Abstract
Early life exposures during times of rapid growth and development are recognized increasingly to impact later life. Epidemiologic studies document an association between exposures at critical windows of susceptibility with outcomes as diverse as childhood and adult obesity, timing of menarche, and risk for hypertension or breast cancer. This paper briefly reviews the theoretical bases for these relationships, utilizing the paradigms of pubertal change and breast cancer and of biobehavioral susceptibility to environmental influences. The long-term sequella of responses to early exposures may impact other adult morbidities and addressing these exposures represent an important challenge for contemporary medicine.
Keywords: developmental plasticity, thrifty phenotype, windows of susceptibility, puberty, obesity
Over the past several decades there has been an increasing awareness that early life events may shape developmental trajectories and thereby impact later health (1). For example, adult diseases, such as breast cancer and ischemic heart disease, are believed to have origins in the early stages of life, and in recent years the study of breast cancer etiology has moved towards studying events during childhood (2). Adolescence has received little attention, despite important behavioral, cognitive, and physical developmental changes that occur during this period. In this rapidly evolving area of study, several frameworks have been forwarded to explain these findings, incorporating diverse disciplines and perspectives, and which impact physical and mental health issues at the individual as well as public health level. These models are not mutually exclusive, yet often emphasize a specific perspective on antecedents or outcomes. This manuscript will review briefly the literature that explores the factors in early life that impact the physiologic changes associated with puberty and how these influence adult morbidity, using breast cancer as a paradigm.
Birth weight perhaps has been the most studied early life factor impacting later health. Both lower and higher birth weight, compared to normal, appear to have implications for short- and long-term outcomes across the life course. The impact of fetal undernutrition has been recognized for several decades. Observations of three cohorts-children born during the Dutch famine of 1944, born within the Hertfordshire district between 1911 and 1930, and selected from the Helsinki Birth Cohort of 1934–44, have led researchers to note the association between small size at birth and during infancy and later increased morbidity and mortality. The increased rates of adverse health outcomes included those for coronary heart disease (3–7) ; stroke (7,8); insulin resistance (9); and type-2 diabetes mellitus (10); adiposity (11,12), especially visceral fat distribution (13); metabolic syndrome (associated with both low birth weight and maternal obesity (14); and osteoporosis (15). The increased rates led some early researchers to hypothesize the “thrifty phenotype” (10), often called the “Barker hypothesis,” as described below.
Nutritional excess during pregnancy has also been linked to adverse outcomes from childhood through adulthood, especially for development of obesity and type 2 diabetes (16). Studies found that maternal triglyceride levels were associated with newborn weight (17) and that the strongest prenatal predictor of pediatric overweight and adiposity is maternal BMI (18). Studies also found positive associations between birth size and cord IGF-1 levels (19,20), as well as cord leptin levels (20), and between birth weight with adolescent height and lower age of menarche (21,22).
Developmental plasticity
Observations of the association between higher infant death rates and adult coronary artery disease in contemporary peers who survived infancy (23) and between infant birth weights and insulin resistance (10) led Barker and colleagues to develop the “thrifty phenotype” hypothesis. That is, the prenatal environment has limited nutritional resources and would lead to metabolic changes, such as insulin resistance, enhanced energy storage, and decreased nephron number, to enhance postnatal success in an anticipated energy-limited environment. However, the infant encounters an imbalance occurs between the prenatal and postnatal environment, with sufficient or even excess caloric exposure, leading to adverse consequences. This is described as a “programmed” effect that results from a permanent or long-term change in structure or function through metabolic imprinting and/or epigenetic changes, acting at critical period of early life. This concept was incorporated into developmental plasticity, defined as variations in developmental pathways that are triggered by environmental events during sensitive periods in development (24), which others call critical windows of sensitivity (25) (or windows of susceptibility). These windows typically occur during periods of rapid growth (25). Several different models have been proposed to explain these findings. The theoretical frameworks include, among others, thrifty genotype (26) as well as thrifty phenotype (10); developmental plasticity (24); ecobiodevelopmental framework (27); life history theory (28); adaptive calibration model; and developmental origins of adult disease (DOAD) (29). A similar perspective is the predictive adaptive response, which is a response to an environmental factor that may not be of immediate benefit but made in expectation of a future environment (30); environment could include not only in utero factors, but also postnatal psychosocial, nutritional, or chemical exposures. These responses carry costs, as suggested by life history theory (28,31); increased allocation of resources to brain growth or energy storage would reduce resources for other traits, such as tissue repair processes. These adaptations are considered the basis of the adverse consequences of fetal undernutrition and maternal overnutrition, leading to the fetal origins of adult disease (32). As discussed below, the adaptations in structure and function are long-term or permanent, and there is increasing evidence that epigenetic mechanisms may be responsible, prompting some to suggest that, rather than a thrifty genotype (26) or thrifty phenotype (10), the underlying mechanism is the thrifty epigenotype, incorporating both hypotheses through proposing epigenetic variations to enhance energy storage and utilization (33). These hypotheses resulted in a renewed interest in exposures that occur at periods of increased susceptibility, such as during fetal development and puberty. For example, Barker noted the relationship between small-for-gestational-age (SGA) status and greater prevalence of adult hypertension (4). Brenner suggested that SGA status may be associated with decreased nephron number (34) and subsequent risk of hypertension. Zendi-Nejad and colleagues reviewed the role of fetal programming on adult hypertension and kidney disease and suggested several explanations, including epigenetic changes, increased apoptosis in the fetal kidney, increased exposure to fetal glucocorticoids, and alterations in the renin-angiotensin system (35).
Pubertal milestones and relative timing of puberty
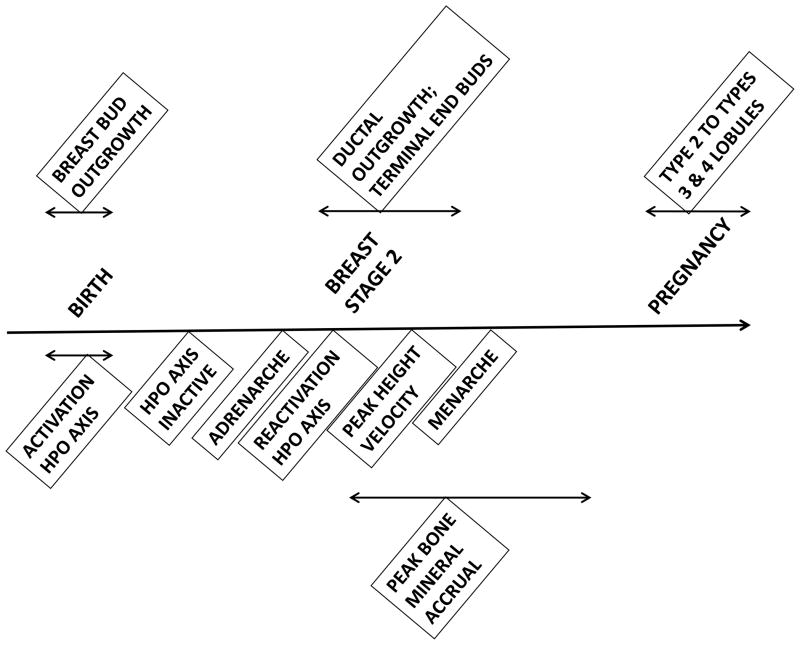
Puberty represents an important developmental window of vulnerability to environmental exposures. Puberty is a time of rapid and profound change, including (re)activation of the hypothalamic-pituitary-gonadal (HPG) and hypothalamic-pituitary-adrenal axes; an acceleration in height velocity and achievement of the pubertal peak height velocity; changes in body composition; the development of secondary sexual characteristics; and the achievement of fertility. The temporal relationships between these events are shown in Figure 1, and compared to timing of breast development. During puberty, there is rapid expansion and differentiation of breast stem cells, as discussed below, which occurs contemporaneously with reactivation of the hypothalamic-pituitary-ovarian axis, the onset of the pubertal growth spurt, and the time of maximal accrual of bone mineral content. The temporal relationships between these factors may suggest shared or underlying biologic mechanisms discussed below.
FIGURE 1.
Pubertal milestones and breast development
The timing of puberty may serve as a sensitive indicator of environmental change (36). There is a four year variability in onset of puberty in girls (37). It is estimated that 61%–75 % of the variation in age at menarche, which is correlated to onset of puberty, is attributable to direct or additive genetic effects (38,39). Recent reviews reported that there are 42 loci associated with timing of puberty but noted that these loci make a small contribution(3.6–6.1%) to the variability of age at onset (40,41).. Greater BMI during childhood is associated with earlier age at menarche (42,43); this association may be related to greater levels of leptin reflecting sufficient energy stores (44) or other mechanisms associated with visceral adiposity (45,46). Parent et al. reviewed other factors that could impact variability in timing of puberty: they include genetic factors and intrauterine environment, as noted above; nutritional intake; climatic exposures; light-dark cycle; and exposure to endocrine-disrupting chemicals (47).
Psychosocial factors and puberty
In addition to metabolic and biologic exposures, studies have linked timing of puberty in girls to adversity in the psychosocial realm. Consistent with evolutionary life history theory, Belsky and colleagues (48) posited that when girls encountered conditions that were not favorable for survival (i.e., environmental stressors), it was generally adaptive for them to become reproductively mature at earlier ages (48). Empirical evidence has confirmed that childhood adversity, accelerates girls’ pubertal development (49). In countries that have adequate nutrition, such as the United States, lower socioeconomic status has been associated with earlier menarche, although effects may vary depending on race/ethnicity (50). In addition, harsh-conflictual family dynamics and poor parent-child attachment predict earlier maturation, while warm and supportive family conditions forecast later puberty among girls (51–54). The absence of a biological father also has been associated with earlier pubertal timing, such that girls with no father in the home prepubertally are about twice as likely to experience menarche prior to 12 years old than those with a father present (52,54–58). Studies of stepfathers have yielded inconsistent findings, and there is no evidence to suggest that a mother’s absence influences puberty; however, the presence of siblings may play a role in delaying menarche (55,59).
The role that prepubertal BMI may play in mediating associations between adverse family factors and girls’ pubertal development is somewhat unclear,. Some studies suggest that the influence of father absence on girls’ pubertal development is mediated by BMI (60) while other studies do not note BMI as a mediator (56,61). Future research is needed to further explore whether BMI or other measures of body composition (e.g., visceral adiposity) may help explain associations between adverse family factors and girls’ pubertal timing.
Pubertal events and breast cancer
Recently, attention has been turned toward the associations between early life events, pubertal changes, and risk for breast cancer in adulthood. Although exposures across the life span have been linked to breast cancer risk (2), the mechanisms underlying the relationship remain unclear. During puberty, mammary growth occurs through exponential cellular proliferation and differentiation, suggesting a stem-like cell with regenerative capacity (62), and the mammary gland undergoes extensive changes. Primary ducts grow and divide with formation of terminal end buds, which further divide into smaller alveolar buds and form the lobule type 1 unit. There is additional growth and differentiation into lobules 2 and 3 throughout puberty and into adulthood (Figure 1). Of note, breast epithelium exhibits maximal proliferative activity during the luteal phase of the menstrual cycle, and the highest level of cell proliferation is observed in undifferentiated lobule type 1 (63). Full differentiation into lobule type 4 occurs as a result of pregnancy, with permanent alterations in gene expression pattern. These pregnancy-associated changes have been hypothesized to result in cells that are more refractory to environmental exposures (64,65), unlike the earlier progenitor cells that are believed to be the cellular target for potential carcinogens and is proposed as the mechanism underlying decreased risk for breast cancer with earlier age at first full-term pregnancy (66). For example, local girls 19 years or younger when the Nagasaki and Hiroshima atom bombs were dropped were more likely than local adults to develop breast cancer (67), suggesting an increased susceptibility for younger women to the effects of radiation.
There are several epidemiologic associations between pubertal events and risk of breast cancer, including age of menarche, growth factors (height and height velocity) and bone mineral density. Epidemiologic studies support up to 30% increased risk with younger age at menarche (2,68–72). A pooled analysis reported that for each year that age of menarche was delayed, the risk of premenopausal breast cancer was reduced by 9%, and risk of postmenopausal breast cancer was reduced by 4% (68). Menarche is one of the most well-established risk factors for breast cancer, in part because the age at which menarche occurred can be recalled years later (73). Young age at onset of menarche is associated with young age at onset of breast development and with young age during the pubertal growth spurt. Young age during the pubertal growth spurt is associated with greater growth velocity (74). Of note, obese and tall children have greater levels of IGF-1 in response to growth hormone than do short and normal-weight children (75), and IGF-1 may mediate the relationship between menarche and breast cancer. A recent study noted that the age at menarche was associated with risk of breast cancer, but not when age at peak growth was included in the analysis: this study found that risk for breast cancer increased 11% for every 5 cm increase in adult height (76). Similarly, if a woman reached her maximum height at or before age 12 years, her risk of breast cancer increased by 1.4 (77). Several studies documented the relationship between greater bone mineral density and later development of breast cancer (78–82). Of note, the majority of bone mineral content is deposited during the teenage years, peaking shortly after the age at peak height velocity (83).
With regard to the concept of “windows of susceptibility,” important factors may expand the window, or lead to more intense exposures. For example, early maturation leads to longer duration of puberty and to a greater peak height velocity. That is, early age at onset of puberty is associated with longer interval between onset of puberty and menarche (84–86) and therefore longer time for completion of puberty (84), with an increased risk for perturbation during cell proliferation and differentiation. Similarly, early age at onset of puberty is associated with greater height velocity (and IGF-1 levels) (84,87); and greater IGF-1 levels are associated with greater premenopausal breast density (88,89), another factor associated with risk of breast cancer. In addition, the risk of breast cancer (and several other cancers) increases with greater height (90), with a 1.17 increased risk for every 10 cm adult height. As noted earlier, greater BMI is associated with earlier maturation in girls, and earlier maturation is associated with higher BMI and increased risk of obesity. An analysis from the Bogalusa Heart Study found that greater childhood BMI was associated with earlier pubertal onset, and earlier puberty with greater BMI as an adult, but the relationship suggested that childhood BMI was the major factor (91). A recent review suggests how IGF-1 might impact risk of cancer (89): the action of IGF-I promotes tumor growth and mitosis, inhibits apoptosis, and induces endothelial growth factor. Multiple reviews discuss the association of obesity and several different cancers (92), and as well as the underlying mechanisms for this association, which include pro-inflammatory cytokines (93).
It is important to consider a life course approach in the care of the adolescent. There is limited evidence, however, for interventions to decrease risk of later breast cancer in children and adolescents. Multiple studies have documented an increased risk of breast cancer with exposure to ionizing radiation during childhood and adolescence, both from survivors of nuclear bombs (94), as well as lower dose exposures (95), including serial radiographs for scoliosis, especially with a family history of breast cancer (96). These studies would suggest that minimizing children and adolescents to ionizing radiation exposure would be beneficial. Another potential exposure is alcohol; in a review of dietary factors and breast cancer, alcohol was the only consistent factor associated with increased risk (97), perhaps mediated through the association of alcohol with increased breast density. Although the literature is somewhat inconsistent, soy intake may be beneficial, with some studies citing intake during childhood (98), or during adulthood (99). Physical activity levels may also be protective, and a case-control study noted that physical activity at ages 14–20 years decreased risk of breast cancer (100). The relationship between hormonal contraceptives and breast cancer is controversial, although risk in younger users with BRCA1 and BRCA2 mutations may be increased modestly (101).
This paper provides a brief review of a rapidly evolving field of inquiry, the developmental basis of adult disease, with an emphasis on the concepts of developmental plasticity and windows of susceptibility. We have focused on one adult disease, breast cancer, to discuss how changes during puberty may be particularly important for later disease. This is especially relevant for health providers of adolescents, who are familiar with a life course model of health, but may be unaware of studies from early life research, or on adult outcomes. Early life factors may impact pubertal changes and predisposition to specific adult morbidities and mortality. The long-term changes may be mediated through epigenetic changes, as well as or resulting in, structural and functional changes to organs and body systems, and are implemented by the developing organism to enhance survival. The long-term sequella of these responses to early exposures may apply to several other adult morbidities and addressing these exposures represent an important challenge for contemporary medicine.
Supplementary Material
Implications and Contributions.
This paper provides an introduction for the concept of windows of susceptibility for health care providers with adolescent patients. It examines the developmental basis for associating early life exposures with adult disorders, utilizing the paradigms of pubertal change and breast cancer and of biobehavioral susceptibility to environmental influences.
Acknowledgments
Supported, in part, by U01 ES-12770 (NCI and NIEHS), and UL1 RR026314 (USPHS); U01 ES019453 and U01 ES012801 (NCI and NIEHS)
Contributor Information
Frank M. Biro, Email: frank.biro@cchmc.org, Rauh Professor, Department of Pediatrics, University of Cincinnati College of Medicine, Director, Division of Adolescent Medicine, Cincinnati Children’s Hospital Medical Center, 3333 Burnet Avenue (ML-4000), Cincinnati OH 45229, P: 513 636-8580, F: 513 636-1129.
Julianna Deardorff, Email: jdeardorff@berkeley.edu, Assistant Professor, Maternal and Child Health Program, King Sweesy and Robert Womack Endowed Chair in Medical Science & Public Health, School of Public Health, 207K University Hall, University of California, Berkeley, California 94720-7360, P: 510 642-7334, F: 510 643-6426.
References
- 1.Godfrey KM, Gluckman PD, Hanson MA. Developmental origins of metabolic disease: Life course and intergenerational perspectives. Trends Endocrinol Metab. 2010;21:199–205. doi: 10.1016/j.tem.2009.12.008. http://dx.doi.org/10.1016/j.tem.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 2.Okasha M, McCarron P, Gunnell D, et al. Exposures in childhood, adolescence and early adulthood and breast cancer risk: a systematic review of the literature. Breast Cancer Res Treat. 2003;78:223–76. doi: 10.1023/A:1022988918755. [DOI] [PubMed] [Google Scholar]
- 3.Andersen LG, Ängquist L, Eriksson JG, et al. Birth weight, childhood body mass index and risk of coronary heart disease in adults: Combined historical cohort studies. PLoS One. 2010;5:e14126. doi: 10.1371/journal.pone.0014126. http://dx.doi.org/10.1016/j.tem.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barker DJP, Osmond C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet. 1986;327:1077–81. doi: 10.1016/s0140-6736(86)91340-1. http://dx.doi.org/10.1016/S0140-6736(86)91340-1. [DOI] [PubMed] [Google Scholar]
- 5.Leon DA, Lithell HO, Vågerö D, et al. Reduced fetal growth rate and increased risk of death from ischaemic heart disease: Cohort study of 15,000 Swedish men and women born 1915–29. BMJ. 1998;317:241–5. doi: 10.1136/bmj.317.7153.241. http://dx.doi.org/10.1136/bmj.317.7153.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Osmond C, Barker D, Winter P, et al. Early growth and death from cardiovascular disease in women. BMJ. 1993;307:1519–24. doi: 10.1136/bmj.307.6918.1519. http://dx.doi.org/10.1136/bmj.307.6918.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rich-Edwards JW, Stampfer MJ, Manson JAE, et al. Birth weight and risk of cardiovascular disease in a cohort of women followed up since 1976. BMJ. 1997;315:396–400. doi: 10.1136/bmj.315.7105.396. http://dx.doi.org/10.1136/bmj.315.7105.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frankel S, Elwood P, Smith GD, et al. Birthweight, body-mass index in middle age, and incident coronary heart disease. Lancet. 1996;348:1478–80. doi: 10.1016/S0140-6736(96)03482-4. http://dx.doi.org/10.1016/S0140-6736(96)03482-4. [DOI] [PubMed] [Google Scholar]
- 9.Fabricius-Bjerre S, Jensen RB, Færch K, et al. Impact of birth weight and early infant weight gain on insulin resistance and associated cardiovascular risk factors in adoelscence. PLoS One. 2011;6:e20595. doi: 10.1371/journal.pone.0020595. Epub 2011 Jun 2. http://dx.doi.org/10.1371/journal.pone.0020595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hales CN, Barker DJP. Type 2 (non-insulin-dependent) diabetes mellitus: The thrifty phenotype hypothesis. Diabetologia. 1992;35:595–601. doi: 10.1007/BF00400248. http://dx.doi.org/10.1007/BF00400248. [DOI] [PubMed] [Google Scholar]
- 11.Kensara OA, Wootton SA, Phillips DI, et al. Fetal programming of body composition: Relation between birth weight and body composition measured with dual-energy X-ray absorptiometry and anthropometric methods in older Englishmen. Am J Clin Nutr. 2005;82:980–7. doi: 10.1093/ajcn/82.5.980. [DOI] [PubMed] [Google Scholar]
- 12.Meas T, Deghmoun S, Armoogum P, et al. Consequences of being born small for gestational age on body composition: An 8-year follow-up study. J Clin Endocrinol Metab. 2008;93:3804–9. doi: 10.1210/jc.2008-0488. http://dx.doi.org/10.1210/jc.2008-0488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rolfe EDL, Loos RJF, Druet C, et al. Association between birth weight and visceral fat in adults. Am J Clin Nutr. 2010;92:347–52. doi: 10.3945/ajcn.2010.29247. http://dx.doi.org/10.3945/ajcn.2010.29247. [DOI] [PubMed] [Google Scholar]
- 14.Boney CM, Verma A, Tucker R, et al. Metabolic syndrome in childhood: Association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics. 2005;115:e290–6. doi: 10.1542/peds.2004-1808. http://dx.doi.org/10.1542/peds.2004-1808. [DOI] [PubMed] [Google Scholar]
- 15.Cooper C, Fall C, Egger P, et al. Growth in infancy and bone mass in later life. Ann Rheum Dis. 1997;56:17–21. doi: 10.1136/ard.56.1.17. http://dx.doi.org/10.1136/ard.56.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heerwagen MJR, Miller MR, Barbour LA, et al. Maternal obesity and fetal metabolic programming: A fertile epigenetic soil. Am J Physiol Regul Integr Comp Physiol. 2010;299:R711–22. doi: 10.1152/ajpregu.00310.2010. http://dx.doi.org/10.1152/ajpregu.00310.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Di Cianni G, Miccoli R, Volpe L, et al. Maternal triglyceride levels and newborn weight in pregnant women with normal glucose tolerance. Diabetic Med. 2005;22:21–5. doi: 10.1111/j.1464-5491.2004.01336.x. http://dx.doi.org/10.1111/j.1464-5491.2004.01336.x. [DOI] [PubMed] [Google Scholar]
- 18.Catalano PM, Farrell K, Thomas A, et al. Perinatal risk factors for childhood obesity and metabolic dysregulation. Am J Clin Nutr. 2009;90:1303–13. doi: 10.3945/ajcn.2008.27416. http://dx.doi.org/10.3945/ajcn.2008.27416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boyne MS, Thame M, Bennett FI, et al. The relationship among circulating insulin-like growth factor (IGF)-I, IGF-binding proteins-1 and -2, and birth anthropometry: A prospective study. J Clin Endocrinol Metab. 2003;88:1687. doi: 10.1210/jc.2002-020633. http://dx.doi.org/10.1210/jc.2002-020633. [DOI] [PubMed] [Google Scholar]
- 20.Vatten LJ, Nilsen ST, Odegård RA, et al. Insulin-like growth factor I and leptin in umbilical cord plasma and infant birth size at term. Pediatrics. 2002;109:1131–5. doi: 10.1542/peds.109.6.1131. http://dx.doi.org/10.1542/peds.109.6.1131. [DOI] [PubMed] [Google Scholar]
- 21.Romundstad PR, Vatten LJ, Nilsen TIL, et al. Birth size in relation to age at menarche and adolescent body size: Implications for breast cancer risk. Int J Cancer. 2003;105:400–3. doi: 10.1002/ijc.11103. http://dx.doi.org/10.1002/ijc.11103. [DOI] [PubMed] [Google Scholar]
- 22.Öberg S, Cnattingius S, Sandin S, et al. Birth weight-breast cancer revisited: Is the association confounded by familial factors? Cancer Epidemiol Biomarkers Prev. 2009;18:2447–52. doi: 10.1158/1055-9965.EPI-09-0123. http://dx.doi.org/10.1158/1055-9965.EPI-09-0123. [DOI] [PubMed] [Google Scholar]
- 23.Barker DJ, Winter PD, Osmond C, et al. Weight in infancy and death from ischaemic heart disease. Lancet. 1989;2:577–80. doi: 10.1016/s0140-6736(89)90710-1. http://dx.doi.org/10.1016/S0140-6736(89)90710-1. [DOI] [PubMed] [Google Scholar]
- 24.Bateson P, Barker D, Clutton-Brock T, et al. Developmental plasticity and human health. Nature. 2004;430:419–21. doi: 10.1038/nature02725. http://dx.doi.org/10.1038/nature02725. [DOI] [PubMed] [Google Scholar]
- 25.Selevan SG, Kimmel CA, Mendola P. Identifying critical windows of exposure for children’s health. Environ Health Perspect. 2000;108:451–5. doi: 10.1289/ehp.00108s3451. http://dx.doi.org/10.1289/ehp.00108s3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neel JV. Diabetes mellitus: A “thrifty” genotype rendered detrimental by “progress”? Am J Hum Genet. 1962;14:353–62. [PMC free article] [PubMed] [Google Scholar]
- 27.Shonkoff JP. Building a new biodevelopmental framework to guide the future of early childhood policy. Child Development. 2010;81:357–67. doi: 10.1111/j.1467-8624.2009.01399.x. [DOI] [PubMed] [Google Scholar]
- 28.Stearns SC. Life history evolution: Successes, limitations, and prospects. Naturwissenschaften. 2000;87:476–86. doi: 10.1007/s001140050763. http://dx.doi.org/10.1007/s001140050763. [DOI] [PubMed] [Google Scholar]
- 29.Barker DJP. The developmental origins of adult disease. J Amer Coll Nutr. 2004;23:588S–95S. doi: 10.1080/07315724.2004.10719428. [DOI] [PubMed] [Google Scholar]
- 30.Gluckman PD, Hanson MA. Developmental origins of disease paradigm: A mechanistic and evolutionary perspective. Pediatr Res. 2004;56:311–7. doi: 10.1203/01.PDR.0000135998.08025.FB. http://dx.doi.org/10.1203/01.PDR.0000135998.08025.FB. [DOI] [PubMed] [Google Scholar]
- 31.de Boo HA, Harding JE. The developmental origins of adult disease (Barker) hypothesis. Aust N Z J Obstet Gynaecol. 2006;46:4–14. doi: 10.1111/j.1479-828X.2006.00506.x. http://dx.doi.org/10.1111/j.1479-828X.2006.00506.x. [DOI] [PubMed] [Google Scholar]
- 32.Barker DJP, Eriksson JG, Forsen T, et al. Fetal origins of adult disease: Strength of effects and biological basis. Int J Epidemiol. 2002;31:1235–9. doi: 10.1093/ije/31.6.1235. http://dx.doi.org/10.1093/ije/31.6.1235. [DOI] [PubMed] [Google Scholar]
- 33.Stöger R. The thrifty epigenotype: An acquired and heritable predisposition for obesity and diabetes? Bioessays. 2008;30:156–66. doi: 10.1002/bies.20700. http://dx.doi.org/10.1002/bies.20700. [DOI] [PubMed] [Google Scholar]
- 34.Brenner B, Garcia D, Anderson S. Glomeruli and blood pressure. Less of one, more the other? Am J Hypertens. 1988;1:335–47. doi: 10.1093/ajh/1.4.335. [DOI] [PubMed] [Google Scholar]
- 35.Zandi-Nejad K, Luyckx VA, Brenner BM. Adult hypertension and kidney disease. Hypertension. 2006;47:502–8. doi: 10.1161/01.HYP.0000198544.09909.1a. http://dx.doi.org/10.1161/01.HYP.0000198544.09909.1a. [DOI] [PubMed] [Google Scholar]
- 36.Buck Louis GM, Gray LEJ, Marcus M, et al. Environmental factors and puberty timing: Expert panel research needs. Pediatrics. 2008;121:S192–207. doi: 10.1542/peds.1813E. http://dx.doi.org/10.1542/peds.1813E. [DOI] [PubMed] [Google Scholar]
- 37.Herman-Giddens ME, Slora EJ, Wasserman RC, et al. Secondary sexual characteristics and menses in young girls seen in office practice: A study from the Pediatric Research in Office Settings Network. Pediatrics. 1997;99:505–12. doi: 10.1542/peds.99.4.505. http://dx.doi.org/10.1542/peds.99.4.505. [DOI] [PubMed] [Google Scholar]
- 38.Kaprio J, Rimpelä A, Winter T, et al. Common genetic influences on BMI and age at menarche. Hum Biol. 1995;67:739–53. [PubMed] [Google Scholar]
- 39.Treloar SA, Martin NG. Age at menarche as a fitness trait: Nonadditive genetic variance detected in a large twin sample. Am J Hum Genet. 1990;47:137–48. [PMC free article] [PubMed] [Google Scholar]
- 40.Elks CE, Perry JR, Sulem P, et al. Thirty new loci for age at menarche identified by a meta-analysis of genome-wide association studies. Nat Genet. 2010;42:1077–85. doi: 10.1038/ng.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.He C, Murabito JM. Genome-wide association studies of age at menarche and age at natural menopause. Molec Cell Endocrinol. 2012 doi: 10.1016/j.mce.2012.05.003. doi.org/10.1016/j.mce.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 42.Freedman DS, Khan LK, Serdula MK, et al. Relation of age at menarche to race, time period, and anthropometric dimensions: The Bogalusa Heart Study. Pediatrics. 2002;110:e43. doi: 10.1542/peds.110.4.e43. http://dx.doi.org/10.1542/peds.110.4.e43. [DOI] [PubMed] [Google Scholar]
- 43.Kaplowitz PB. Link between body fat and the timing of puberty. Pediatrics. 2008;121:S208–17. doi: 10.1542/peds.2007-1813F. http://dx.doi.org/10.1542/peds.2007-1813F. [DOI] [PubMed] [Google Scholar]
- 44.Burt Solorzano CM, McCartney CR. Obesity and the pubertal transition in girls and boys. Reproduction. 2010;140:399–410. doi: 10.1530/REP-10-0119. http://dx.doi.org/10.1530/REP-10-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ahmed ML, Ong KK, Dunger DB. Childhood obesity and the timing of puberty. Trends Endocrinol Metabol. 2009;20:237–42. doi: 10.1016/j.tem.2009.02.004. http://dx.doi.org/10.1016/j.tem.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 46.Jasik CB, Lustig RH. Adolescent obesity and puberty: The “perfect storm”. Ann N Y Acad Sci. 2008;1135:265–79. doi: 10.1196/annals.1429.009. http://dx.doi.org/10.1196/annals.1429.009. [DOI] [PubMed] [Google Scholar]
- 47.Parent AS, Teilmann G, Juul A, et al. The timing of normal puberty and the age limits of sexual precocity: Variations around the world, secular trends, and changes after migration. Endocr Rev. 2003;24:668–93. doi: 10.1210/er.2002-0019. http://dx.doi.org/10.1210/er.2002-0019. [DOI] [PubMed] [Google Scholar]
- 48.Belsky J, Steinberg L, Draper P. Childhood experience, interpersonal development, and reproductive strategy: An evolutionary theory of socialization. Child Dev. 1991;62:647–70. doi: 10.1111/j.1467-8624.1991.tb01558.x. http://dx.doi.org/10.2307/1131166. [DOI] [PubMed] [Google Scholar]
- 49.Ellis BJ. Timing of pubertal maturation in girls: An integrated life history approach. Psychol Bull. 2004;130:920–58. doi: 10.1037/0033-2909.130.6.920. http://dx.doi.org/10.1037/0033-2909.130.6.920. [DOI] [PubMed] [Google Scholar]
- 50.Braithwaite D, Moore DH, Lustig RH, et al. Socioeconomic status in relation to early menarche among black and white girls. Cancer Causes Control. 2009;20:713–20. doi: 10.1007/s10552-008-9284-9. http://dx.doi.org/10.1007/s10552-008-9284-9. [DOI] [PubMed] [Google Scholar]
- 51.Belsky J, Steinberg L, Houts RM, et al. The development of reproductive strategy in females: Early maternal harshness --> earlier menarche --> increased sexual risk taking. Dev Psychol. 2010;46:120–8. doi: 10.1037/a0015549. http://dx.doi.org/10.1037/a0015549. [DOI] [PubMed] [Google Scholar]
- 52.Ellis BJ, McFadyen-Ketchum S, Dodge KA, et al. Quality of early family relationships and individual differences in the timing of pubertal maturation in girls: A longitudinal test of an evolutionary model. J Pers Soc Psychol. 1999;77:387. doi: 10.1037//0022-3514.77.2.387. http://dx.doi.org/10.1037/0022-3514.77.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saxbe DE, Repetti RL. Brief report: Fathers’ and mothers’ marital relationship predicts daughters’ pubertal development two years later. J Adolesc. 2009;32:415–23. doi: 10.1016/j.adolescence.2008.06.009. http://dx.doi.org/10.1016/j.adolescence.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 54.Tither JM, Ellis BJ. Impact of fathers on daughters’ age at menarche: A genetically and environmentally controlled sibling study. Dev Psychol. 2008;44:1409–20. doi: 10.1037/a0013065. http://dx.doi.org/10.1037/a0013065. [DOI] [PubMed] [Google Scholar]
- 55.Bogaert AF. Age at puberty and father absence in a national probability sample. J Adolesc. 2005;28:541–6. doi: 10.1016/j.adolescence.2004.10.008. http://dx.doi.org/10.1016/j.adolescence.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 56.Deardorff J, Ekwaru JP, Kushi LH, et al. Father absence, body mass index, and pubertal timing in girls: Differential effects by family income and ethnicity. J Adolesc Health. 2011;48:441–7. doi: 10.1016/j.jadohealth.2010.07.032. http://dx.doi.org/10.1016/j.jadohealth.2010.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Quinlan RJ. Father absence, parental care, and female reproductive development. Evol Hum Behav. 2003;24:376–90. http://dx.doi.org/10.1016/S1090-5138(03)00039-4. [Google Scholar]
- 58.Romans S, Martin J, Gendall K, et al. Age of menarche: The role of some psychosocial factors. Psychol Med. 2003;33:933–9. doi: 10.1017/s0033291703007530. http://dx.doi.org/10.1017/S0033291703007530. [DOI] [PubMed] [Google Scholar]
- 59.Milne FH, Judge DS. Brothers delay menarche and the onset of sexual activity in their sisters. Proc Biol Sci. 2011;278:417–23. doi: 10.1098/rspb.2010.1377. http://dx.doi.org/10.1098/rspb.2010.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ellis BJ, Essex MJ. Family environments, adrenarche, and sexual maturation: a longitudinal test of a life history model. Child Dev. 2007;78:1799–1817. doi: 10.1111/j.1467-8624.2007.01092.x. [DOI] [PubMed] [Google Scholar]
- 61.Bogaert AF. Menarche and father absence in a national probability sample. J Biosoc Sci. 2008;40:623–36. doi: 10.1017/S0021932007002386. http://dx.doi.org/10.1017/S0021932007002386. [DOI] [PubMed] [Google Scholar]
- 62.Visvader JE. Keeping abreast of the mammary epithelial hierarchy and breast tumorigenesis. Genes Dev. 2009;23:2563–77. doi: 10.1101/gad.1849509. http://dx.doi.org/10.1101/gad.1849509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Russo J, Hu YF, Yang X, et al. Developmental, cellular, and molecular basis of human breast cancer. J Natl Cancer Inst Monogr. 2000;27:17–37. doi: 10.1093/oxfordjournals.jncimonographs.a024241. [DOI] [PubMed] [Google Scholar]
- 64.Russo J, Mailo D, Hu YF, et al. Breast differentiation and its implication in cancer prevention. Clin Cancer Res. 2005;11:931s–6s. [PubMed] [Google Scholar]
- 65.Russo J, Moral R, Balogh GA, et al. The protective role of pregnancy in breast cancer. Breast Cancer Res. 2005;7:131–42. doi: 10.1186/bcr1029. http://dx.doi.org/10.1186/bcr1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Reya T, Morrison SJ, Clarke MF, et al. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–11. doi: 10.1038/35102167. http://dx.doi.org/10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 67.Land CE, Tokunaga M, Koyama K, et al. Incidence of female breast cancer among atomic bomb survivors, Hiroshima and Nagasaki, 1950–1990. Radiat Res. 2003;160:707–17. doi: 10.1667/rr3082. http://dx.doi.org/10.1667/RR3082. [DOI] [PubMed] [Google Scholar]
- 68.Clavel-Chapelon F. Differential effects of reproductive factors on the risk of pre- and postmenopausal breast cancer. Results from a large cohort of French women. Brit J Cancer. 2002;86:723–7. doi: 10.1038/sj.bjc.6600124. http://dx.doi.org/10.1038/sj.bjc.6600124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Garland M, Hunter DJ, Colditz GA, et al. Menstrual cycle characteristics and history of ovulatory infertility in relation to breast cancer risk in a large cohort of US women. Am J Epidemiol. 1998;147:636–43. doi: 10.1093/oxfordjournals.aje.a009504. [DOI] [PubMed] [Google Scholar]
- 70.Kelsey JL, Gammon MD, John EM. Reproductive factors and breast cancer. Epidemiol Rev. 1993;15:36–47. doi: 10.1093/oxfordjournals.epirev.a036115. [DOI] [PubMed] [Google Scholar]
- 71.Peeters PHM, Verbeek ALM, Krol A, et al. Age at menarche and breast cancer risk in nulliparous women. Breast Cancer Res Treat. 1995;33:55–61. doi: 10.1007/BF00666071. http://dx.doi.org/10.1007/BF00666071. [DOI] [PubMed] [Google Scholar]
- 72.Petridou E, Syrigou E, Toupadaki N, et al. Determinants of age at menarche as early life predictors of breast cancer risk. Int J Cancer. 1996;68:193–8. doi: 10.1002/(SICI)1097-0215(19961009)68:2<193::AID-IJC9>3.0.CO;2-T. http://dx.doi.org/10.1002/(SICI)1097-0215(19961009)68:2<193::AID-IJC9>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 73.Cooper R, Blell M, Hardy R, et al. Validity of age at menarche self-reported in adulthood. J Epidemiol Community Health. 2006;60:993–7. doi: 10.1136/jech.2005.043182. http://dx.doi.org/10.1136/jech.2005.043182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Biro FM, McMahon RP, Striegel-Moore R, et al. Impact of timing of pubertal maturation on growth in black and white female adolescents: The National Heart, Lung, and Blood Institute Growth and Health Study. J Pediatr. 2001;138:636–43. doi: 10.1067/mpd.2001.114476. http://dx.doi.org/10.1067/mpd.2001.114476. [DOI] [PubMed] [Google Scholar]
- 75.Bouhours-Nouet N, Gatelais F, Boux de Casson F, et al. The insulin-like growth factor-I response to growth hormone is increased in prepubertal children with obesity and tall stature. J Clin Endocrinol Metab. 2007;92:629–35. doi: 10.1210/jc.2005-2631. http://dx.doi.org/10.1210/jc.2005-2631. [DOI] [PubMed] [Google Scholar]
- 76.Ahlgren M, Melbye M, Wohlfahrt J, et al. Growth patterns and the risk of breast cancer in women. Int J Gynecol Cancer. 2006;16 (Suppl 2):569–75. doi: 10.1111/j.1525-1438.2006.00698.x. http://dx.doi.org/10.1111/j.1525-1438.2006.00698.x. [DOI] [PubMed] [Google Scholar]
- 77.Li CI, Littman AJ, White E. Relationship between age maximum height is attained, age at menarche, and age at first full-term birth and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2007;16:2144–9. doi: 10.1158/1055-9965.EPI-07-0242. http://dx.doi.org/10.1158/1055-9965.EPI-07-0242. [DOI] [PubMed] [Google Scholar]
- 78.Cauley JA, Lucas FL, Kuller LH, et al. Bone mineral density and risk of breast cancer in older women: The Study of Osteoporotic Fractures Research Group. JAMA. 1996;276:1404–8. http://dx.doi.org/10.1001/jama.1996.03540170048031. [PubMed] [Google Scholar]
- 79.Hadji P, Gottschalk M, Ziller V, et al. Bone mass and the risk of breast cancer: The influence of cumulative exposure to oestrogen and reproductive correlates. Results of the Marburg breast cancer and osteoporosis trial (MABOT) Maturitas. 2007;56:312–21. doi: 10.1016/j.maturitas.2006.09.005. http://dx.doi.org/10.1016/j.maturitas.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 80.Kalder M, Jäger C, Seker-Pektas B, et al. Breast cancer and bone mineral density: The Marburg Breast Cancer and Osteoporosis Trial (MABOT II) Climacteric. 2011:1–10. doi: 10.3109/13697137.2011.557754. [DOI] [PubMed] [Google Scholar]
- 81.Kuller LH, Cauley JA, Lucas L, et al. Sex steroid hormones, bone mineral density, and risk of breast cancer. Environ Health Perspect. 1997;105:593–9. doi: 10.1289/ehp.97105s3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang Y, Kiel DP, Kreger BE, et al. Bone mass and the risk of breast cancer among postmenopausal women. N Engl J Med. 1997;336:611–7. doi: 10.1056/NEJM199702273360903. http://dx.doi.org/10.1056/NEJM199702273360903. [DOI] [PubMed] [Google Scholar]
- 83.Baxter-Jones ADG, Faulkner RA, Forwood MR, et al. Bone mineral accrual from 8 to 30 years of age: An estimation of peak bone mass. J Bone Miner Res. 2011;26:1729–39. doi: 10.1002/jbmr.412. http://dx.doi.org/10.1002/jbmr.412. [DOI] [PubMed] [Google Scholar]
- 84.Biro FM, Huang B, Crawford PB, et al. Pubertal correlates in black and white girls. J Pediatr. 2006;148:234–40. doi: 10.1016/j.jpeds.2005.10.020. http://dx.doi.org/10.1016/j.jpeds.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 85.de Ridder CM, Thijssen JH, Bruning PF, et al. Body fat mass, body fat distribution, and pubertal development: A longitudinal study of physical and hormonal sexual maturation of girls. J Clin Endocrinol Metab. 1992;75:442–6. doi: 10.1210/jcem.75.2.1639945. http://dx.doi.org/10.1210/jc.75.2.442. [DOI] [PubMed] [Google Scholar]
- 86.Marti-Henneberg C, Vizmanos B. The duration of puberty in girls is related to the timing of its onset. J Pediatr. 1997;131:618–21. doi: 10.1016/s0022-3476(97)70073-8. http://dx.doi.org/10.1016/S0022-3476(97)70073-8. [DOI] [PubMed] [Google Scholar]
- 87.Sandhu J, Smith GD, Holly J, et al. Timing of puberty determines serum insulin-like growth factor-I in late adulthood. J Clin Endocrinol Metab. 2006;91:3150–7. doi: 10.1210/jc.2005-2318. http://dx.doi.org/10.1210/jc.2005-2318. [DOI] [PubMed] [Google Scholar]
- 88.Byrne C, Colditz GA, Willett WC, et al. Plasma insulin-like growth factor (IGF) I, IGF-binding protein 3, and mammographic density. Cancer Res. 2000;60:3744–8. [PubMed] [Google Scholar]
- 89.Renehan AG, Zwahlen M, Minder C, et al. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: Systematic review and meta-regression analysis. Lancet. 2004;363:1346–53. doi: 10.1016/S0140-6736(04)16044-3. http://dx.doi.org/10.1016/S0140-6736(04)16044-3. [DOI] [PubMed] [Google Scholar]
- 90.Green J, Cairns BJ, Casabonne D, et al. Height and cancer incidence in the Million Women Study: Prospective cohort, and meta-analysis of prospective studies of height and total cancer risk. Lancet Oncol. 2011;12:785–94. doi: 10.1016/S1470-2045(11)70154-1. http://dx.doi.org/10.1016/S1470-2045(11)70154-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Freedman D, Khan L, Serdula M, et al. The relation of menarcheal age to obesity in childhood and adulthood: The Bogalusa heart study. BMC Pediatrics. 2003;3:3. doi: 10.1186/1471-2431-3-3. http://dx.doi.org/10.1186/1471-2431-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Renehan AG, Tyson M, Egger M, et al. Body-mass index and incidence of cancer: A systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–78. doi: 10.1016/S0140-6736(08)60269-X. http://dx.doi.org/10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 93.Lorincz AM, Sukumar S. Molecular links between obesity and breast cancer. Endocr Relat Cancer. 2006;13:279–92. doi: 10.1677/erc.1.00729. http://dx.doi.org/10.1677/erc.1.00729. [DOI] [PubMed] [Google Scholar]
- 94.Tokunaga M, Norman JE, Jr, Asano M, et al. Malignant breast tumors among atomic bomb survivors, Hiroshima and Nagasaki, 1950–74. J Natl Cancer Inst. 1979;62:1347–1359. [PubMed] [Google Scholar]
- 95.Shore RE. Low-dose radiation epidemiology studies: status and issues. Health Phys. 2009;97:481–86. doi: 10.1097/HP.0b013e3181ab98d9. [DOI] [PubMed] [Google Scholar]
- 96.Ronckers CM, Doody MM, Lonstein JE, et al. Multiple diagnostic X-rays for spine deformities and risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2008;17:605–13. doi: 10.1158/1058/1055-9965.EPI-07-2628. [DOI] [PubMed] [Google Scholar]
- 97.Michels KB, Mohllajee AP, Roset-Bahmanyar E, et al. Diet and breast cancer: A review of the prospective observational studies. Cancer. 2007;109:2712–49. doi: 10.1002/cncr.22654. [DOI] [PubMed] [Google Scholar]
- 98.Korde LA, Wu AH, Fears T, et al. Childhood soy intake and breast cancer risk in Asian American women. Cancer Epidemiol Biomarkers Prev. 2009;18:1050–59. doi: 10.1158/1055-9965.EPI-08-0405. [DOI] [PubMed] [Google Scholar]
- 99.Lee SA, Shu XO, Li H, et al. Adolescent and adult soy food intake and breast cancer risk: results from the Shanghai Women’s Health Study. Am J Clin Nutr. 2009;89:1920–26. doi: 10.3945/ajcn.2008.27361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kruk J. Lifetime physical activity and the risk of breast cancer: A case-control study. Cancer Detect Prev. 2007;31:18–28. doi: 10.1016/j.cdp.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 101.Brohet RM, Goldgar DE, Easton DF, et al. Oral contraceptives and breast cancer risk in the international BRCA1/2 carrier cohort study: a report from EMBRACE, GENEPSO, GEO-HEBON, and the IBCCS Collaborating Group. J Clin Oncol. 2007;25:3831–36. doi: 10.1200/JCO.2007.11.1179. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.