Abstract
Objective
Autoantibodies to glutamate decarboxylase (GAD65Ab) are found in patients with autoimmune neurological disorders and patients with type 1 diabetes. The correct diagnosis of GAD65Ab-associated neurological disorders is often delayed by the variability of symptoms and a lack of diagnostic markers. We hypothesize that the frequency of neurological disorders with high GAD65Ab titers is significantly higher than currently recognized.
Methods
We analyzed GAD65Ab titer, inhibition of GAD65 enzyme activity, and pattern of GAD65Ab epitopes in a cohort of type 1 diabetes patients (n=100) and correlated our findings with neurological symptoms and diseases.
Results
Fourty-three percent (43/100) of the patients had detectable GAD65Ab titers (median=400 U/ml, range: 142–250,000U/ml). The GAD65Ab titers in 10 type 1 diabetes patients exceeded the 90th percentile of the cohort (2,000–250,000 U/ml). Sera of these 10 patients were analyzed for their GAD65Ab epitope specificity and their ability to inhibit GAD65 enzyme activity in vitro. GAD65Ab of five patients inhibited the enzyme activity significantly (by 34–55%). Three of these patients complained of muscle stiffness and pain, which was documented in two of these patients.
Conclusions
Based on our findings we suggest that neurological disorders with high GAD65Ab titers are more frequent in type 1 diabetes patients than currently recognized.
Keywords: GAD65, neurological disorder, epitopes, radioligand binding assay, Stiff Person Syndrome, cerebellar ataxia
Introduction
Autoantibodies to the smaller isoform of glutamate decarboxylase (GAD65) are found in the majority of patients with type 1 diabetes (frequency: 80%), patients with neurological disorders, such as Stiff Person Syndrome (frequency: 70–80%) (SPS), cerebellar ataxia (frequency: 30–60%), intractable epilepsy (frequency: 10–30%) (for review see (1)) and Batten disease (frequency: 100%) (2). GAD65Ab in these different diseases show distinct antibody titers, epitope specificities, tissue distributions, and capacities to inhibit GAD65 enzyme activity. Patients with GAD65Ab-associated neurological disorders often have GAD65Ab titers that exceed those found in type 1 diabetes patients by at least 100-fold (3). In patients with SPS and cerebellar ataxia GAD65Ab can be detected in the patients’ peripheral blood and cerebrospinal fluid (CSF), while GAD65Ab in type 1 diabetes patients are restricted to the circulating blood levels (4, 5). GAD65Ab epitope pattern in GAD65-associated neurological disorders differ from those in type 1 diabetes patients (6), and 83% of GAD65Ab-positive SPS sera inhibit the enzyme activity of GAD65, an uncommon characteristic for type 1 diabetes patients (7).
GAD65-associated neurological disorders such as SPS are considered rare diseases affecting one person per million in the general population (8). SPS is often associated with other autoimmune diseases and 30% of SPS patients are also diagnosed with type 1 diabetes (9). However, the prevalence of SPS in type 1 diabetes patients is thought to be as low as 1/10,000 (10).
The identification of patients with GAD65Ab-associated neurological disorders is hampered by a wide range of mild and non-specific symptoms such as phobias (11, 12), depression (13), and muscle aches and pain during the early stages of the neurological disease. The variability of symptoms (14–16) hinders the correct diagnosis of these conditions and previously used strict diagnosis criteria are not always helpful in clinical practice (17). We hypothesize that the prevalence of GAD65Ab-associated neurological disorders is currently underestimated. The aim of this study was to establish the prevalence of GAD65Ab with antibody characteristics found in GAD65Ab-associated neurological diseases in a cohort of type 1 diabetes patients. We chose this cohort based on the known association of type 1 diabetes and other autoimmune diseases and the relatively higher frequency of autoimmune neurological disorders in patients with type 1 diabetes (18–20).
Methods
Patient cohort
Samples from patients with type 1 diabetes analyzed in this study (n=100) were collected at the University of Washington in Seattle, Washington between 2007 and 2009. Along with the blood samples, surveys were conducted regarding age at onset of diabetes, duration of disease, and diabetes management. Inclusion criteria for this study were determined by a clinical diagnosis of type 1 diabetes with subcutaneous insulin treatment. Exclusion criteria were: ages younger than 18 years at sampling, serious illnesses affecting the immune system, and medications suppressing the immune system. Median age at onset was 16 years (range: 2–62), median duration of disease was 25 years (range: 2–60), and median age at sampling time was 42 years (range: 18–85). Fifty-six patients were female. Local institutional ethics committee approval and subjects’ consent was obtained prior to collection of all serum samples.
GAD65Ab Radioligand binding assay (RBA)
Recombinant human [35S]-GAD65 was produced in an in vitro coupled transcription/translation system with SP6 RNA polymerase and nuclease treated rabbit reticulocyte lysate (Promega, Madison, WI, USA) as described previously (21). The in vitro translated [35S]-GAD65 was kept at −70°C and used within 2 weeks.
Sera (2.5μl) were incubated with [35S]-GAD65 (25,000 of TCA precipitable radioactivity). After an overnight incubation at 4°C, antibody-bound [35S]-GAD65 was separated from unbound antigen by precipitation with Protein A Sepharose (Invitrogen, Carlsbad, CA). The immunoprecipitated radioactivity was counted using a Wallac Microbeta Liquid Scintillation Counter (Perkin Elmer Life and Analytical Sciences, Inc, Boston, MA). A standard curve consisting of dilutions of the international WHO standard for GAD65Ab was included for the calculation of the antibody levels. The cut-off value for positivity (135 U/ml) was determined as the 99th percentile of a healthy control cohort (n=50).
Epitope-specific Radioligand Binding assay (ES-RBA)
The capacity of the recombinant Fab (rFab) to inhibit GAD65 binding by human serum GAD65Ab was tested in a competitive ES-RBA as described (22). Sera were analyzed at a dilution representing half maximal binding to GAD65 as established by serial dilutions. All sera and a positive control were analyzed for their binding to GAD65 in the presence of rFab b96.11 and b78. b96.11 shares its GAD65Ab epitope specificity with the majority of type 1 diabetes patients (22), while b78 is a prototypical monoclonal antibody for GAD65Ab epitope specificities in SPS patients (6).
Non-competed GAD65 binding was established by no addition of rFab. The cutoff for specific competition was determined as >15% by using a control rFab. Each sample was measured in triplicates, and the mean value was calculated. Binding of GAD65Ab to GAD65 in the presence of rFab was expressed as follows: GAD65Ab cpm in the presence of rFab (competed) / GAD65Ab cpm in the absence of rFab (non-competed) x100. A higher binding to GAD65 in the presence of an rFab indicates a lower proportion of GAD65Ab binding to the respective epitope.
Definition of high GAD65Ab titer
Sera with high GAD65Ab levels were defined by us by two criteria: 1) their GAD65Ab titer exceeded that of the median GAD65Ab titer of the entire cohort (119U/ml) by at least 10-fold, and 2) successful displacement of binding to radiolabled GAD65 by 100µg/ml recombinant GAD65 was observed only when serum samples containing GAD65Ab that were not displaced in the presence of 100µg/ml recombinant GAD65 following the procedure previously described (23) (data not shown). Each serum sample with a GAD65Ab binding level of 135 U/ml or higher was analyzed for binding to radiolabeled GAD65 in the presence of 100µg/ml recombinant human GAD65 to determine the level of unspecific binding. We found that binding to radiolabeled GAD65 in all but ten samples was successfully displaced (binding was reduced by more than 50%) with 100µg/ml GAD65. These ten samples had GAD65Ab levels of ≥2000 U/ml and were subsequently reanalyzed at their half-maximal binding concentration, at which their binding to radiolabeled GAD65 was successfully displaced. The cut-off for high GAD65Ab titer of ≥2000 U/ml is in agreement with other reports (24).
GAD65 enzyme activity assay
GAD65 enzyme activity was measured by the 14CO2-trapping method described previously (25). Recombinant human GAD65 (100 ng) was incubated with or without the indicated amounts of serum for 1 hr at room temperature. The enzymatic reaction was initiated by the addition of 0.56 mM L-glutamate and 0.018 µCi 14C-glutamate (Amersham Life Science Inc, Arlington Heights, IL) and allowed to continue for 2 hours at 37°C with gentle agitation. During incubation, released 14CO2 was captured on filter paper (Kontes, Vineland, NJ) soaked in 50 µl 1 M NaOH. After the incubation, the absorbed radioactivity was determined in a Wallac Microbeta Liquid Scintillation Counter. The results are presented as follows: % residual activity = cpm in the presence of serum/cpm in the absence of serum × 100.
Statistical analyses
Binding of GAD65Ab to GAD65 in the presence of rFab was expressed as follows: cpm of [35S]-GAD65 bound in the presence of rFab/cpm of [35S]-GAD65 in the absence of rFab ×100.
All samples were analyzed in triplicate determinations and the intra-assay average coefficient of variation was 5% with the highest value of 13% and the lowest being 0.04%.
Results
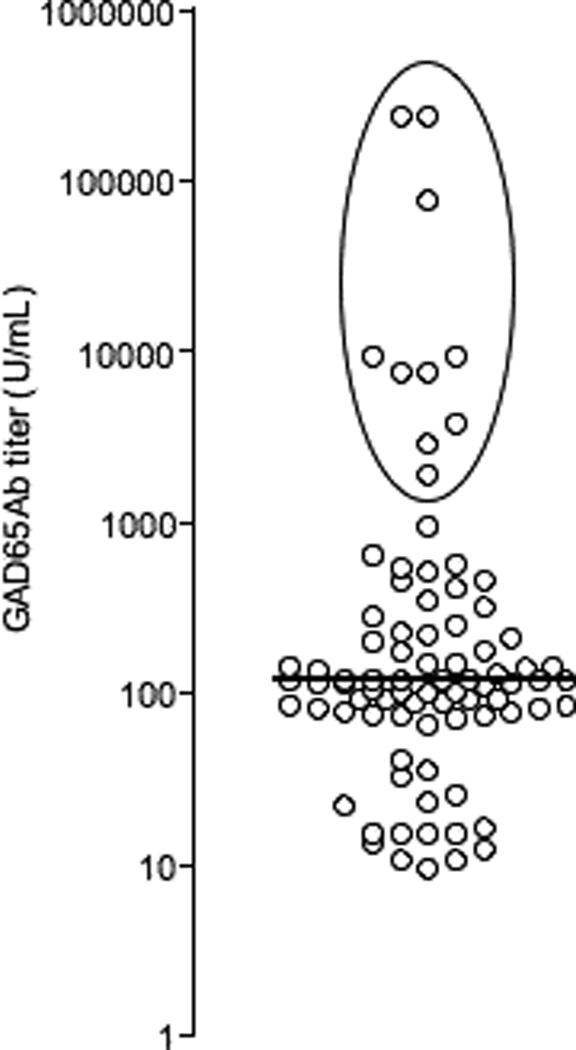
We found detectable GAD65Ab in 43% (43/100) of the patients (median titer of GAD65Ab-positive patients=400 U/ml, range: 142–250,000U/ml). Ten of these patients had GAD65Ab titers ≥2,000 U/ml (2,000–250,000 U/ml) (Figure 1). No significant correlations between GAD65Ab titer, age at onset, duration, gender, or age at sampling were observed (data not shown). Presence of other autoimmune diseases, as reported by the patients, in these two groups is shown in Table 2.
Figure 1. Analysis of GAD65Ab in 100 type 1 diabetes patients.
GAD65Ab titers are shown on a logarithmical scale, patients, whose sera exceeded a GAD65Ab titer of 2,000U/ml, are presented in the circled area. Median GAD65Ab titer is indicated.
Table 2.
Self-reported autoimmune diseases besides type 1 diabetes in patients with GAD65Ab <2000 U/ml and ≥2000U/ml.
| GAD65Ab titer (U/ml) |
|
|---|---|
| GAD65Ab titers ≥2000U/ml | |
| Hypothyrodism | 2897 |
| Hypothyrodism | 7020 |
| Hashimoto's thyroditis, Pernicious Anemia, Fibromyalgia | 11279 |
| GAD65Ab <2000U/ml | |
| Graves’ disease | 1421 |
| Hashimoto’s thyroditis | 364 |
| Hashimoto’s thyroditis, celiac disease | 115 |
| Hashimoto’s thyroditis | 161 |
The ten patients with GAD65Ab titers ≥2,000 U/ml were further evaluated for their GAD65Ab epitope pattern and their serum’s ability to inhibit GAD65 enzyme activity.
Epitope pattern
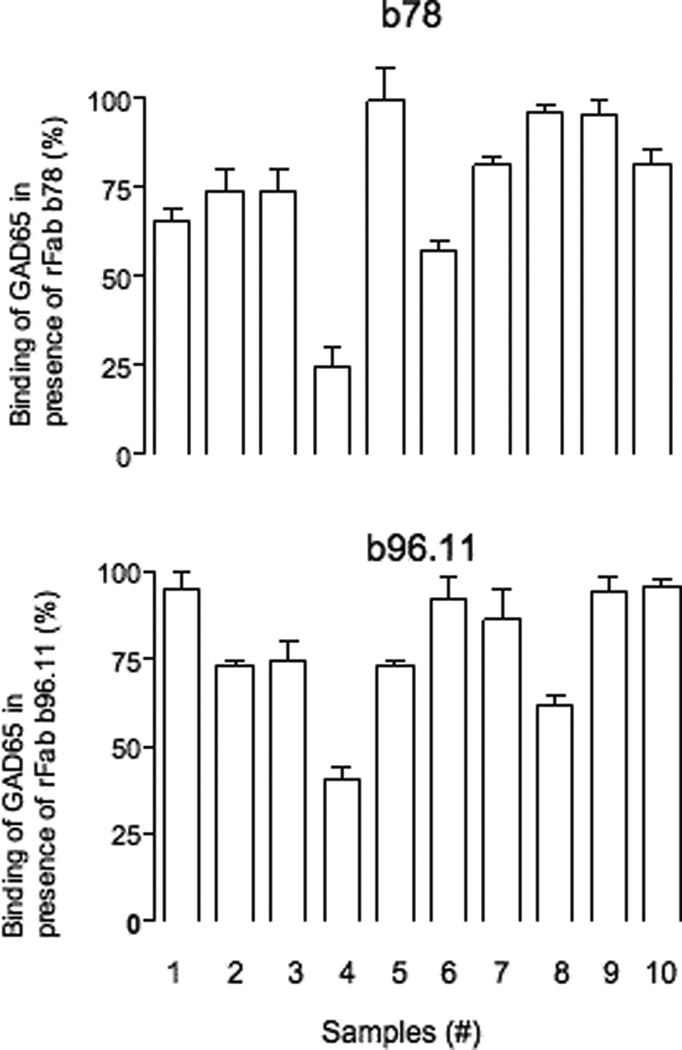
Patients’ sera were diluted to their half-maximal binding activity and analyzed for binding to GAD65 in the presence of rFab b96.11 or b78 (Table 3). Six of the 10 sera showed drastically reduced binding to radiolabeled GAD65 in the presence of rFab b96.11 (26–75% inhibition). Four sera showed substantially reduced GAD65 binding in the presence of rFab b78 (16–42% inhibition).
Table 3.
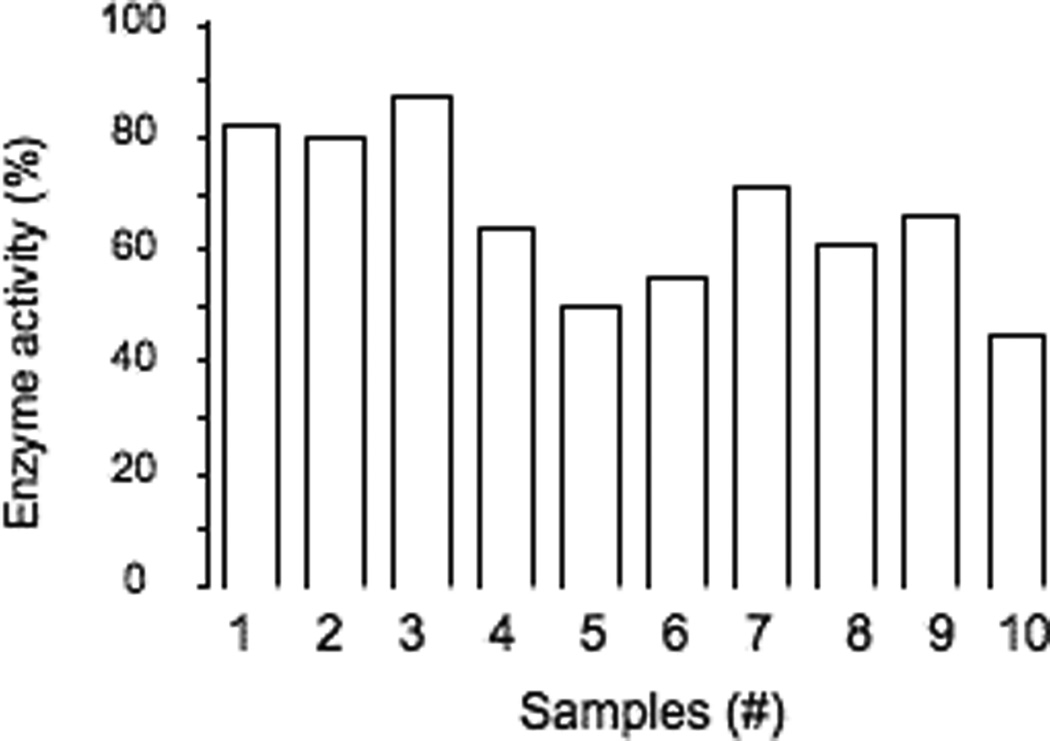
Summary of GAD65Ab characteristics in samples with high GAD65Ab titer.
| Sample (#) | Inhibition by rFab b78 (%) |
Inhibition by rFab b96.11 (%) |
Enzyme inhibition (%) |
GAD65Ab titer (U/ml) |
|---|---|---|---|---|
| 1 | 26 (±2) | 28 (±4) | 39 (±4) | 250000 |
| 2 | 0 (±5) | 0 (±5) | 29 (±1) | 10000 |
| 3 | 4 (±0) | 38 (±3) | 36 (±7) | 10000 |
| 4 | 16 (±0) | 13 (±2) | 45 (±3) | 8000 |
| 5 | 3 (±2) | 56 (±5) | 50 (±7) | 4000 |
| 6 | 0 (±2) | 26 (±0) | 13 (±1) | 3000 |
| 7 | 1 (±3) | 75 (±5) | 20 (±5) | 2000 |
| 8 | 4 (±4) | 26 (±2) | 18 (±9) | 8000 |
| 9 | 42 (±2) | 7 (±6) | 55 (±6) | 250000 |
| 10 | 34 (±4) | 7 (±4) | 34 (±5) | 80000 |
Enzyme inhibition
Patients’ sera were tested for their capacity to inhibit GAD65 enzyme activity (Table 3). GAD65 enzyme activity was significantly inhibited (inhibition by 34–55%) for 5/10 patients. Three of these five sera also showed significant reduction in binding to GAD65 in the presence of rFab b78.
Clinical evaluation of neurological symptoms
Three of the ten patients complained of generalized muscle stiffness and pain, and/or arthritis pain. One of the patients (#9) was a 54-year-old woman with type 1 diabetes for >27 years. Approximately 20 years ago, she developed diffuse body aches and was subsequently diagnosed with fibromyalgia. About four years ago she developed increased stiffness, particularly in her neck. She was found to have cervical stenosis and her symptoms improved for a few months following surgical decompression. Subsequently, diffuse discomfort in her hands, legs, neck, and back recurred. She could not “straighten" her shoulders or back and felt that her neck was chronically flexed forward. The intensity of her symptoms waxed and waned. Overall, she had diminished mobility and was no longer able to participate in vigorous exercise. She was started on 5 mg of diapzepam (Valium) and immediately felt an improvement in her sleep and the stiffness affecting her neck and shoulders. Her stiffness continues to be improved by low doses of diazepam (4 to 6 mg per day). Her examination was normal except for tightness in her trapezius and cervical muscles with slight reduction in neck mobility, mild symmetrical reduction in sharp-dull sensation to her mid-calves and decreased sharp-dull sensation in her left hand in a median nerve distribution suggestive of a differential diagnosis that would include carpal tunnel syndrome.
Another patient was a 44-year-old man (#5). He developed type 1 diabetes >25 years ago. Subsequently, he noticed a tendency to have cold hands and feet. In his mind, he was always tensing in an effort to combat this cold feeling. He gradually noticed a chronic stiffness affecting shoulders, forearms, posterior thighs, low back, and, to a lesser extent, his calves. This tightness was nearly always present, and the patient recalled it to have been most severe when he was in his late 20s to, early 30s. He thought the tension was less severe more recently but also wondered if this was because he had simply learned how to manage it more effectively. His muscular tightness resulted in difficulties with competitive athletic activities such as playing soccer. He could do this at a slow pace, but if he competed more vigorously he developed severe spasms in his abdominal region, which could compromise his ability to breathe freely. He thought that his symptoms were better during sleep. He did not have pain with his muscular stiffness except very rarely when he might have brief lower calf pain. He had no symptoms in his hands and his handwriting and ability to play the piano were unaffected. He was tried on diazepam at night (5 mg), and noticed that he slept much easier for the first time in years and was somewhat less stiff during the day. His examination was normal except for a slightly stiff gait with decreased associated movements, and slightly tense trapezius and cervical muscles.
The last patient (#10) reported neurological symptoms, but did not undergo neurological evaluation or treatment. As such, we presume that this patient had SPS.
The clinical parameters of these three patients are summarized in Table 1.
Table 1.
Clinical parameters of three type 1 diabetes patients meeting both physical and immunological criteria for GAD65Ab-associated autoimmune neurological disorders.
| Sample (#) |
Age at onset |
Age at sampling (years) |
Gender | Related diagnosis |
Complaints about pain and muscle stiffness |
|---|---|---|---|---|---|
| 5 | 20 | 45 | Male | Yes | |
| 9 | 14 | 51 | Female | Fibromyalgia | Yes |
| 10 | 62 | 85 | Female | Yes |
None of the other patients in this cohort reported neurological autoimmune diseases or associated symptoms.
Discussion and Conclusions
We analyzed GAD65Ab in 100 type 1 diabetes patients to identify GAD65Ab specificities in patients with high GAD65Ab titers. Although the median duration of disease in our cohort was 25 years, we were able to detect significant GAD65Ab titers in 43% of the patients, confirming earlier observations of persistent GAD65Ab titers in long-standing diabetes (26, 27). Ten of these patients had GAD65Ab exceeding the typical GAD65Ab titer in type 1 diabetes patients by 10-fold, a characteristic of patients with GAD65-associated neurological disorders (3). We identified three patients with high GAD65Ab titers and inhibition of GAD65 enzyme activity, who suffer neurological symptoms, such as muscle stiffness and pain.
It is of importance to note that neither assay alone could positively identify these patients. While all three patients had high GAD65Ab titer (inclusion criteria), and inhibited GAD65 enzyme activity, GAD65Ab of only 2 of the 3 patients recognized the b78-defined GAD65Ab epitope, which has been associated with SPS (6). Moreover, one patient, who met all three criteria (high GAD65Ab titer, recognition of the b78-defined epitope, and inhibition of GAD65 enzyme activity), had no neurological symptoms. We hypothesize that in such patients, GAD65 enzyme inhibiting antibodies are present only in the periphery and not in the CSF. CSF was not available for any of the patients to test our hypothesis. In a previous study we did not observe significant differences in GAD65Ab titers in CSF obtained from SPS patients with or without type 1 diabetes (unpublished results).
High GAD65Ab titers have been described in other cohorts of diabetes patients. In a recent report very high GAD65Ab titers (above 20,000 U/ml) were detected in 3/23 type 1 diabetes patients, 7/290 type 2 diabetes patients, and 4/57 patients with unspecified diabetes (23). The authors suggested that the prolonged exposure of these patients to GAD65 was involved in these uncharacteristically high GAD65Ab titers. The neurological status of these patients was not evaluated. In another study, GAD65Ab titers above 2,000 U/ml were detected in only 0.8% of analyzed type 1 diabetes patients (24). None of these patients showed neurological symptoms. Neither study investigated the epitope pattern of the associated GAD65Ab or enzyme inhibition. It is unclear what caused the difference in frequencies of high GAD65Ab samples in these studies.
The lack of a detailed neurological examination of the patients presents a major flaw of our study. In future studies we plan to include thorough neurological exams evaluating stiffness, rigidity or increased tone and painful spasms and electromyography (EMG) performed by a physician familiar with the findings of SPS to validate our hypothesis of higher prevalence of neurological symptoms in patients with increased GAD65Ab titers. However our data suggest that measurement of GAD65Ab in type 1 diabetes patients in conjunction with neurological exams may identify patients with underlying neurological diseases such as SPS and aid in their successful treatment.
Acknowledgments
The study was performed as independent research sponsored by the National Institutes of Health (DK26190, and DK17047), and a Basic Science Award from the American Diabetes Association to CSH.
Abbreviations
- cpm
counts per minute
- CSF
cerebrospinal fluid
- EMG
electromyography
- ES-RBA
Epitope-specific Radioligand Binding assay
- GAD65
glutamate decarboxylase
- GAD65A
autoantibodies to GAD65
- RBA
Radioligand binding assay
- SPS
Stiff Person Syndrome
- TCA
Trichloroacetic Acid
- WHO
World Health Organization
- U/ml
Units per ml
- rFab
recombinant Fab
References
- 1.Ali F, Rowley M, Jayakrishnan B, et al. Stiff-person syndrome (SPS) and anti-GAD-related CNS degenerations: protean additions to the autoimmune central neuropathies. J Autoimmun. 2011;37(2):79–87. doi: 10.1016/j.jaut.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 2.Chattopadhyay S, Ito M, Cooper JD, et al. An autoantibody inhibitory to glutamic acid decarboxylase in the neurodegenerative disorder Batten disease. Hum Mol Genet. 2002;11(12):1421–1431. doi: 10.1093/hmg/11.12.1421. [DOI] [PubMed] [Google Scholar]
- 3.Kim J, Namchuk M, Bugawan T, et al. Higher autoantibody levels and recognition of a linear NH2-terminal epitope in the autoantigen GAD65, distinguish stiff-man syndrome from insulin-dependent diabetes mellitus. J Exp Med. 1994;180(2):595–606. doi: 10.1084/jem.180.2.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vianello M, Tavolato B, Armani M, Giometto B. Cerebellar ataxia associated with anti-glutamic acid decarboxylase autoantibodies. Cerebellum. 2003;2(1):77–79. doi: 10.1080/14734220309432. [DOI] [PubMed] [Google Scholar]
- 5.Dalakas MC, Li M, Fujii M, Jacobowitz DM. Stiff person syndrome: quantification, specificity, and intrathecal synthesis of GAD65 antibodies. Neurology. 2001;57(5):780–784. doi: 10.1212/wnl.57.5.780. [DOI] [PubMed] [Google Scholar]
- 6.Raju R, Foote J, Banga JP, et al. Analysis of GAD65 Autoantibodies in Stiff-Person Syndrome Patients. J Immunol. 2005;175(11):7755–7762. doi: 10.4049/jimmunol.175.11.7755. [DOI] [PubMed] [Google Scholar]
- 7.Dinkel K, Meinck H, Jury KM, Karges W, Richter W. Inhibition of gamma-aminobutyric acid synthesis by glutamic acid decarboxylase autoantibodies in stiff-man syndrome. Annals of Neurology. 1998;44(2):194–201. doi: 10.1002/ana.410440209. [DOI] [PubMed] [Google Scholar]
- 8.Raju R, Hampe CS. Immunobiology of stiff-person syndrome. Int Rev Immunol. 2008;27(1–2):79–92. doi: 10.1080/08830180701883240. [DOI] [PubMed] [Google Scholar]
- 9.Solimena M, De Camilli P. Autoimmunity to glutamic acid decarboxylase (GAD) in Stiff-Man syndrome and insulin-dependent diabetes mellitus. Trends Neurosci. 1991;14(10):452–457. doi: 10.1016/0166-2236(91)90044-u. [DOI] [PubMed] [Google Scholar]
- 10.Orija IB, Gupta M, Zimmerman RS. Graves' disease and stiff-person (stiff-man) syndrome: case report and literature review. Endocr Pract. 2005;11(4):259–264. doi: 10.4158/EP.11.4.259. [DOI] [PubMed] [Google Scholar]
- 11.Henningsen P, Meinck HM. Specific phobia is a frequent non-motor feature in stiff man syndrome. Journal of Neurology, Neurosurgery and Psychiatry. 2003;74(4):462–465. doi: 10.1136/jnnp.74.4.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ameli R, Snow J, Rakocevic G, Dalakas MC. A neuropsychological assessment of phobias in patients with stiff person syndrome. Neurology. 2005;64(11):1961–1963. doi: 10.1212/01.WNL.0000163984.71993.FE. [DOI] [PubMed] [Google Scholar]
- 13.Culav-Sumic J, Bosnjak I, Pastar Z, Jukic V. Anxious depression and the stiff-person plus syndrome. Cogn Behav Neurol. 2008;21(4):242–245. doi: 10.1097/WNN.0b013e318185e6d2. [DOI] [PubMed] [Google Scholar]
- 14.O'Sullivan EP, Behan LA, King TF, Hardiman O, Smith D. A case of stiff-person syndrome, type 1 diabetes, celiac disease and dermatitis herpetiformis. Clin Neurol Neurosurg. 2009;111(4):384–386. doi: 10.1016/j.clineuro.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 15.Kono S, Miyajima H, Sugimoto M, et al. Stiff-person syndrome associated with cerebellar ataxia and high glutamic acid decarboxylase antibody titer. Intern Med. 2001;40(9):968–971. doi: 10.2169/internalmedicine.40.968. [DOI] [PubMed] [Google Scholar]
- 16.Bilic E, Sepec BI, Vranjes D, et al. Stiff-person syndrome in a female patient with type 1 diabetes, dermatitis herpetiformis, celiac disease, microcytic anemia and copper deficiency Just a coincidence or an additional shared pathophysiological mechanism? Clin Neurol Neurosurg. 2009;111(7):644–645. doi: 10.1016/j.clineuro.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 17.McKeon A, Robinson MT, McEvoy KM, et al. Stiff-man syndrome and variants: clinical course, treatments, and outcomes. Arch Neurol. 2012;69(2):230–238. doi: 10.1001/archneurol.2011.991. [DOI] [PubMed] [Google Scholar]
- 18.Honnorat J, Saiz A, Giometto B, et al. Cerebellar ataxia with anti-glutamic acid decarboxylase antibodies: study of 14 patients. Arch Neurol. 2001;58(2):225–230. doi: 10.1001/archneur.58.2.225. [DOI] [PubMed] [Google Scholar]
- 19.Bayreuther C, Hieronimus S, Ferrari P, Thomas P, Lebrun C. Auto-immune cerebellar ataxia with anti-GAD antibodies accompanied by de novo late-onset type 1 diabetes mellitus. Diabetes Metab. 2008;34(4 Pt 1):386–388. doi: 10.1016/j.diabet.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 20.Saiz A, Arpa J, Sagasta A, et al. Autoantibodies to glutamic acid decarboxylase in three patients with cerebellar ataxia, late-onset insulin-dependent diabetes mellitus, and polyendocrine autoimmunity. Neurology. 1997;49(4):1026–1030. doi: 10.1212/wnl.49.4.1026. [DOI] [PubMed] [Google Scholar]
- 21.Hampe CS, Hammerle LP, Bekris L, et al. Recognition of Glutamic Acid Decarboxylase (GAD) by Autoantibodies from Different GAD Antibody-Positive Phenotypes. J Clin Endocrinol Metab. 2000;85(12):4671–4679. doi: 10.1210/jcem.85.12.7070. [DOI] [PubMed] [Google Scholar]
- 22.Padoa CJ, Banga JP, Madec AM, et al. Recombinant Fabs of human monoclonal antibodies specific to the middle epitope of GAD65 inhibit type 1 diabetes-specific GAD65Abs. Diabetes. 2003;52(11):2689–2695. doi: 10.2337/diabetes.52.11.2689. [DOI] [PubMed] [Google Scholar]
- 23.Hansson I, Lynch KF, Hallmans G, Lernmark A, Rolandsson O. High-titer GAD65 autoantibodies detected in adult diabetes patients using a high efficiency expression vector and cold GAD65 displacement. Autoimmunity. 2010;44(2):129–136. doi: 10.3109/08916934.2010.482117. [DOI] [PubMed] [Google Scholar]
- 24.Saiz A, Blanco Y, Sabater L, et al. Spectrum of neurological syndromes associated with glutamic acid decarboxylase antibodies: diagnostic clues for this association. Brain. 2008;131(Pt 10):2553–2563. doi: 10.1093/brain/awn183. [DOI] [PubMed] [Google Scholar]
- 25.Hampe CS, Hammerle LP, Falorni A, Robertson J, Lernmark A. Site-directed mutagenesis of K396R of the 65 kDa glutamic acid decarboxylase active site obliterates enzyme activity but not antibody binding. FEBS Lett. 2001;488(3):185–189. doi: 10.1016/s0014-5793(00)02429-7. [DOI] [PubMed] [Google Scholar]
- 26.Borg H, Marcus C, Sjoblad S, Fernlund P, Sundkvist G. Islet cell antibody frequency differs from that of glutamic acid decarboxylase antibodies/IA2 antibodies after diagnosis of diabetes. Acta Paediatr. 2000;89(1):46–51. doi: 10.1080/080352500750029059. [DOI] [PubMed] [Google Scholar]
- 27.Decochez K, Tits J, Coolens JL, et al. High frequency of persisting or increasing islet-specific autoantibody levels after diagnosis of type 1 diabetes presenting before 40 years of age. The Belgian Diabetes Registry. Diabetes Care. 2000;23(6):838–844. doi: 10.2337/diacare.23.6.838. [DOI] [PubMed] [Google Scholar]