Abstract
Objective
The knowledge that one carries the apolipoprotein E (APOE) ε4 allele risk factor for Alzheimer’s disease was recently found to have little short-term psychological risk. The authors investigated the impact of knowledge of carrying the risk allele on subjective ratings of memory and objective memory test performance of older adults.
Method
Using a nested case-control design, the authors administered objective verbal and visual memory tests and self-rating scales of memory function to 144 cognitively normal older adults (ages 52–89) with known APOE genotype who knew (ε4+, N=25; ε4−, N=49) or did not know (ε4+, N=25; ε4−, N=45) their genotype and genetic risk for Alzheimer’s disease prior to neuropsychological evaluation.
Results
Significant genotype-by-disclosure interaction effects were observed on several memory rating scales and tests of immediate and delayed verbal recall. Older adults who knew their ε4+ genotype judged their memory more harshly and performed worse on an objective verbal memory test than did ε4+ adults who did not know. In contrast, older adults who knew their ε4− genotype judged their memory more positively than did ε4− adults who did not know, but these groups did not differ in objective memory test performance.
Conclusions
Informing older adults that they have an APOE genotype associated with an increased risk of Alzheimer’s disease can have adverse consequences on their perception of their memory abilities and their performance on objective memory tests. The patient’s knowledge of his or her genotype and risk of Alzheimer’s disease should be considered when evaluating cognition in the elderly.
The ε4 allele of the apolipoprotein E (APOE) gene located on chromosome 19 is the single most important susceptibility gene for dementia and Alzheimer’s disease (1–3). Recent research and debate have focused on the risks, benefits, and general ethics of disclosing APOE genotype to older adults. Results of a recent study (4) suggest that disclosure has few adverse emotional risks. Groups of asymptomatic older adults with a living or deceased parent with Alzheimer’s disease who were randomly assigned to a disclosure group informed of their APOE gene status or a non-disclosure group who did not receive this information did not differ in levels of depression or anxiety during a year of follow-up. This was true regardless of whether disclosure revealed ε4+ or ε4− gene status. Despite the relatively low emotional impact of APOE genotype disclosure, some argue that few benefits are to be gained by informing asymptomatic adults that they may be at risk for a disease that cannot be prevented and for which available treatments offer only limited utility (5).
A question that has not been addressed is that of how knowledge of one’s APOE genotype might affect subjective self-judgment of memory functioning. Because the devastating impact of Alzheimer’s disease on the ability to remember is widely known, older adults who know they have a genetic risk for the disease might be more likely to have lower subjective ratings of their memory than those who do not have the risk or do not know their genotype. Consistent with this possibility, studies have shown that older adults with a positive family history of Alzheimer’s disease rate their memory functioning lower than those without a family history (6, 7); however, participants in these studies were aware of their family history, so the impact of being at risk as opposed to the knowledge of that risk cannot be separated. No studies have been conducted of self-ratings of memory and knowledge of APOE gene status, but one recent study (8) found no difference in subjective memory judgments made by ε4+ versus ε4− participants who were unaware of their genotype.
It is also possible that knowledge of genetic risk for Alzheimer’s disease could influence objective memory performance. Knowledge of possession of a characteristic associated with poor cognitive performance can lead to lowered self-efficacy beliefs (i.e., belief in one’s capability to produce a given level of performance), which may result in underperformance on objective memory tests as a result of low confidence, reduced effort, or lack of perseverance (9). Studies have shown, for example, that activation of negative stereotypes about aging lead to decreased self-efficacy beliefs related to memory ability and decreased memory test performance in older adults (10; see also reference 11). Conversely, higher self-efficacy regarding memory ability is associated with better verbal memory test performance in elderly men (12). These results suggest that memory test performance might be altered in normal elderly individuals to the extent that knowledge of APOE genotype leads them to question or to have confidence in their memory ability.
To address these issues, we obtained subjective memory ratings and tested memory performance in groups of cognitively normal older adults with known APOE genotype who were either informed (with appropriate genetic counseling) or not informed of their genotype prior to memory evaluation. We hypothesized that older adults with knowledge of their ε4+ status would judge their memory more harshly and have worse objective memory test performance than those without that knowledge. In contrast, older adults with knowledge of their ε4− status would judge their memory more positively and have better objective memory test performance than those without that knowledge.
Method
Participants
Participants were neurologically intact adults 52 to 89 years of age (N=144). They were recruited through media advertisements and public lectures calling for cognitively normal older volunteers. Individuals with a positive history of alcoholism, drug abuse, learning disability, or serious neurological or psychiatric illness were excluded. All participants were tested with a global mental status scale, the Dementia Rating Scale (13), and those who scored less than 130 out of 144 were excluded (see reference 14).
Genetic Testing and Disclosure
Participants were genotyped for APOE using a polymerase chain reaction-based method (2). Fifty participants (34.7%) were ε4+ (ε2/ε4, N=4; ε3/ε4, N=43; ε4/ε4, N=3) and 94 (65.3%) were ε4− (ε2/ε2, N=1; ε2/ε3, N=19; ε3/ε3, N=74).
The study was carried out in two phases using a nested case-control design. In the first phase, APOE genotype was disclosed, after the informed consent process, to 74 participants (the “informed” group) who were enrolled in a study of cognitive and neuroimaging changes associated with normal aging (N=70) or in a pilot study of the impact of genetic screening on anxiety, health care utilization, and implementation of advance directives for health care (N=4). At the time of enrollment, these participants were told that APOE genotyping would be performed and that they would be informed of the results unless they chose not to be informed. Fifteen participants enrolled in this manner chose not to be informed. Disclosure was performed by an experienced genetic counselor (a licensed social worker or psychologist), who informed the participant of his or her APOE genotype. Counseling included information about the level of risk incurred from having a particular genotype, placing this risk in perspective by comparing it with other well-known medical risks, answering questions about Alzheimer’s disease and genetic risk, describing the limitations of APOE genotype testing, and dealing with any psychological or emotional distress related to the test results. Systematic counseling occurred only at the time of disclosure, although additional counseling was offered if needed. None of the participants requested additional counseling. The subjective and objective memory evaluation that is the focus of the present study followed genotype disclosure by an average of 8.19 months (SD=5.78, range=1–24).
In the second phase, a group of 70 participants who had never been informed of their APOE genotype was recruited to match the informed group on age, years of education, Dementia Rating Scale score, and APOE genotype distribution. These participants were recruited either into the study of normal aging described above at a point when genotype was no longer disclosed (N=16; genotype disclosure was stopped for all participants in this study toward the end of recruitment) or as healthy control participants at the University of California, San Diego (UCSD) Alzheimer’s Disease Research Center (ADRC), where genotype was never routinely disclosed (N=39). At the time of enrollment, these participants were told that APOE genotyping would be performed and that they would not be informed of the results. The 15 participants from the study of normal aging who had chosen not to be informed were included in this group. Thus, approximately half of the APOE ε4+ and ε4− participants knew their gene status at the time memory was evaluated, resulting in four groups that were compared on the outcome measures (see Table 1).
TABLE 1.
Demographic and Clinical Characteristics of Participants With (ε4+) or Without (ε4−) the APOE ε4 Allele Who Knew (Informed) or Did Not Know (Not Informed) Their APOE Genotypea
| Participants Without APOE ε4 Allele (ε4−) | Participants With APOE ε4 Allele (ε4+) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Not Informed (N=45) | Informed (N=49) | Not Informed (N=25) | Informed (N=25) | ||||||||
| N | Mean | SD | N | Mean | SD | N | Mean | SD | N | Mean | SD | |
| Age (years) | 45 | 75.0 | 6.7 | 49 | 72.9 | 5.1 | 25 | 72.1 | 8.3 | 25 | 72.6 | 5.9 |
| Education (years) | 45 | 15.9 | 2.9 | 49 | 15.2 | 2.2 | 25 | 15.8 | 2.5 | 25 | 16.0 | 2.4 |
| Mini-Mental State Examination | 26 | 29.6 | 0.7 | 49 | 29.2 | 0.8 | 20 | 29.5 | 0.7 | 25 | 29.4 | 0.7 |
| Dementia Rating Scale | 45 | 140.4 | 2.8 | 49 | 140.7 | 2.9 | 25 | 141.4 | 2.0 | 25 | 140.6 | 3.1 |
| Geriatric Depression Scale | 44 | 5.0 | 4.8 | 48 | 4.6 | 4.2 | 23 | 3.5 | 4.3 | 23 | 3.3 | 2.9 |
| N | % | N | % | N | % | N | % | |||||
| Male | 23 | 51 | 20 | 41 | 10 | 40 | 11 | 44 | ||||
No significant differences between groups on any variable.
The inclusion and exclusion criteria were identical for the normal aging study and the ADRC healthy control group. The normal aging study, the small pilot study, and the ADRC study were each reviewed and approved by the UCSD institutional review board. In all cases, informed consent was obtained from each participant prior to enrollment after the study had been fully described to them.
Procedure
Participants completed widely used and sensitive objective tests of verbal and visual memory, two validated and well-standardized scales for subjective self-assessment of memory functioning, and the Geriatric Depression Scale (15). Memory testing was carried out in a quiet, well-lit room by a trained psychometrist blind to participants’ genotype and knowledge of that genotype. Participants completed the subjective memory assessment scales at home and returned them by mail.
Objective memory tests
The logical memory subtest of the Wechsler Memory Scale–Revised (16) requires participants to remember two brief stories that are read aloud to them. Recall is assessed immediately after each story is read and again after a 30-minute delay. Each story consists of 25 ideas, and the number of ideas correctly recalled is scored for a possible total of 50 points. Immediate and delayed recall are scored separately. The Rey-Osterrieth Complex Figure Test (17) requires participants to copy a complex abstract line drawing as precisely as possible, then to immediately reproduce the figure from memory. After a 30-minute delay filled with unrelated verbal activity, participants again draw the figure from memory. The immediate and delayed recall drawings are scored for the presence and accuracy of each element of the complex figure (18). The total possible score for each drawing is 20.
Subjective memory scales
The short form of the Metamemory in Adulthood Questionnaire (19) includes 15 items that ask participants to rate their current everyday memory abilities on 5-point Likert scales (1=never, 5=always). Items collapse into two scales evaluating participants’ subjective impressions of their memory ability in specific domains (capacity) and of changes in their memory ability across time (change). Higher scores on these scales indicate, respectively, better subjective memory and less self-perceived decline in memory across time.
The short form of the Memory Functioning Questionnaire (20, 21) contains 46 items that are each rated on a 7-point Likert scale (1=never, 7=always). Items collapse into five primary scales that assess subjective ratings of current compared with past memory functioning (retrospective functioning); how often problems arise in specific memory situations (frequency of forgetting); how often memory problems occur while reading books and newspapers (forgetting when reading); skill at recalling past events (past events); and how often external memory aids are relied upon to aid memory (mnemonics usage). Across all scales, items tally such that higher scores correspond to better subjective memory functioning.
Statistical Analysis
Univariate one-way analysis of variance (ANOVA) was used to compare age, years of education, and mental status test scores across the four groups of participants. Post hoc pairwise group comparisons were made with Student’s t tests as necessary. Gender distribution was compared across the four groups using the chi-square test. Two-way multivariate ANOVA (MANOVA) was used to examine the effects of genotype and disclosure status on the seven subjective rating measures. Because some participants inadvertently failed to answer one or more items on one or more subjective scales, complete data for all seven of the subjective measures used in the MANOVA were available for 122 of the 144 participants. Follow-up two-way (genotype-by-disclosure status) ANOVAs were carried out on the individual subjective measures that showed significant effects in the MANOVA. Post hoc pairwise group comparisons were made with Student’s t tests. Separate two-way ANOVAs were used to examine the effects of genotype and disclosure status on the four objective memory test measures. Post hoc pairwise group comparisons were made with Student’s t tests. Because groups were matched, by design, on age, level of education, and mental status scores and did not significantly differ on these factors, these variables were not covaried in the MANOVA or ANOVA tests. Nor was the interval between disclosure and objective and subjective memory testing, since this was only relevant for the informed group and there were no significant correlations between the duration of the interval and any of the objective or subjective memory scores.
Partial eta-squared (ηp2) was used to measure effect sizes when analyses used ANOVA, and Cohen’s d when analyses used the t test. Previous studies comparing cognitively normal young and older individuals (e.g., reference 22) or cognitively normal older individuals and those with mild cognitive impairment (e.g., references 23, 24) produced effect sizes of 0.80 or greater on the objective and subjective memory tests used in the present study. A sample of approximately 25 participants per group provides a power of 0.80 to detect these or similar effect sizes with p<0.05 (two-tailed); thus, alpha was set at 0.05 (two-tailed). Analyses were conducted with SPSS 19.0 (IBM, Armonk, N.Y.).
Because of experimenter error, 30 participants were not tested with the logical memory subtest. The participant groups that completed this test contained 23 APOE ε4+ participants who knew their genotype status and 23 who did not, and 30 ε4− participants who knew their gene status and 38 who did not. All analyses were repeated with only these 114 participants. The groups remained similar (i.e., not significantly different) on demographic and mental status variables, and the pattern of results on the genotype-by-disclosure status MANOVA and ANOVA tests for the subjective and objective memory measures was identical to that of the full groups. Therefore, only the results obtained with the full groups are presented.
Results
Demographic and Clinical Comparisons
The participant groups did not differ significantly in age, years of education, Mini-Mental State Examination (MMSE) score, or Dementia Rating Scale score (Table 1). There was no difference in gender distribution among the groups. A genotype-by-disclosure status between-groups ANOVA of scores on the Geriatric Depression Scale revealed no significant effect of genotype or disclosure status, and no genotype-by-disclosure status interaction effect.
Subjective Memory Comparisons
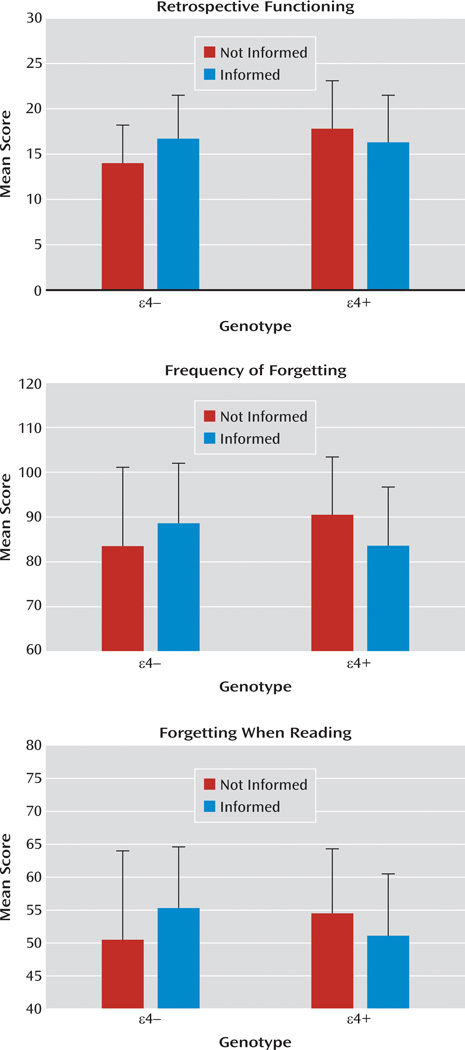
Memory self-rating scores are summarized in Table 2. A genotype-by-disclosure status MANOVA that included all seven subjective memory measures as dependent variables revealed no significant effect of genotype or disclosure status, but a significant genotype-by-disclosure status interaction effect (F=2.7, df=7,112, p<0.05). Follow-up ANOVAs with each subjective memory measure revealed significant genotype-by-disclosure status interaction effects for the capacity scale of the Metamemory in Adulthood Questionnaire (F=9.3, df=1, 137, p<0.01; ηp2=0.064) and for the retrospective functioning scale (F=6.2, df=1, 137, p=0.01; ηp2=0.043), frequency of forgetting scale (F=4.5, df=1, 131, p<0.05; ηp2=0.033), and forgetting when reading scale (F=6.9, df=1, 131, p<0.05; ηp2=0.050) of the Memory Functioning Questionnaire (Figures 1 and 2). Participants who knew they were ε4− rated their memory functioning higher than those who did not know they were ε4− on the Metamemory in Adulthood Questionnaire capacity scale (t=2.31, df=90, p=0.023; d=0.48) and on the Memory Functioning Questionnaire retrospective functioning scale (t=2.81, df=89, p=0.006; d=0.59) and forgetting when reading scale (t=2.08, df=85, p=0.041; d=0.44). Participants who knew they were ε4+ rated their memory worse on the capacity scale than those who did not know they were ε4+ (t=2.03, df=47, p=0.048; d=0.58). These subgroups did not differ on other subjective memory rating scales. Comparisons limited to subgroups who did not know their genotype showed that ε4+ participants rated their memory better than ε4−participants on the retrospective functioning scale (t=3.24, df=65, p=0.002; d=0.79) and the capacity scale (t=2.20, df=65, p=0.03; d=0.58), but not on the frequency of forgetting and forgetting when reading scales.
TABLE 2.
Scores on the Metamemory in Adulthood Questionnaire and Memory Functioning Questionnaire and on Verbal and Visual Memory Tests for Participants With (ε4+) or Without (ε4−) the APOE ε4 Allele Who Knew (Informed) or Did Not Know (Not Informed) Their APOE Genotype
| Participants Without APOE ε4 Allele (ε4−) | Participants With APOE ε4 Allele (ε4+) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Not Informed (N=45) | Informed (N=49) | Not Informed (N=25) | Informed (N=25) | |||||||||
| Measure | N | Mean | SD | N | Mean | SD | N | Mean | SD | N | Mean | SD |
| Metamemory in Adulthood Questionnaire | ||||||||||||
| Capacitya | 44 | 27.8 | 6.5 | 48 | 30.5 | 4.7 | 24 | 31.2 | 5.2 | 25 | 28.0 | 6.4 |
| Change | 44 | 15.9 | 4.9 | 49 | 16.7 | 4.1 | 24 | 17.0 | 5.4 | 25 | 16.9 | 5.5 |
| Memory Functioning Questionnaire | ||||||||||||
| Retrospective functioninga | 42 | 14.3 | 4.1 | 49 | 16.7 | 4.7 | 25 | 18.3 | 5.8 | 25 | 15.9 | 4.7 |
| Frequency of forgettinga | 43 | 84.5 | 17.0 | 44 | 88.9 | 13.4 | 24 | 90.5 | 14.7 | 24 | 83.4 | 13.3 |
| Forgetting when readinga | 41 | 50.5 | 13.1 | 46 | 55.5 | 8.8 | 24 | 54.9 | 8.4 | 24 | 50.7 | 9.3 |
| Past events | 45 | 18.1 | 4.9 | 49 | 19.9 | 4.5 | 23 | 19.2 | 4.6 | 25 | 18.3 | 4.3 |
| Mnemonics usage | 44 | 22.8 | 9.5 | 48 | 25.1 | 8.4 | 24 | 23.1 | 11.4 | 25 | 20.3 | 7.4 |
| Verbal memory tests | ||||||||||||
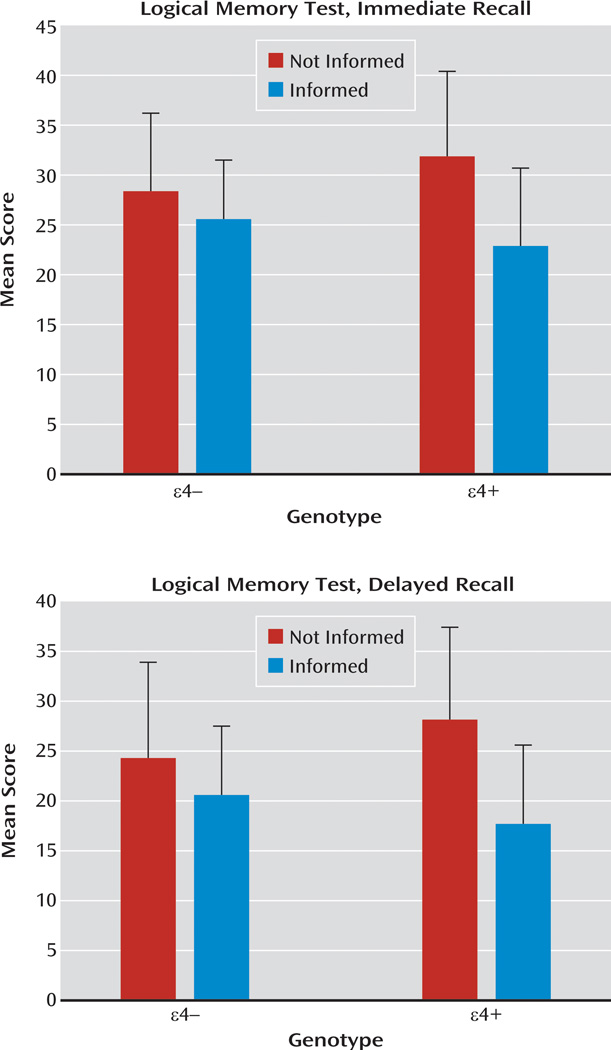
| Logical memory subtest, immediate recalla | 38 | 28.4 | 7.8 | 30 | 25.6 | 5.9 | 23 | 31.9 | 8.5 | 23 | 22.9 | 7.8 |
| Logical memory subtest, delayed recalla | 38 | 24.3 | 9.6 | 30 | 20.6 | 6.9 | 23 | 28.1 | 9.3 | 23 | 17.7 | 7.9 |
| Visual memory tests | ||||||||||||
| Rey-Osterrieth Complex Figure Test, immediate recall | 45 | 9.3 | 3.7 | 49 | 9.7 | 3.1 | 25 | 9.7 | 2.7 | 25 | 10.6 | 2.6 |
| Rey-Osterrieth Complex Figure Test, delayed recall | 45 | 9.0 | 4.0 | 49 | 9.9 | 2.8 | 25 | 9.0 | 3.1 | 25 | 10.1 | 2.4 |
Significantly different between groups, p<0.05.
FIGURE 1.
Scores on the Capacity Scale of the Metamemory in Adulthood Questionnaire for Participants With (ε4+) or Without (ε42) the APOE ε4 Allele Who Knew (Informed) or Did Not Know (Not Informed) Their APOE Genotypea
a Error bars represent standard deviation.
FIGURE 2.
Memory Functioning Questionnaire Scores for Participants With (ε4+) or Without (ε42) the APOE ε4 Allele Who Knew (Informed) or Did Not Know (Not Informed) Their APOE Genotypea
a Error bars represent standard deviation.
Objective Memory Comparisons
The mean scores achieved on the objective memory tests are listed in Table 2. Separate genotype-by-disclosure status between-groups ANOVAs of scores for the immediate and delayed conditions of the Rey-Osterrieth Complex Figure Test revealed no significant main effects or interaction effects. In contrast, genotype-by-disclosure status between-groups ANOVAs of scores for the logical memory subtest revealed a significant main effect of disclosure status for immediate (F=16.9, df=1, 110, p<0.001; ηp2=0.133) and delayed (F=18.7, df=1, 110, p<0.001; ηp2=0.145) recall, no significant main effects of genotype, and significant genotype-by-disclosure status interaction effects for both immediate (F=4.7, df=1, 110, p<0.05; ηp2=0.041) and delayed (F=4.3, df=1, 110, p<0.05; ηp2=0.037) recall (Figure 3). Participants who knew they were ε4+ performed significantly worse than those who did not know they were ε4+ in both immediate (t=3.76, df=44, p=0.001; d=1.11) and delayed (t=4.11, df=44, p<0.001; d=0.80) recall, whereas recall did not differ significantly in ε4−participants who knew or did not know their genotype. There were no significant differences in immediate or delayed recall scores achieved by ε4+ and ε4− participants who did not know their genotype.
FIGURE 3.
Scores on Immediate and Delayed Recall From the Logical Memory Subtest for Participants With (ε4+) or Without (ε42) the APOE ε4 Allele Who Knew (Informed) or Did Not Know (Not Informed) Their APOE Genotypea
a Error bars represent standard deviation.
Discussion
Our findings indicate that disclosure of an APOE genotype associated with an increased risk of Alzheimer’s disease to cognitively normal older adults can have adverse consequences on subjective self-ratings of their memory abilities and on their performance on objective tests of memory. Consistent with our hypotheses, older adults with knowledge of their APOE ε4+ status tended to judge their memory more harshly and performed worse on an objective verbal memory test than did ε4+ adults without that knowledge. On the other hand, older adults with knowledge of their APOE ε4− status judged their memory more positively than did those who did not know that they were ε4−, although these two groups did not differ in objective memory test performance. This interaction was apparent despite the fact that there was no significant difference in the objective memory test performance of ε4+ and ε4− individuals who did not know their genotype. Furthermore, ε4+ individuals who did not know their genotype had higher, not lower, memory self-ratings on some scales (e.g., the Memory Functioning Questionnaire retrospective functioning scale) than did ε4−individuals who did not know their genotype. The lack of differences (or differences in the unexpected direction) in memory performance or ratings in those who did not know their genotype supports the contention that the different patterns observed in ε4+ and ε4− participants who knew their genotype was related to that knowledge. The main effect of disclosure on objective memory test performance occurred because knowledge of being ε4− did not enhance objective memory performance, unlike the enhancing effect this knowledge had on subjective memory ratings. However, the main effect of disclosure should not affect the expression of the observed interaction effect of genotype by disclosure status, since performance on the memory test was not near floor or ceiling levels in any of the four groups.
Consistent with the results of Green et al. (4), we found no significant differences in scores on the Geriatric Depression Scale among the ε4+ and ε4−participants who knew or did not know their genotype. This does not rule out the possibility that poorer performance and self-ratings could be related to greater anxiety or test-related distress in those who knew they were ε4+, but neither of these psychological conditions was elevated by knowledge of an APOE ε4+ genotype in a previous study (4). The different patterns of response to knowledge of APOE genotype in ε4+ and ε4− individuals cannot be attributed to differences in demographic characteristics, since the participant groups did not differ significantly in age, level of education, or gender distribution, nor to differences in overall cognitive ability (i.e., MMSE, Dementia Rating Scale scores).
Worse performance on objective memory tests in ε4+ older adults who knew their genotype compared with those who did not know could be related to lower self-efficacy beliefs regarding memory in those with knowledge that they are at increased risk for Alzheimer’s disease. Previous studies have shown that memory performance of older adults is related to their beliefs about their memory abilities (11, 12, 25) or can be influenced by manipulating those beliefs (10). It may be the case that those who knew they were at risk for Alzheimer’s disease had lowered expectations that resulted in reduced persistence and effort in performing memory tasks (26) or a reduced allocation of time to memory tasks that were perceived as difficult (27). These possibilities are consistent with the pattern of subjective memory ratings we observed and with the fact that Metamemory in Adulthood Questionnaire capacity scale ratings were significantly correlated with immediate (r=0.27; p=0.004) and delayed (r=0.29; p=0.002) logical memory subtest scores across the entire sample, regardless of actual genotype or knowledge of genotype.
Although knowledge of having a genotype associated with an increased risk of Alzheimer’s disease may adversely affect older adults’ beliefs about their memory abilities and their performance on certain memory tasks, these effects might be reduced by psychological interventions. A number of studies have shown that negative stereotypes regarding cognitive abilities can be overcome to improve performance (10, 28, 29). Interventions designed to mitigate negative self-perception of cognitive abilities (10) might be particularly useful in reducing the adverse effects of knowledge of APOE ε4+ genotype in older individuals.
Several limitations of this study should be noted. First, the study design did not include random assignment of participants to disclosure or non-disclosure groups. This could introduce bias (e.g., confounding by indication). That is, the group who agreed to learn their genotype may have been more motivated by memory concerns than the group who did not learn their genotype. This limits our ability to make strong inferences about cause-and-effect relationships. However, the matching procedure we used ensured that the four groups were similar in demographic characteristics and global cognitive abilities and likely did not differ in their motivation. We believe the primary difference was that the informed groups accepted the opportunity to know their APOE genotype and the other groups (for the most part) were never offered that opportunity. Second, it is possible that even though the ε4+ and ε4− groups scored similarly on the Dementia Rating Scale, subtle cognitive differences existed between the groups, since those who are ε4+ and therefore at greater risk of Alzheimer’s disease are more likely to already have cerebral Alzheimer’s pathology. Such subtle cognitive deficits could be evident on rigorous objective memory tests or associated with a sense of subjective cognitive impairment. It should be noted, however, that there was no main effect of genotype on any of the objective or subjective memory measures examined. Third, the effect of knowledge of APOE genotype on objective memory performance was evident on a verbal memory test but not on a visual memory test. Although this modality-specificity is consistent with the results of studies that compared older adults with a positive or a negative family history of dementia and Alzheimer’s disease (12), it is difficult to determine whether this is a reliable finding without more extensive verbal and visual memory testing. Finally, the results need to be replicated with a larger sample given that the potentially spurious finding of higher subjective memory ratings in ε4+ than in ε4− not-informed participants suggests a possible sampling bias.
Despite these limitations, our results indicate that informing older adults that they have an APOE genotype associated with an increased risk of Alzheimer’s disease can have adverse consequences on their perception of their memory abilities and on their performance on objective memory tests. Similar consequences might be expected if other indices of an increased risk of Alzheimer’s disease are disclosed (e.g., neuroimaging or CSF biomarkers of preclinical Alzheimer’s disease [30]). Such knowledge could have a serious clinical impact by increasing the likelihood of false positive diagnosis of dementia or mild cognitive impairment in those who know they are APOE ε4+, or it could distort the results of clinical trials of primary prevention of Alzheimer’s disease if participants with knowledge of their APOE ε4+ status are overrepresented in an unbalanced way in either the placebo or the treatment arm. Thus, clinicians and researchers should consider patients’ knowledge of their genotype or knowledge of possession of other Alzheimer’s biomarkers when evaluating older adults who may or may not be at risk for developing dementia.
Acknowledgments
Dr. Galasko has served on advisory panels for Elan Pharmaceuticals and Neurophage and on data and safety monitoring boards for clinical trials for Balance Pharmaceuticals, Elan Pharmaceuticals, Janssen Immunotherapy, and Pfizer. Dr. Salmon has served as a consultant for Bristol-Myers Squibb.
Supported by National Institute on Aging grants P50-AG 05131, R01-AG 012674, and K24-AG 026431 and an Alzheimer’s Centers of California grant from the State of California.
The authors thank Lisa Snyder, L.C.S.W., of the University of California, San Diego (UCSD), for providing genetic counseling. They also gratefully acknowledge the volunteer participants and staff of the UCSD Shiley-Marcos Alzheimer’s Disease Research Center.
Footnotes
The other authors report no financial relationships with commercial interests.
References
- 1.Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 2.Saunders AM, Strittmatter WJ, Schmechel D, George-Hyslop PH, Pericak-Vance MA, Joo SH, Rosi BL, Gusella JF, Crapper-MacLachlan DR, Alberts MJ, Hulette C, Crain B, Goldgaber D, Roses AD. Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer’s disease. Neurology. 1993;43:1467–1472. doi: 10.1212/wnl.43.8.1467. [DOI] [PubMed] [Google Scholar]
- 3.Strittmatter WJ, Weisgraber KH, Huang DY, Dong LM, Salvesen GS, Pericak-Vance MA, Schmechel DE, Saunders AM, Goldgaber D, Roses AD. Binding of human apolipoprotein E to synthetic amyloid beta peptide: isoform-specific effects and implications for late-onset Alzheimer disease. Proc Natl Acad Sci USA. 1993;90:8098–8102. doi: 10.1073/pnas.90.17.8098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Green RC, Roberts JS, Cupples LA, Relkin NR, Whitehouse PJ, Brown T, Eckert SL, Butson M, Sadovnick AD, Quaid KA, Chen C, Cook-Deegan R, Farrer LA REVEAL Study Group. Disclosure of APOE genotype for risk of Alzheimer’s disease. N Engl J Med. 2009;361:245–254. doi: 10.1056/NEJMoa0809578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kane RA, Kane RL. Effect of genetic testing for risk of Alzheimer’s disease. N Engl J Med. 2009;361:298–299. doi: 10.1056/NEJMe0903449. [DOI] [PubMed] [Google Scholar]
- 6.LaRue A, Small G, McPherson S, Komo S, Matsuyama SS, Jarvik LF. Subjective memory loss in age-associated memory impairment: family history and neuropsychological correlates. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 1996;3:132–140. [Google Scholar]
- 7.McPherson S, La Rue A, Fitz A, Matsuyama S, Jarvik LF. Self-reports of memory problems in relatives of patients with probable Alzheimer’s disease. Int Psychogeriatr. 1995;7:367–376. doi: 10.1017/s1041610295002110. [DOI] [PubMed] [Google Scholar]
- 8.Weaver Cargin J, Collie A, Masters C, Maruff P. The nature of cognitive complaints in healthy older adults with and without objective memory decline. J Clin Exp Neuropsychol. 2008;30:245–257. doi: 10.1080/13803390701377829. [DOI] [PubMed] [Google Scholar]
- 9.Bandura A. Social Foundations of Thought and Action: A Social Cognitive Theory. New York: Prentice-Hall; 1986. [Google Scholar]
- 10.Levy B. Improving memory in old age through implicit self-stereotyping. J Pers Soc Psychol. 1996;71:1092–1107. doi: 10.1037//0022-3514.71.6.1092. [DOI] [PubMed] [Google Scholar]
- 11.Desrichard O, Kopetz C. A threat in the elder: the impact of task instructions, self-efficacy, and performance expectations on memory performance in the elderly. Eur J Soc Psychol. 2005;35:537–552. [Google Scholar]
- 12.Seeman T, McAvay G, Merrill S, Albert M, Rodin J. Self-efficacy beliefs and change in cognitive performance: MacArthur Studies of Successful Aging. Psychol Aging. 1996;11:538–551. doi: 10.1037//0882-7974.11.3.538. [DOI] [PubMed] [Google Scholar]
- 13.Mattis S. Dementia Rating Scale (DRS) Odessa, Fla: Psychological Assessment Resources; 1988. [Google Scholar]
- 14.Monsch AU, Bondi MW, Salmon DP, Butters N, Thal LJ, Hansen LA, Wiederholt WC, Cahn DA, Klauber MR. Clinical validity of the Mattis Dementia Rating Scale in detecting dementia of the Alzheimer type: a double cross-validation and application to a community-dwelling sample. Arch Neurol. 1995;52:899–904. doi: 10.1001/archneur.1995.00540330081018. [DOI] [PubMed] [Google Scholar]
- 15.Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982–1983;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 16.Wechsler D. The Wechsler Memory Scale–Revised. San Antonio, Tex: Psychological Corp; 1987. [Google Scholar]
- 17.Osterrieth PA. Le test de copie d’une figure complexe. Arch Psychol. 1944;30:206–356. [Google Scholar]
- 18.Stern RA, Singer EA, Duke LM, Singer NG, Morey CE, Daughtrey EW, Kaplan E. The Boston Qualitative Scoring System for the Rey-Osterreith Complex Figure: description and interrater reliability. Clin Neuropsychol. 1994;8:309–322. [Google Scholar]
- 19.Dixon RA, Hultsch DF, Hertzog C. The Metamemory in Adulthood (MIA) questionnaire. Psychopharmacol Bull. 1988;24:671–688. [PubMed] [Google Scholar]
- 20.Gilewski MJ, Zelinski EM. Memory Functioning Questionnaire (MFQ) Psychopharmacol Bull. 1988;24:665–670. [PubMed] [Google Scholar]
- 21.Gilewski MJ, Zelinski EM, Schaie KW. The Memory Functioning Questionnaire for assessment of memory complaints in adulthood and old age. Psychol Aging. 1990;5:482–490. doi: 10.1037//0882-7974.5.4.482. [DOI] [PubMed] [Google Scholar]
- 22.Hultsch DF, Hertzog C, Dixon RA. Age differences in metamemory: resolving the inconsistencies. Can J Psychol. 1987;41:193–208. doi: 10.1037/h0084153. [DOI] [PubMed] [Google Scholar]
- 23.Alladi S, Arnold R, Mitchell J, Nestor PJ, Hodges JR. Mild cognitive impairment: applicability of research criteria in a memory clinic and characterization of cognitive profile. Psychol Med. 2006;36:507–515. doi: 10.1017/S0033291705006744. [DOI] [PubMed] [Google Scholar]
- 24.Rabin LA, Paré N, Saykin AJ, Brown MJ, Wishart HA, Flashman LA, Santulli RB. Differential memory test sensitivity for diagnosing amnestic mild cognitive impairment and predicting conversion to Alzheimer’s disease. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2009;16:357–376. doi: 10.1080/13825580902825220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seeman TE, Rodin J, Albert M. Self-efficacy and cognitive performance in high-functioning older individuals: Mac-Arthur Studies of Successful Aging. J Aging Health. 1993;5:455–474. [Google Scholar]
- 26.Bandura A. Self-Efficacy: The Exercise of Control. New York, WH: Freeman; 1997. [Google Scholar]
- 27.Gardiner M, Luszcz MA, Bryan J. The manipulation and measurement of task-specific memory self-efficacy in younger and older adults. Int J Behav Dev. 1997;21:209–227. [Google Scholar]
- 28.Cohen GL, Garcia J, Purdie-Vaughns V, Apfel N, Brzustoski P. Recursive processes in self-affirmation: intervening to close the minority achievement gap. Science. 2009;324:400–403. doi: 10.1126/science.1170769. [DOI] [PubMed] [Google Scholar]
- 29.Miyake A, Kost-Smith LE, Finkelstein ND, Pollock SJ, Cohen GL, Ito TA. Reducing the gender achievement gap in college science: a classroom study of values affirmation. Science. 2010;330:1234–1237. doi: 10.1126/science.1195996. [DOI] [PubMed] [Google Scholar]
- 30.Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, Iwatsubo T, Jack CR, Jr, Kaye J, Montine TJ, Park DC, Reiman EM, Rowe CC, Siemers E, Stern Y, Yaffe K, Carrillo MC, Thies B, Morrison-Bogorad M, Wagster MV, Phelps CH. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]