantity of RNA. A different format for barcoding and multiplexing in the 5′ linker adapter is required because the HiSeq machine’s colony-calling algorithm now demands significant base diversity in the first 4 bases sequenced, as evidenced by our previous 4-base barcode immediately downstream sequencing primer #1 yield 1/5th of the theoretical maximum number of reads (~60 million versus ~300 million, Fig. 1E). The introduction of three random nucleotides before a 5 bp barcode sequence creates sufficient color diversity for the Hi-Seq colony-calling algorithm to approach sufficient number of quality-passing reads (Fig. 1E, 1F).
The steps below highlight additional specific modifications to include in other previously described small RNA library construction procedures.
Previously we relied upon chemical adenylation as the most cost-effective method to generate pre-adenylated 3′ Linker adaptors, but the organic chemistry steps are not routine procedures for molecular biologists. We now employ enzymatic adenylation of the 3′ Linker adaptor with the Mth RNA ligase in the 5′-Adenylation Kit sold by New England Biolabs, which nearly quantitatively adds an adenylated moiety to the 5′ end of a monophosphorylated oligonucleotide.
Although gel-purified RNAs from passive elution in NaCl is typically concentrated by ethanol precipitation with glycogen carrier in siliconized tubes, for very small samples we have observed decreased loss of RNA by using the RNA Clean & Concentrator kit from Zymo Research, following the manufacturer’s protocol for total RNA (>17 nt). We also recommend increasing the volume of ethanol 3X for the mix in the first two steps of the standard manufacturer’s protocol. Elutions are with 20 μl of dH2O.
The 5′ Linker adaptor ligation reaction components and conditions remain the same as previous protocols except the use of the new barcoded 5′ Linker adaptors for the Hi-Seq sequencer. When one has included the new chimeric RNA/DNA markers of 18 and 34 nt length with internal deoxy-uracils as spiked-in radiolabeled markers to facilitate visualizing the shift of ligated products via gel-electrophorese and phosphorimaging, there is the optional post-reaction step of adding 5 units of uracil DNA glycosylase to the 5′ Linker ligation reaction and incubating for 20 minutes at 37°C.
Reverse transcription (RT) with Superscript III reverse transcriptase and PCR with Phusion polymerase can be directly performed from ¼ or ½ of the 5′ Linker ligation reaction without the need of purifying the ligated molecules from unligated molecules, following the same protocol as previous methods (4, 5, 15–18). However, a common problem in amplifying a library that began from minute starting RNA is that linker-linker dimers can dominate in the final PCR, regardless of whether one performs or skips the gel purification of the ligated products after the 5′ Linker adapter ligation reaction. By reducing the number of PCR cycles (no more than 15 cycles) in the initial amplification rounds from the RT reaction, this PCR sample can be gel purified on a 4% low melting temperature agarose gel for the desired 95 to 115 nt long amplicons from the linker-linker dimer that is ~80 nt long.
From this purification, an additional 15 cycles of PCR can be applied to generate enough material for small scale cloning with a standard PCR cloning vector like in the Zero-Blunt TOPO PCR cloning kit followed by Sanger sequencing verification. Purified PCR-amplified DNA can be suitably quantitated for concentration with the Quant-iT Pico-green dsDNA assay kit.
4. NOTES AND COMMENTARY
-
4.1
Female flies that have been fattened up with yeast prior to ovary dissection yield larger egg chambers and facilitate the manual dissection of ovaries, which in turn enhances the harvest of follicle cells. Our enrichment procedure significantly depletes the oocytes and nurse cells in the Drosophila egg chamber, presumably from loss of structural integrity after trypsinization, however all cell-cycle stages of follicle cells are isolated in our procedure. For more extensive purifications of specific staged follicle cells, see (19, 20).
-
4.2
Although sequencing chemistry for the Illumina platform continues to improve in providing longer read lengths to over 100 nt, the fidelity and quality of reading the first couple of bases extending from a sequencing or indexing primer are still considerably higher than readings towards the end of the molecule. Thus, we have chosen to place our linker barcode sequence at the first set of bases to be read by the small RNA sequencing primer #1, in contrast to other methodologies that place the barcode sequence in the 3′ Linker (12, 13).
On the Illumina Genome Analyzer, the platform uses approximately 8 bases of read information to fine tune the assign and distinguish the “molecule colonies” on the flow cell lane, and our original 4 base barcode was suitable for multiplexing. However, the Hi-Seq platform only utilizes the initial 4–6 bases, and thus finds the original 4 base barcode too low in complexity to distinguish colonies, thus drastically reducing the theoretical numbers of quality passing reads by 3 fold (Fig. 1F).
-
4.3
The boronate affinity gel was first described as a means to resolve and detect modifications on transfer RNAs (9). These authors noted the limitation of the boronate gel in terms of overloading the capacity of the gel and the maximum resolving abilities for shorter RNAs. In our experiments, we found the maximum capacity of a 1 mm thick APB gel with a 1cm wide well was 2 μg total RNA after which the retention of unmodified RNAs was skewed. When dealing with minute tissue samples, however, total RNA is typically limiting, such as where a single follicle cell enriched sample might typically yield 5 μg of total RNA.
References
- 1.Lau NC, Lim LP, Weinstein EG, Bartel DP. Science. 2001;294:858–62. doi: 10.1126/science.1065062. [DOI] [PubMed] [Google Scholar]
- 2.Lee RC, Ambros V. Science. 2001;294:862–4. doi: 10.1126/science.1065329. [DOI] [PubMed] [Google Scholar]
- 3.Lagos-Quintana M, Rauhut R, Lendeckel W, Tuschl T. Science. 2001;294:853–8. doi: 10.1126/science.1064921. [DOI] [PubMed] [Google Scholar]
- 4.Lau NC, Robine N, Martin R, Chung WJ, Niki Y, Berezikov E, Lai EC. Genome Res. 2009;19:1776–85. doi: 10.1101/gr.094896.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lau NC, Ohsumi T, Borowsky M, Kingston RE, Blower MD. EMBO J. 2009;28:2945–58. doi: 10.1038/emboj.2009.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lau NC. Curr Protoc Mol Biol. 2008;Chapter 26(Unit 26):7. doi: 10.1002/0471142727.mb2607s81. [DOI] [PubMed] [Google Scholar]
- 7.Saito K, Sakaguchi Y, Suzuki T, Siomi H, Siomi MC. Genes Dev. 2007;21:1603–8. doi: 10.1101/gad.1563607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horwich MD, Li C, Matranga C, Vagin V, Farley G, Wang P, Zamore PD. Curr Biol. 2007;17:1265–72. doi: 10.1016/j.cub.2007.06.030. [DOI] [PubMed] [Google Scholar]
- 9.Igloi GL, Kossel H. Methods Enzymol. 1987;155:433–48. doi: 10.1016/0076-6879(87)55029-7. [DOI] [PubMed] [Google Scholar]
- 10.Babiarz JE, Ruby JG, Wang Y, Bartel DP, Blelloch R. Genes Dev. 2008;22:2773–85. doi: 10.1101/gad.1705308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiang HR, Schoenfeld LW, Ruby JG, Auyeung VC, Spies N, Baek D, Johnston WK, Russ C, Luo S, Babiarz JE, Blelloch R, Schroth GP, Nusbaum C, Bartel DP. Genes Dev. 2010;24:992–1009. doi: 10.1101/gad.1884710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hafner M, Landgraf P, Ludwig J, Rice A, Ojo T, Lin C, Holoch D, Lim C, Tuschl T. Methods. 2008;44:3–12. doi: 10.1016/j.ymeth.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hafner M, Renwick N, Farazi TA, Mihailovic A, Pena JT, Tuschl T. Methods. 2012 doi: 10.1016/j.ymeth.2012.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruby JG, Stark A, Johnston WK, Kellis M, Bartel DP, Lai EC. Genome Res. 2007;17:1850–64. doi: 10.1101/gr.6597907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ro S, Yan W. Methods Mol Biol. 2010;629:273–85. doi: 10.1007/978-1-60761-657-3_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thomas MF, Ansel KM. Methods Mol Biol. 2010;667:93–111. doi: 10.1007/978-1-60761-811-9_7. [DOI] [PubMed] [Google Scholar]
- 17.Havecker ER. Methods Mol Biol. 2011;732:55–68. doi: 10.1007/978-1-61779-083-6_5. [DOI] [PubMed] [Google Scholar]
- 18.Donovan WP, Zhang Y, Howell MD. Methods Mol Biol. 2011;744:159–73. doi: 10.1007/978-1-61779-123-9_11. [DOI] [PubMed] [Google Scholar]
- 19.Claycomb JM, Benasutti M, Bosco G, Fenger DD, Orr-Weaver TL. Dev Cell. 2004;6:145–55. doi: 10.1016/s1534-5807(03)00398-8. [DOI] [PubMed] [Google Scholar]
- 20.Bryant Z, Subrahmanyan L, Tworoger M, LaTray L, Liu CR, Li MJ, van den Engh G, Ruohola-Baker H. Proc Natl Acad Sci U S A. 1999;96:5559–64. doi: 10.1073/pnas.96.10.5559. [DOI] [PMC free article] [PubMed] [Google Scholar]