Abstract
Interactions between neurons and their targets of innervation influence many aspects of neural development. To examine how synaptic activity interacts with neurotrophic signaling, we determined the effects of blocking neuromuscular transmission on survival and axonal outgrowth of ciliary neurons from the embryonic chicken ciliary ganglion. Ciliary neurons undergo a period of cell loss due to programmed cell death between embryonic Days (E) 8 and 14 and they innervate the striated muscle of the iris. The nicotinic antagonist d-tubocurarine (dTC) induces an increase in branching measured by counting neurofilament-positive voxels (NF-VU) in the iris between E14–17 while reducing ciliary neuron survival. Blocking ganglionic transmission with dihyro-β-erythroidin and α-methyllycacontine does not mimic dTC. At E8, many trophic factors stimulate neurite outgrowth and branching of neurons placed in cell culture; however, at E13, only GDNF stimulates branching selectively in cultured ciliary neurons. The GDNF-induced branching at E13 could be inhibited by BDNF. Blocking ret signaling in vivo with a dominant negative (dn)ret decreases survival of ciliary and choroid neurons at E14 and prevents dTC induced increases in NF-VU in the iris at E17. Blocking TRKB signaling with dn TRKB increases NF-VU in the iris at E17 and decreases neuronal survival at E17, but not at E14. Thus, RET promotes survival during programmed cell death in the ciliary ganglion and contributes to promoting branching when synaptic transmission is blocked while TRKB inhibits branching and promotes maintenance of neuronal survival. These studies highlight the multifunctional nature of trophic molecule function during neuronal development.
Keywords: GDNF, BDNF, d-tubocurarine, iris, ciliary
INTRODUCTION
Neuronal activity plays an important role in coordinating innervation of targets with neuronal survival. Chronic blockade of neuromuscular transmission with nicotinic acetylcholine receptor (nAChR) antagonists increases intramuscular axonal branching and promotes neuronal survival (Pittman and Oppenheim, 1979; Dahm and Landmesser, 1988; Landmesser, 1992; Oppenheim et al., 2000). Increased axonal branching and neuronal survival is also observed in transgenic mice where synaptogenesis on muscle is altered by null mutations in choline acetyltransferase (Brandon et al., 2003), rapsyn (Gautam et al., 1995), or muscle specific kinase (DeChiara et al., 1996). These studies suggest that interfering with neuromuscular transmission enhances retrograde signaling between muscle and nerve, which, in turn, promotes axonal branching, endplate formation, and neuronal survival.
Two trophic molecules that likely mediate activity-dependent interactions are glial cell line-derived neurotrophic factor (GDNF; (Nguyen et al., 1998) and brain derived neurotrophic factor (BDNF; (Streppel et al., 2002). Both GDNF and BDNF promote survival and induce branching of motor neurons in a number of animal models. BDNF is also responsible for activity-driven remodeling of axonal inputs in the visual cortex (Hanover et al., 1999; Huang et al., 1999), induces branching of hippocampal neurons (Danzer et al., 2002), and enhances outgrowth of corticospinal neurons (Ozdinler and Macklis, 2006). Signaling of GDNF is mediated by the tyrosine kinase receptor RET, together with the GFRalpha1 co-receptor, while BDNF activates signaling through the tyrosine kinase receptor TRKB.
To understand the effects of blocking neuromuscular transmission on trophic interactions, we used the avian ciliary ganglion because of its simplicity and well-defined trophic dependence. The ciliary ganglion consists of two populations of neurons, ciliary and choroid, that are innervated by cholinergic inputs from the accessory oculomotor nucleus (Marwitt et al., 1971). Ciliary neurons are cholinergic, have myelinated axons, and innervate the iris and ciliary muscle, which commence development as smooth muscle, but transition into striated muscle between E11–15 (Pilar et al., 1987; Link and Nishi, 1998b). Choroid neurons are cholinergic, unmyelinated, innervate the vasculature of the choroid, and are distinguished from ciliary neurons by the expression of somatostatin-like immunoreactivity (DeStefano et al., 1993; Bunker and Nishi, 2002; Hruska et al., 2007). Thus, the two subpopulations can be readily counted in vivo and their axonal projections are segregated in vivo (Bunker and Nishi, 2002). Both populations undergo a period of cell loss between E8–14 that is mediated by signaling through α7 containing nicotinic receptors (nAChRs; Hruska et al., 2007) as well as trophic interactions with targets. CNTF (Finn and Nishi, 1996; Finn et al., 1998) and GDNF (Hashino et al., 2001) support ciliary neuron survival in cell culture and enhance axonal outgrowth in vitro and in vivo (Hashino et al., 2001). In addition, ciliary ganglion also neurons express TRKB and respond to BDNF (Zhou et al, 2004).
Here we show that chronic paralysis of chicken embryos with d-tubocurarine (dTC) induces an increase in branching of axons in the iris while nicotinic antagonists that block ganglionic transmission without blocking neuromuscular transmission fail to do so. Using an in vitro assay, we identify GDNF and BDNF as candidate factors involved in mediating this effect. By blocking RET and TRKB signaling with dominant negative receptors, we show that signaling through these receptor systems has distinct effects on axonal branching and neuronal survival. These studies illustrate the multifunctional nature of neurotrophic signaling during development.
METHODS
White leghorn chicken (Gallus gallus domesticus) embryos, embryonic Days (E) 8, 13, 14, 15, or 17 of either sex were used for all experiments. All embryos were immediately decapitated with sharp scissors after opening the egg. For retroviral experiments, pathogen-free White Leghorn eggs (Charles River, Wilmington, MA) were used. White Leghorn eggs (Oliver Merrill & Sons, Concord, NH) were used for all other experiments. Embryos were staged according to Hamburger and Hamilton (Hamburger and Hamilton, 1951; Hamburger and Hamilton, 1992).
Cell Culture Bioassay For Branching
Ciliary ganglia obtained from E8 embryos were dissociated as previously described unless otherwise noted (Nishi, 1996). Neurons were plated at a density of 1000–2000 neurons per polylysine/laminin-coated coverslip in complete medium consisting of a modified L-15 medium (Mains and Patterson, 1973) containing 10% (v/v) heat-inactivated horse serum (Invitrogen, Carlsbad, CA), 6 mg/mL glucose, 20 U/mL penicillin, 20 mg/mL streptomycin, and 2 mM glutamine. Conditions that promoted adhesion with a basal level of branching were 0.4 μg laminin/coverslip and 1 ng/mL of chicken CNTF (chCNTF; a bacterially expressed [his]6chCNTFyyy purified in the Nishi lab (Finn et al., 1998). After 10 h, the cultures were incubated while alive for 30 min in mAb Q211 IgM ascites (1:3000, a generous gift from Dr. Herman Rohrer, Max Planck Institute for Brain Research, Frankfurt Germany), which recognizes a neuronal specific ganglioside that is the tetanus toxin receptor (Rohrer and Thoenen, 1987), then washed and fixed in Zamboni’s fixative (4% paraformaldehyde, 15% picric acid in 0.1M phosphate buffer, pH 7.4) for 20 min at room temperature. Cover slips were washed and incubated overnight at 4°C in blocking solution (10% (v/v) heat-inactivated horse serum (Invitrogen, Carlsbad, CA), 0.5% (w/v) Triton-X 100 (Sigma-Aldrich, Carlsbad, St. Louis, MO) in phosphate buffered saline (PBS). Anti-mouse Alexa 488 (1:750, Molecular Probes, Carlsbad, CA) was added for 1 hr at room temperature. Coverslips were washed and mounted on slides using Permafluor (Fisher Scientific, Pittsburg, PA).
E13 ciliary ganglia were dissociated by incubating in Earle’s Balanced Salt Solution (EBSS; Invitrogen) containing 1 mg/mL collagenase, Type 2 (Cat #4176, Worthington, Lakewood, NJ), 1 mg/mL hyaluronidase (Cat #2592, Worthington), and 10 mg/mL bovine serum albumin (Sigma, St. Louis, MO) for 15 min at 37°C. The activity of the proteases was terminated by dilution, the supernatant was removed, and ganglia were incubated in EBSS containing 3 mg/mL trypsin (Cat #3703, Worthington) for 3 min at 37°C. Ganglia were triturated until completely dissociated. Following dissociation, cells were preplated in complete medium in a 60 mm tissue culture dish for 30 min at 37°C in order to remove adherent cells. E13 neurons were plated at a density of 6000 neurons per coverslip. Each coverslip was coated with 2 μg of laminin to promote adhesion and the neurons survived in the absence of trophic support. After 21 h, the neurons were fixed and blocked as described above with the exception that neurons were labeled with rabbit anti-neurofilament 150 (1:500, Millipore, Billerca, MA) to facilitate identification of neurites and rat anti-somatostatin (1:100, Accurate Chemical and Scientific Corporation, Westbury, NY), to identify choroid neurons.
Neurotrophic factors were tested for branch inducing activity at concentrations that were at least 10x above saturation based on previously published dose response curves in E8 ciliary ganglion neurons: 10 ng/mL brain derived neurotrophic factor (BDNF), 10 ng/mL of neurotrophin-3 (NT-3), 10 ng/mL of transforming growth factor β1 (TGF-β1), 10 ng/mL of ciliary neurotrophic factor (CNTF), 10 ng/mL glial cell derived neurotrophic factor (GDNF) or 20 ng/mL of nerve growth factor (NGF). All growth factors were purchased from R&D systems, Minneapolis, MN. In E13 ciliary neurons, 10 ng/mL BDNF, 10 ng/mL TGF-β1, 10 ng/mL of CNTF, 5 ng/mL GDNF, 10 ng/mL of NGF, 10 ng/mL IGF-I, and 10 ng/mL IGF-II were used (all growth factors purchased from R&D systems, Minneapolis, MN). In both E8 and E13 cultures, d-tubocurarine (dTC, Sigma-Aldrich, St. Louis, MO), a nicotinic antagonist, was added at a concentration of 4.8 μM. This concentration of dTC is equivalent to 2 mg of dTC added per day in ovo, assuming that the drug distributes throughout the volume of the egg (60 mL). All factors were added to the medium at the time of plating. As stated above, E8 neurons were grown for 10 h prior to staining and fixation for analysis, while E13 neurons were grown for 21 h.
Total neurite length of all neurites and the total number of branch points per neuron were quantified with the investigator blinded to the samples using a Nikon Eclipse E600 equipped with epifluorescence, an automatic stage driver, video camera, and computer using Neurolucida® (MBF Bioscience, Williston, VT). To ensure consistency, only neurons that were not in contact with other neurons and had a neurite longer than three cell body lengths were quantified. Furthermore, a branch was defined and counted only if it extended 10 μm or longer. Experiments were done in triplicate and repeated a minimum of three times. Statistical analysis was done using a one-way or two-way ANOVA with Bonferroni post-test.
Drug Treatment In Ovo
The egg was windowed at E3 and the opening was covered with glass cover slip sealed with sterile vacuum grease. For all experiments, 100 μL of solution was applied to the chorioallantonic membrane of the embryo every 24 h from E8–E16. Drug dosages were: 0.9% (w/v) sodium chloride solution alone or containing 2 mg dTC, 1.25 μg α-bungarotoxin (αbtx; Sigma-Aldrich, St. Louis, MO) or a combination of 44 μg dihydro-β-erythroidine (DHβE, Sigma Aldrich, St. Louis, MO) and 0.1 μg methyllycaconitine (MLA, Sigma-Aldrich, St. Louis, MO). The drug doses of both dTC and αbtx have been well documented to silence cholinergic transmission and neuromuscular activity during development [Pittman and Oppenheim, 1979; Usiak and Landmesser, 1999; Oppenheim et al., 2000; Bunker and Nishi, 2002); Supporting Information Fig. 1(A)]. The cocktail of DHβE/MLA completely blocks ganglionic transmission in the ciliary ganglion (Liu et al., 2006) and we confirmed that this dosage of DHβE/MLA did not affect motility of the embryo [no significant difference in the number of kicks per minute when compared to the saline controls; Supporting Information Fig. 1(B)]. Windowed eggs were kept in a 37°C incubator until the tissue was harvested. Embryos were euthanized by rapid decapitation immediately after removal from the egg.
Immunohistochemistry of Iris Whole Mounts
Eyes were removed at E14 and E17 and fixed for 1–2 h at room temperature in Zamboni’s fixative and washed thoroughly in PBS. The iris was dissected from the anterior chamber of the eye and placed in blocking solution (PBS containing 10% (v/v) horse serum, 0.5% Triton X-100, and 0.1% sodium azide) overnight at 4°C, then incubated in rabbit anti-neurofilament 150 (1:500; Chemicon, Temecula, CA) overnight at 4°C. Secondary antibodies were anti-rabbit Cy3, anti-mouse TRITC IgG2b or anti-mouse Alexa 488 IgG1 (all secondary antibodies: 1:750, Molecular Probes Invitrogen, Carlsbad CA) and incubated overnight at room temperature, shaking. The tissue was washed with PBS and mounted in permaflour. The entire volume of the iris was imaged using 2.5 μm steps by a Nikon Eclipse E800 Fluorescence Microscope and Nikon C1 confocal system. For image presentation, the Z stacks were flattened to create a maximum intensity projection image using IMAGTRAK® (Freeware, created by Peter K. Stys).
Immunohistochemistry of Iris Sections
Eyes were isolated and fixed as described above. The anterior chamber was dissected out and equilibrated in 30% sucrose in PBS overnight at 4°C. Irises used for quantification of axons were cut in the same half using the incoming ciliary nerve for orientation, embedded in MICROM cryo-embedding compound (Richard Allen Scientific, Kalama-zoo, MI) and sectioned using a Microm HM 580 cryostat. Irises were cut in 30 μm sections in the sagittal plane, to visualize ciliary axons in cross section. The sections were placed on Superfrost Plus® slides (Fisher Scientific, Pitts-burgh, PA), dried at room temperature for 3–4 h, baked for 60 min at 40°C, and carefully immersed in PBS to rinse off the residual embedding medium. The slides were blocked overnight at 4°C in blocking solution. Sections were labeled with rabbit anti-neurofilament for 6–8 h at 4°C and incubated in secondary antibody anti-rabbit Cy3 overnight at 4°C. The sections were mounted and the entire thickness of the iris section was imaged using a C1 Nikon confocal with 2-μm steps through the Z-axis. Image parameters (exposure, gain and laser strength) were kept constant across all sections collected for analysis. Images were processed using IMAGTRAK® (freeware created by Peter K. Stys) and Volocity®(Improvision, England).
Quantification of Axonal Branching Using Volocity®
Analyses were performed with the observer blinded to the experimental condition. Branching was quantified in iris sections through the entire Z plane of the iris section using the image analysis software package, Volocity® (Perkin-Elmer, Waltham, MA) to count the number of neurofilament positive voxels (three-dimensional pixels). The iris was readily identified in cross section because of its unique structure—it is thin at the ciliary margin, thickens in the middle and becomes thinner again at the tip that defines the margin of the pupil. The intensity threshold for identifying neurofilament positive voxels was set empirically as the level at which staining of neurofilament above background could be discerned. Only voxels at this level or above were counted and these typically represented 1–8% of the total number of voxels, depending upon the number of axonal profiles, the strength of staining and the level of the background staining. The number of neurofilament positive voxels per section was normalized to the sum of all voxels in the same color channel to normalize for differences in background staining, antibody penetration, and intensity of the secondary antibody from sections taken from different animals. This normalized number was multiplied by 1000 to yield the total number of normalized voxel units (NF-VU). The overall thickness of the iris sections in all experiments was also evaluated and was not statistically different from animal to animal. Statistical analysis was done using a one-way or a two-way ANOVA and a Bonferroni post test as stated in the figure legends.
RNA Extraction From Iris Tissue and Ciliary Ganglia
Tissues were removed from embryos at E15 and E17 and immediately frozen on dry ice. RNA was extracted from irises pooled from three to nine embryos. Ciliary ganglia were rapidly isolated at E15 and E17 and pooled from both eyes of three embryos; three separate extractions were performed for the analyses reported. RNA was extracted according to manufacturer’s instructions using TriReagent (Molecular Research Center, Cincinnati, OH). The pellet was dried and resuspended in 20 μL of RNAse free water. RNA quality and quantity were determined using a NanoDrop (NanoDrop Technologies, Wilmington, DE). Complementary DNA (cDNA) was generated using equal amounts of RNA by reverse transcriptase using Oligo dTs and SuperScript II Reverse Transcriptase (Invitrogen, Carlsbad, CA). cDNA was stored at 4°C until used for real-time PCR.
Real-Time PCR
Real-time PCR primers and probes were designed as described in Taqman Universal PCR Master Mix protocol (Applied Biosystems, Foster City, CA; Table 1). The primers and probes were designed to span exon boundaries to prevent amplification of genomic DNA, with the one exception, Gallus BDNF (Accession number NM 001031616), which contains only one exon. The BDNF primers were tested on both DNAse (to remove genomic DNA) and non-DNAse-treated RNA to ensure similar amplification efficiencies (data not shown). The expression of BDNF, GDNF (Accession number AF176017), and CNTF (Accession number Z48168) were normalized to the expression of chicken ribosomal protein S17 (CHRPS, Accession number NM204217). All primers (Table 1) were designed using the http://frodo.wi.mit.edu/primer3 website, secondary structure was checked using MacVector® (Accelrys Burlington, MA) and purchased from Sigma Genosys (Woodland, TX) or Operon Biotechnologies (Huntsville, AL). The product size for all primers fell within the range of 100–150 bps. The probes were labeled on the 5′end with the 6-FAM reporter dye and on the 3′ end with a black hole quencher. Analysis and quantification were done using the ABI Prism 7700 sequence detector, Neuroscience COBRE Facility (University of Vermont, College of Medicine, Burlington, VT) and arbitrary units were calculated relative to CHRPS as an endogenous control. The PCR products from each set of primers were run on a 1% agarose gel to check for primer dimers (data not shown). Primer efficiency was evaluated using serial 1:10 dilutions of cDNA and the primers were validated if the slope fell between −3.3 and −3.8. Statistical analysis was done either using a Student’s t-test or ANOVA with a Bonferroni post test as indicated in the figure legends.
Table 1.
Real-Time PCR Primers and Probes for Indicated Gallus Transcripts
| Gene | Forward Primer (5′–3′) | Reverse Primer (5′–3′) | Probe (5′fam–3′BHQ) |
|---|---|---|---|
| Chrps | AACGACTTCCACACCAACAA | CTTCATCAGGTGGGTGACAT | CGCCATCATCCCCAGCAAGA |
| BDNF | AGCCCAGTGAGGAAAACAAG | ACTCCTCGAGCAGAAAGAGC | TACACATCCCGAGTCATGCTGAGCA |
| GDNF | TATGGGATGTCGTGGCTGT | GCCCCTCTACCACTGAAGG | TCTCCACCTCACCCCTGCCC |
| CNTF | CACTGGGTCTGCCTGAGA | GAAGTGTCCTGGGTTAGTAGCA | CTGCTGACAGGCACAGGGGCTT |
Quantification of Neuronal Number Using Design-Based Stereology
Design-based stereology was used to count the number of ciliary and choroid neurons as previously described (Bunker and Nishi, 2002) with the following modifications. Serial sections of E14 and E17 ciliary ganglia were cut at 30 μm using a Microm HM 580 cryostat and placed on Superfrost Plus® slides. Rat anti-somatostatin (Cat # YMC1020, Accurate Chemical and Scientific Corporation, Westbury, NY) was diluted 1:100 and mouse anti-Islet-1 (Hybridoma Supernatant Clone 39.405, Developmental Studies Hybridoma Bank, Iowa City, Iowa), a culture supernatant concentrated by ammonium sulfate precipitation in the Nishi lab, was diluted 1:250. Estimation of neuronal number was performed using the Optical Fractionator probe in Stereo Investigator® (MBF Bioscience, Williston, VT) as previously described (Lee et al., 2001). The number of ciliary neurons was calculated by subtracting the number of choroid neurons, as indicated by somatostatin-like immunoreactivity, from the total number of neurons. Statistical analysis was done using either a Student’s t-test or ANOVA with a Bonferroni post-test as indicated.
Retroviral Production
The dominant negative RET (dnRET) sequence was generated using the mouse RET DNA sequence (Accession number AF209436) with a truncation at amino acid position 2331, to remove the kinase domain (generous gift from Dr Felix Eckenstein, University of Vermont, Burlington, VT) and subcloned into RCASBP(A) retroviral vector by the Neuroscience COBRE Facility (University of Vermont, College of Medicine, Burlington VT). The dnTRKB construct was generated by truncating the Gallus TRKB sequence to remove the entire kinase domain and subcloned into RCASBP(A) retroviral vector (generous gift from Dr. Karina Cramer, University of California, Irvine). The open construct did not contain a transgene. Stable virus was generated by transfection of a RCASBP(A) dnRET, RCASBP(A) dnTRKB or RCASBP(A) open construct using the TransIT®-LT1 reagent according to manufacturer’s instructions (Mirus, Madison, WI) in chicken DF-1 cells (American Type Cell Culture Collection, Manassas, VA). Prior to collection of the virus, infected DF-1 cells were tested for expression of the appropriate proteins (Supporting Information Fig. 2). Virus was collected, concentrated, and titered as previously described (Morgan and Fekete, 1996; Lee et al., 2001; Hruska et al., 2007). Viral titer used was 109 infective particles per mL. Injections were done using a Nanoject II® (Drummond Scientific Company, Broomall, PA) and glass electrodes. A hole was cut into the egg at the appropriate stage (HH stage 8–9 or stage 14) and the hole was covered by a glass coverslip, sealed with vacuum grease until ready for the viral injection. Virus, resuspended with 4% Fast Green to aid in visualization, was injected in the neural tube of the developing embryos (57 nl). The embryos were then placed in a 37°C incubator until the tissue was harvested at the appropriate age. RCASBP retroviral vectors are used with the permission of Dr. Stephen Hughes (National Cancer Institute), who engineered these vectors (http://home.ncifcrf.gov/hivdrp/RCAS/requests.html).
RESULTS
Chronic Blockade of Neuromuscular Transmission Increases Branching of Axons in the Iris
We first determined whether axonal branching is altered in the avian iris after blocking neuromuscular transmission. Chicken embryos were treated with the paralytic, nicotinic acetylcholine receptor (nAChR) antagonist, d-tubocurarine (dTC), which blocks transmission of signals from the ciliary neurons to the striated muscle of the chicken iris. To monitor whether the dTC dose given was actually paralytic, we counted the number of kicks observed in treated and control embryos [Supporting Information Fig. 2(A)]. As reported previously (Pittman and Oppenheim, 1979), 2 mg/day of dTC was sufficient to reduce the number of movements by more than 90%. Iris whole mounts from E17 dTC and saline-treated embryos were stained for neurofilament immunoreactivity and the axons from the three ciliary nerves that enter the iris and ciliary muscle were readily visualized (Fig. 1). In dTC-treated embryos, the ciliary nerves are defasciculated in the ciliary muscle, but the iris appears to have many more axons (compare axons in the iris at the arrows in Fig. 1(A–D)]. At higher magnification, visual inspection reveals many more branch points of neurofilament immunoreactivity in the iris after dTC treatment [see arrows in Fig. 1(B, E)]. No new nerves are observed entering the iris after dTC treatment [note ciliary nerves entering in Fig. 1(A) with Fig. 1(D)]. When the iris is cut in the sagittal plane directly through the lens, the axons in the iris can be seen in cross-section; thus, neurofilament staining appears as punctate profiles throughout the thickness of the tissue and more profiles are seen in the dTC-treated iris [compare Fig. 1(C) with 1(F)]. Occasionally, axon bundles cut in a tangential plane can also be seen. In general, the number of axonal profiles increases after dTC treatment.
Figure 1.
Blocking neuromuscular transmission with dTC induces an increase in axonal branching in the iris Irises from embryos treated with saline or 2 mg/day dTC from E8–E16, removed at E17, fixed, and stained with anti-neurofilament, which is observed as white fibers. A: Montage of 10 images taken with a ×20 objective from a saline treated embryo. B: High magnification of the axons in the iris from the saline-treated embryo. Arrows point to individual axons that do not branch. C: Cross section of the iris from a different embryo showing the axons and axons bundles in profile. D: Montage of 10 images taken with a ×20 objective from a dTC-treated embryo. When the axons traveling in the iris at comparable distances from the primary ciliary nerve are examined (see arrows in A and D, there are many more axons in the dTC-treated iris. E: High magnification of axons in the iris of the dTC-treated embryo. Arrows point to axons that branch. F: Cross section of the iris from a different embryo but taken from the same radial position as the cross section in (C). Note many more axons and axon bundles are here (F) in comparison to the profiles in (C). Iris whole mounts and cross sections were imaged using a Nikon C1 confocal with a 2.5 or a 2 μm Z step, respectively. For (A, C D, and F) calibration bar = 200μm, for (B and E) calibration bar = 40μm, I, Iris; CM, ciliary muscle.
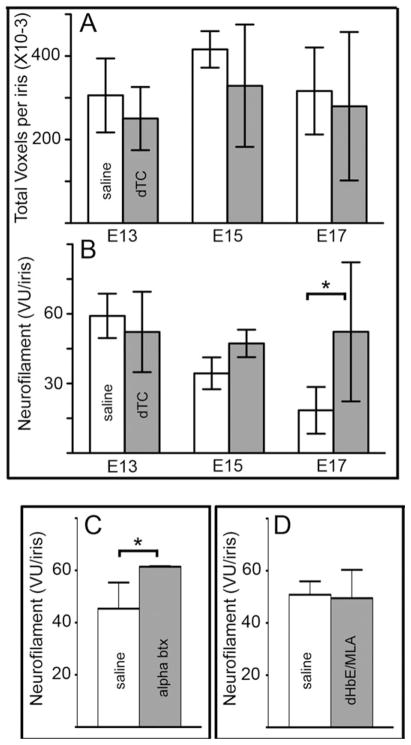
To quantify changes in the number of axons in the iris, we used Volocity®, an image analysis program, to determine the number of neurofilament positive voxels units (NF-VU) throughout a cross-section of iris taken. The total number of unthresholded voxels, which represents background staining and therefore an approximation of the volumes measured from sample to sample, did not differ significantly between saline and dTC-treated irises [Fig. 2(A)]. However, the number of thresholded, normalized voxels representing true neurofilament immunoreactivity (NF-VU) steadily decreases from E13 to E17 in saline-treated controls [Fig. 2(B)], which corresponds to the period during which the iris muscle changes from smooth to striated muscle (Link and Nishi, 1998a,b). This normal decrease in NF-VU may represent a process of pruning; however, in dTC-treated animals, the NF-VU per iris remains high. Thus, by E17, after the iris transition into striated muscle is complete (Pilar et al., 1987; Link and Nishi, 1998a), dTC-treated embryos have nearly 3-fold more NF-VU than saline-treated controls [Fig. 2(B)].
Figure 2.
Quantification of axonal number in irises using neurofilament-immunoreactivity Irises from animals treated with saline (light bars) versus 2 mg/day dTC (dark bars) starting at E7 were removed at E13, E15, and E17, fixed, sectioned, and stained with anti-neurofilament. Neurofilament immunoreactivity throughout the thickness of the section was imaged using a confocal microscope and the voxels were quantified as described in methods. A: Total number of unthresholded voxels represents background staining and does not differ significantly across the same samples. B: After counting only pixels above a threshold established by the background staining, the normalized neurofilament voxel units (VU) per iris section at different ages shows that axonal number in the iris declines with age and that dTC prevents the decline (P < 0.05). The error bars represent standard deviation. Statistical analysis was done using a two-way ANOVA with a Bonferroni post test (n = 3–6 iris sections, each from a different animal). C: Embryos were treated daily from E8–E16 with saline, 1.25 μg αbungarotoxin (αbtx) and processed as above at E17. Alpha btx, a nicotinic antagonist that binds to muscle nAChRs and α7 containing neuronal nAChRs completely blocks neuromuscular transmission but does not prevent transmission through the ciliary ganglion. A significant increase in axonal branching occurred in embryos treated with αbtx, *P < 0.05 (Student’s t-test, n = 3–5 for each condition). D: Embryos treated with saline or 44 μg dihydro-β-erythroidin (DHβE) + 0.1 μg αmethylycacontine (MLA) from E8–E16 and harvested at E17. This combination of nicotinic antagonists completely block transmission through the ciliary ganglion, but does not block neuromuscular transmission (see Supporting Information Fig. 2). No significant difference was observed between embryos treated with saline and embryos treated with DHβE +MLA (Student’s t-test). All error bars represent standard deviation.
To determine if dTC acts to enhance branching in the iris through its ability to block neuromuscular transmission or through its ability to antagonize neuronal nAChRs that mediate ganglionic transmission, we tested other nicotinic antagonists. Ciliary ganglion neurons express two major types of nAChRs: α7 subunit containing homomers and α3* heteromers that contain α3, α5, β4 and sometimes β2 (Liu and Berg, 1999). First, embryos were treated with 1.25 μg per day of αbungarotoxin (αbtx), which blocks muscle specific nAChRs as well as neuronal α7 nAChRs. Application of αbtx increased NF-VU in E17 iris over controls [Fig. 2(C)]. Secondly, a cocktail of 4 μM DHβE and 20 nM of MLA known to block ganglionic transmission completely by antagonizing both α3* and α7 containing nAChRs (Liu et al., 2006) did not change NF-VU over controls [Fig. 2(D)]. We also confirmed that this cocktail that does not paralyze movement by counting the number of kicks per minute observed in drug-treated animals [Supporting Information Fig. 2(B)]. Thus, two paralytic drugs that act by blocking nerve transmission to muscle, dTC and αbtx, cause increases in NF-VU, whereas a cocktail of nAChR antagonists that block cholinergic transmission through the ganglion without paralysis do not.
A specific increase in NF-VU due to dTC treatment could be caused either by more branching and axonal outgrowth per neuron, or it could result from enhanced neuronal survival, which would contribute many more axons. In the ciliary ganglion, target-dependent neuronal cell loss occurs from E8–E13, with neuronal number stabilizing after E14 (Landmesser and Pilar, 1974; Lee et al, 2001); therefore, we counted the number of ciliary and choroid neurons from saline and dTC-treated embryos at E14 using design-based stereology (Hruska et al., 2007). Chronic treatment with dTC reduces the number of ciliary neurons two-fold, but has no effect on survival of choroid neurons (Fig. 3). This result suggests that the higher number of NF-VU in the dTC-treated irises is due to increased branching and outgrowth of axons from the remaining ciliary neurons.
Figure 3.
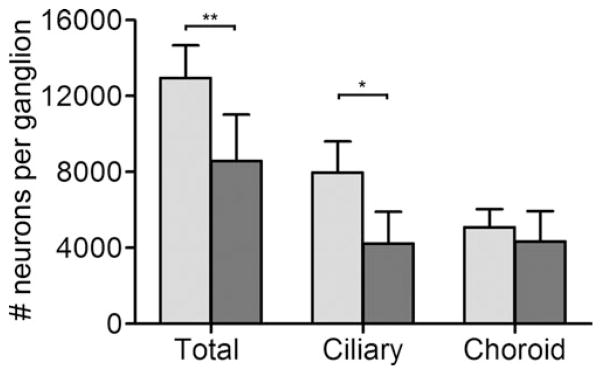
dTC treatment decreases survival of ciliary but not choroid neurons. Neurons were counted in E14 ciliary ganglia using design-based stereology after embryos were treated daily with saline (light bars) or 2 mg/day of the pan-nAChR antagonist, dTC (dark bars). Choroid neurons were distinguished from ciliary neurons by somatostatin immunoreactivity and all neurons were identified using the neuronal nuclei-specific antibody Islet-1. Error bars represent standard deviation. Statistical analysis was done using a Student’s t-test comparing drug treatments in each group (n = 6–9 ganglia, each ganglion from a different embryo, *p < 0.05, ***p < 0.001). The effect of dTC contrasts with that of αbtx, which enhances choroid and ciliary neuron survival by blocking neuronal α7 containing nAChRs (Bunker, 2002 #6450; Hruska, 2007 #7112).
Candidate Molecules that Induce Neurite Branching
We evaluated candidate neurotrophic factors for their ability to influence the degree of branching and neurite length from neurons isolated from dissociated ciliary ganglia and plated at a low density on coverslips. Neurites were visualized using immunofluorescence, traced, and quantified at a fixed time after plating (10 h for E8 neurons; 21 h for E13 neurons). At E8, neurons are beginning to extend axons into their respective target tissues, neuronal number has yet to be stabilized (Landmesser and Pilar, 1974), and there are no known markers for distinguishing between choroid and ciliary neurons. In contrast, at E13, the number of neurons in the ganglion is nearly stable (Landmesser and Pilar, 1974), functional synaptic contacts have been established with target tissues (Pilar et al., 1987), choroid neurons can be identified by somatostatin-like immunoreactivity (Epstein et al., 1988; Coulombe and Nishi, 1991), and low density cultures can be readily established with minimal non-neuronal cell contamination (at older ages, neurons fail to adhere to laminin and prefer to adhere to non-neuronal cells; R. Nishi, unpublished observation).
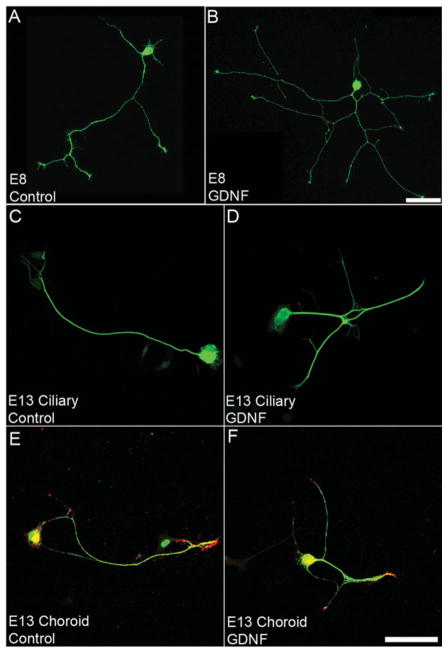
In E8 ciliary ganglion neurons, GDNF, TGF-β1, CNTF, and NGF induce a two-fold increase in the number of branch points and total neurite length [Fig. 4(A, B) and Table 2], whereas NT-3, BDNF, and dTC have no significant effect (Table 2). In contrast, E13 neurons respond to only GDNF and CNTF: GDNF induces a two-fold increase in the number of branch points per ciliary neuron [Fig. 4(C, D)], and this response is not seen in choroid neurons [Fig. 4(E, F) and Table 2], whereas CNTF induces a two- and three-fold increase in the number of branch points of choroid and ciliary neurons, respectively (Table 2). At E13, none of the factors tested promotes an increase in total neurite length (Table 2). Likewise, dTC has no direct effect on either branching or total neurite length (Table 2). Note that profound changes in the complexity of neurite morphology can be observed with a two-fold increase in the number of branch points [compare Fig. 4(A) to (B) and Fig. 4(C) to 4(D)]. All factors were tested at concentrations 10x above the level of saturation typically observed in cell culture-based assays; therefore, we were reasonably sure that a lack of effect was truly a negative result. In fact, it is unlikely that factors that produce effects at significantly higher levels (e.g., 100x saturation) are working through the expected signaling system because these very high concentrations could be producing a nonspecific activation of related receptors.
Figure 4.
GDNF induces branching of E8 and E13 ciliary ganglion neurons in vitro. Representative images of E8 (AB) and E13 (C–F) ciliary ganglion neurons cultured without the addition of factors (Control, A, C, E) or with the addition of 10 ng/ml of GDNF (B, D, F). E8 neurons were grown for 10 h and live labeled with Q211, a neuronal specific ganglioside. E13 neurons were cultured for 21 h and double labeled with neurofilament (green), to label axons, and somatostatin (red), to label choroid neurons. Calibration bar = 50 μm.
Table 2.
Effects of Factors in Cell Culture on Neurite Outgrowth and Branching of Ciliary Ganglion Neurons at E8 Versus E13
| Factor | E8 Ciliary Ganglion
|
E13 Ciliary
|
E13 Choroid
|
|||
|---|---|---|---|---|---|---|
| #BP | NL (mm) | #BP | NL (mm) | #BP | NL (mm) | |
| Control | 7 ± 0.5 | 575 ± 20 | 2.3 ± 0.2 | 800 ± 35 | 2.9 ± 0.3 | 743 ± 82 |
| dTC | 9 ± 0.6 | 754 ± 33 | 1.6 ± 0.3 | 554 ± 91 | 2.4 ± 0.4 | 704 ± 66 |
| IGF-I | ND | ND | 1.8 ± 0.6 | 621 ± 89 | 2.0 ± 0.3 | 730 ± 90 |
| IGF-II | ND | ND | 1.7 ± 0.3 | 594 ± 60 | 2.5 ± 0.3 | 665 ± 50 |
| NT-3 | 9.4 ± 0.7 | 709 ± 35 | ND | ND | ND | ND |
| BDNF | 9.9 ± 0.6 | 736 ± 34 | 2.4 ± 0.3 | 694 ± 65 | 3.1 ± 0.4 | 832 ± 74 |
| TGFβ | 10 ± 0.7* | 846 ± 41* | 2.4 ± 0.5 | 800 ± 97 | 2.4 ± 0.4 | 673 ± 87 |
| NGF | 11.3 ± 0.7* | 882 ± 37* | 3.3 ± 0.4 | 926 ± 73 | 3.1 ± 0.5 | 598 ± 67 |
| GDNF | 11 ± 0.6* | 800 ± 35* | 3.8 ± 0.4+ | 795 ± 39 | 3.7 ± 0.5 | 734 ± 82 |
| CNTF | 11 ± 0.6* | 891 ± 61* | 5.7 ± 0.5+ | 1020 ± 76 | 4.9 ± 0.5⋄ | 805 ± 81 |
E8 or E13 neurons were isolated and grown on coverslips for 10 h (E8) or 21 h (E13) in the presence of the indicated growth factors. Neurites were labeled and the total length of all neurites (NL) per neuron as well as the number of branch points (BP) were quantified as described in methods. At E8, there are no markers for distinguishing ciliary from choroid neurons. At E13, choroid neurons were identified by somatostatin-like immunoreactivity (SOM-IR); ciliary neurons were negative for SOM-IR. Concentrations of growth factors were: 4.8 × 10−6M dTC, 10 ng/mL IGF-I, 10 ng/mL IGF-II, 10 ng/mL NT-3, 10 ng/mL BDNF, 10 ng/mL GDNF, 10 ng/mL TGF-β, 10 ng/mL CNTF, 1 ng/mL NGF (For E8), and 10 ng/mL NGF (for E13). Experiments were done in triplicate and repeated a minimum of three times. Statistical analysis was done by comparing BP and NO after addition of the individual neurotrophic factor to control with a one way ANOVA with Bonferroni post test. ND, not done. All values that were significantly different from untreated controls are bolded.
E8 neurons, n = 52–63 Branching: p < 0.01 for TGF-β, p < 0.001 for NGF, CNTF and GDNF; Outgrowth: p < 0.01 for GDNF, p < 0.001 for TGF-b, NGF, and CNTF.
E13 ciliary neurons, n = 23–143 #BP: p < 0.05 for GDNF, p < 0.01 for CNTF.
E13 choroid neurons, n = 33–144 #BP: p < 0.01 for CNTF.
Because BDNF did not promote neurite branching in E8 or E13 ciliary neurons (Table 2), but has been linked to the maturation and stabilization of axonal territories by neuronal activity (Hu et al., 2005), we tested whether BDNF could inhibit the effects of a branching factor (Fig. 5). BDNF inhibited GDNF-induced neurite branching in E13 ciliary [Fig. 5(A–D)]. Since GDNF expression did not induce branching of E13 choroid neurons, we did not expect, nor did we observe, any changes in branching of choroid neurons cultured with BDNF + GDNF [Fig. 5(D)]. The total neurite length in these cultures was unaffected in either population of neurons [Fig. 5(E)].
Figure 5.
BDNF inhibits GDNF induced branching in E13 ciliary neurons. Neurons from dissociated E13 embryos were cultured for 21 h in the presence of no factor (A, control), 10 ng/mL of GDNF (B) or 10 ng/mL GDNF+10 ng/mL BDNF (C). Cultures were double-labeled with antineurofilament and anti-somatostatin to identify ciliary (lacking somatostatin-immunoreactivity) and choroid (somatostatin-immunoreactive) neurons. The number of branch points (D) and total neurite length (E) per ciliary neuron and choroid neuron were quantified using Neurolucida®. Each experiment was done in triplicate and repeated three times. Statistical analysis was done using a one way ANOVA with a Bonferroni post test to compare treatments in ciliary and choroid neurons, separately. GDNF produces a significantly higher number of branch points per ciliary neuron above control unsupplemented medium and the addition of BDNF inhibits the effect of GDNF (*p < 0.05, n = 49–52 cells). These effects are not seen in choroid neurons. Error bars represent standard error of the mean. Calibration bar = 50 μm.
These assays show that branching and neurite outgrowth of ciliary neurons to growth factors decreases between E8 and E13, and that CNTF and GDNF (or family members that activate the same receptor signaling pathways) are candidate neurotrophic molecules in vivo that induce increased branching in the iris caused by blocking neuromuscular transmission between E13 and E17. In contrast, BDNF (or neurotrophins that activate the same receptor signaling pathways) could act to stabilize axonal territories in the developing iris by inhibiting branching. Therefore, we chose to focus on these three growth factors for further study.
Expression of Candidate Neurotrophic Factors In Vivo
The relative levels of GDNF, CNTF, and BDNF mRNA in irises from embryos treated with dTC or saline were determined using quantitative real-time PCR. In saline-treated embryos, the level of GDNF mRNA decreases two-fold between E15 and E17. In contrast, with dTC treatment the expression of GDNF mRNA does not drop, but remains high throughout the developmental window [Fig. 6(A)]. The levels of CNTF and BDNF mRNA in the iris between dTC-and saline-treated embryos are unchanged by dTC treatment across the same developmental window [Fig. 6(B–C)]. Thus, the enhanced expression of GDNF in the iris after dTC treatment together with its ability to promote branching of E13 neurons in cell culture makes GDNF a possible branching factor whose expression is controlled by neuromuscular transmission.
Figure 6.

The expression of GDNF mRNA is increased in iris from dTC-treated embryos at E17. The relative expression of (A) GDNF, (B) CNTF, or (C) BDNF mRNA in E15 and E17 iris tissue after treatment with saline (light bars) or 2 mg/day of dTC (dark bars) from E8–E16 was quantified with real-time PCR using Taqman probes. The mRNA expression was normalized to chicken ribosomal protein s17 (Chrps). Each bar represents the mean normalized arbitrary units (AU) of the indicated number of irises, which were separately extracted for total RNA. Each iris represents a different animal. Each qPCR run was performed using duplicate samples of template from each iris. The error bars represent standard deviation. Statistical analysis was done using a two way ANOVA with a Bonferroni post test (*p < 0.05, E15 saline vs. E17 saline, ***p < 0.001, E17 saline vs. E17 dTC).
Retroviral Expression of Dominant Negative RET
To block signaling induced by GDNF and related family members, we expressed a dominant negative (dn) RET (constructed by deleting the tyrosine kinase domain and generously provided by F.P. Eckenstein, University of Vermont; Supporting Information Fig. 2). The efficacy of the dnRET construct was tested in E8 ciliary ganglion neurons. Infected neurons were identified by immunoreactivity for the viral gag (Fig. 7). The branching induced by GDNF [Fig. 7(A) vs. (B)] is blocked in dnRET infected neurons, but not in open RCASBP(A) infected neurons [compare Fig. 7(D) to 7(B); (E)]. Interestingly, there is also a significant decrease in total neurite length of E8 neurons from both the control and GDNF-treated cultures from dnRET infected embryos [Fig. 7(F)]. Thus, we proceeded to use the dnRET construct to block GDNF family member signaling in vivo.
Figure 7.
GDNF enhanced branching is reduced in E8 ciliary neurons when ret signaling is blocked HH St 8–9 embryos were injected with open control (A, B) or dn ret (C, D) retroviral constructs, embryos were harvested on E8 and cultured with no addition (A, C) or with 10 ng/mL GDNF (B, D) for 10 h. Q211 was used to label axons and anti-p27 gag to label infected neurons. The number of branch points (E) and total neurite length (F) per virally infected neurons was quantified with no addition (light bars) and with the addition of 10 ng/mL GDNF (dark bars) using Neurolucida3. Experiment was done in triplicate, n = 16–20. Error bars represent standard error of the mean. Statistical analysis was done using a two way ANOVA with a Bonferroni post test (**p < 0.01, ***p < 0.001). Calibration bar = 50 μm.
Embryos infected with dnRET or open RCASBP(A) were treated with either saline or dTC from E8–16 and harvested at E17. As expected, open RCASBP(A) infected embryos responded as previously observed with uninfected embryos-dTC-treated irises had twice the number of NF-VU than saline-treated controls [compare light colored bars in Fig. 8(A)]; however, when expressing dnRET, the NF-VU of dTC-treated irises dropped to the levels seen in saline-treated animals [compare dark bar of dTC-treated embryo to light and dark bars of saline-treated embryos in Fig. 8(A)]. In addition, dnRET caused a significant decrease in survival in saline-treated embryos, with a trend towards doing so in dTC-treated embryos [Fig. 8(B)]. Thus, RET is responsible for the increase in NF-VU caused by dTC treatment and is clearly necessary for optimal neuronal survival during the period of target innervation.
Figure 8.
Blocking signaling through RET prevents dTC mediated increases in the number of axon profiles in the iris and reduces ciliary neuron survival HH St 8–9 embryos were infected with open (light bars) or dnRET (dark bars) retroviral constructs and treated daily with saline or 2 mg/day of dTC from E8–E16. Irises and ciliary ganglia were removed at E17 and processed as described in methods. Ciliary ganglia from the same embryos were also removed and processed for design-based stereology as described in methods. A: Quantification of NF-VU per iris section. B: Number of ciliary neurons per ganglion. Error bars represent standard deviation. Statistical analysis was done using a two-way ANOVA with a Bonferroni post test (axonal branching: *p < 0.05; ciliary neuron counts: **p < 0.01). Error bars represent standard deviation.
Retroviral Expression of dnTRKB
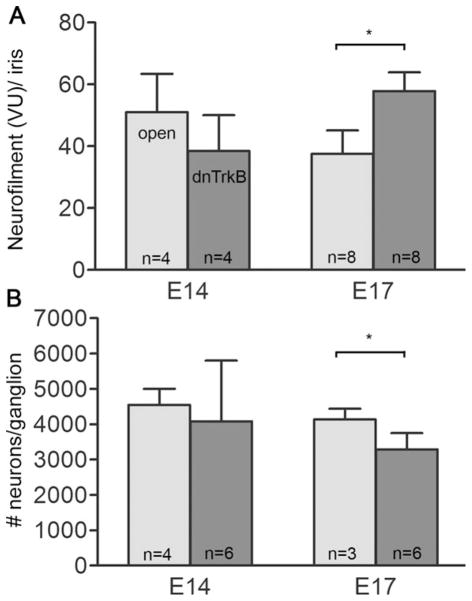
To test whether TRKB inhibits axonal branching in vivo, as we previously demonstrated in vitro, we infected embryos with a construct previously shown to act as a dominant negative TRKB in chicken embryos (Turner et al., 2006; Supporting Information Fig. 2). At E17, there is a significant increase in NF-VU in iris sections from dnTRKB infected embryos [Fig. 9(A)]. When the survival of ciliary neurons was examined at E14, the number of ciliary neurons between open and dnTRKB infected embryos was unchanged [Fig. 9(B)], showing that activation of TRKB is not required to prevent normal programmed cell death, which occurs between E8–14. However, at E17, the number of ciliary neurons from dnTRKB infected embryos was significantly decreased compared to ciliary neurons from open RCASBP(A) infected embryos [Fig. 9(B)], and this decrease became very pronounced at later stages (data not shown). This suggests that TRKB is necessary for survival of mature ciliary ganglion neurons.
Figure 9.
Blocking TRKB signaling increases axonal profiles in iris and decreases survival of ciliary neurons HH St 8–9 embryos were infected with open (light bars) or dnTrkB (dark bars) retroviral constructs. Irises and ciliary ganglia were removed at E17 and processed as described in methods. A: Quantification of NFVU per iris section. B: Number of ciliary neurons per ganglion. Error bars represent standard deviation. Statistical analysis was done using a two-way ANOVA with a Bonferroni post test (*p < 0.05).
DISCUSSION
The principal finding of this study is that the neurotrophic factor receptors RET and TRKB coordinate survival and axonal outgrowth of developing ciliary neurons that innervate the striated muscle of the avian iris. RET is necessary for neuronal survival during the period of target innervation in vivo as well as for axonal branching enhanced by blocking neuromuscular transmission. In contrast, TRKB inhibits branching and is necessary for maintenance of mature neurons. Thus, our studies demonstrate that neurotrophic signaling plays multifunctional roles in coordinating interactions between target tissues and the neurons that innervate them.
Branching or extent of axonal outgrowth within a target tissue in vivo is challenging to quantify. Previous studies reporting enhanced axonal branching after neuromuscular blockade have used qualitative criteria (Brandon et al., 2003) or quantified synaptogenesis by counting postsynaptic clusters of nicotinic acetylcholine receptors (Pittman and Oppenheim, 1979; Dahm and Landmesser, 1988). We chose to quantify neurofilament immunoreactivity in order to follow actual branching or the extent of neurite outgrowth without limiting the analysis to synaptogenesis. While it is possible to stain whole mounts of avian iris, the actual tracing of axons throughout the entire volume of the iris is labor-intensive and prone to user error, thereby precluding the analysis of a sufficient number of samples necessary to achieve statistical significance in experimental manipulations. Instead, we relied on sampling that quantified the number of NF-VU throughout the entire volume of a representative iris cross-section chosen by external markers to be reproducible from animal to animal. Based on our observations, most fibers run parallel to the surface of the iris, circling its structure while coursing throughout its thickness to synapse on myofibers; thus, a cross-section of these fibers would show more neurofilament staining if more fibers were entering the structure, or if more fibers were branching while in the structure. Our method of quantification was able to detect differences in NF-VU when embryos were paralyzed with either dTC or αbtx, but only after the period of time when the iris muscle is completely composed of striated muscle myotubes (Pilar et al., 1987; Link and Nishi, 1998a). Furthermore, the same differences were not detected in embryos treated with a cocktail of dHβE and MLA known to block ganglionic transmission, thus the increased NF-VU in the iris was selectively observed only when the embryos were paralyzed.
The relative contribution of neuronal survival to axonal branching is important to monitor. For example, an increase in NF-VU within the iris could be due to enhanced axonal outgrowth or branching or simply due to an increased number of ciliary neurons. While this may be true for αbtx treatment because αbtx increases neuronal survival (Bunker and Nishi, 2002), dTC increases NF-VU by the same magnitude while causing a loss of ciliary neurons. Interestingly, this suggests a homeostatic mechanism that regulates the number of axons allowed to enter and arborize in the iris. Paralysis with dTC increases the density of NF-VU in the iris, even when the total number of neurons innervating the iris is reduced. This expansion of innervation into denervated areas was previously observed when the ciliary nerve was axotomized at E7–8 (Pilar et al., 1980). One caveat of our study is that NF-VU cannot distinguish among ciliary, sympathetic, or sensory fibers. We stained iris sections with an antibody against chicken tyrosine hydroxylase to identify noradrenergic fibers and failed to detect immunoreactivity in the iris, although fibers were found encircling the ciliary musculature (data not shown). In addition, it has been estimated that only 15% of the axons in the ciliary nerve are sensory (Pilar et al., 1980), thus the majority of the changes in NF-VU we observed by selective blockade of neuromuscular transmission were most likely in ciliary axons.
Our studies in cell culture with acutely isolated neurons at two stages of development (prior to target innervation (E8) and after target innervation (E13) established a matrix of responsiveness to candidate trophic molecules. These results complement and add to studies that previously showed that CNTF, GDNF, and neurturin, but not artemin and persephin, stimulate neurite outgrowth from explanted E8 ciliary ganglia, while E16 explants only responded to CNTF (Hashino et al., 2001). However, these previous studies did not quantify total neurite length or the extent of branching of individual neurons, nor did they examine the responsiveness of ciliary versus choroid neurons. At E8, when initial outgrowth into target tissues occurs, ciliary ganglion neurons respond to a large number of candidate trophic factors, including GDNF, TGF-β, CNTF, and NGF. Thus, even when RET is blocked, neurite outgrowth can be stimulated by TGF-β, CNTF, and NGF. In contrast, by E13, neurons do not require trophic support and axonal outgrowth and branching become selective for CNTF and GDNF with only GDNF able to affect branching independently of stimulating increased neurite length.
Consistent with this ability of E13 ciliary neurons to respond to GDNF by enhanced branching, we see an increase in GDNF mRNA in the iris in dTC-treated embryos, and dnRET prevents the enhanced branching in the iris caused by dTC. The RET receptor mediates signaling of GDNF family members GDNF, neurturin, persephin, and artmemin and has been implicated in a wide variety of trophic functions (Airaksinen and Saarma, 2002; Ernsberger, 2008; Paratcha and Ledda, 2008). Others have shown that GDNF and neurturin support survival and promote axonal outgrowth of embryonic ciliary ganglion neurons in cell culture (Buj-Bello et al., 1995; Hashino et al., 2001); and we complement these studies in vivo by showing that dnRET enhances cell death without preventing initial axonal outgrowth into the iris, but prevents surviving neurons from responding to signals generated by the muscle when neuromuscular transmission is blocked. While these results may seem paradoxical (how can an increase in GDNF caused by dTC result in a decrease of ciliary neuron survival), it can be explained by the temporal coordination of growth factor sensitivity. Ciliary neurons at E8 require CNTF or GDNF for survival, but, after neuronal number stabilizes at E13–14, neurons do not require trophic support (Nishi, unpublished observations). In fact, GDNF protein levels in the eye decline to undetectable levels by E12 (Hashino et al., 2001). Our studies here show that dTC prevents this decline in GDNF in the iris, allowing it to induce branching between E15 and E17. In other studies, overexpression of GDNF in murine neonatal skeletal muscle induces hyperinnervation in the gastroncemius muscle without affecting neuronal number (Nguyen et al., 1998), and overexpression of GDNF in adult mice increases the size of the motor unit three to eight fold (Keller-Peck et al., 2001).
The dissociation of axonal sprouting from survival observed with blocking neuromuscular transmission of ciliary neurons with dTC contrasts to that found in spinal cord motor neurons. In the spinal cord, dTC rescues motor neurons while it enhances axonal branching in muscle (Pittman and Oppenheim, 1979; Oppenheim et al., 2000), and the same is observed for embryonic muscle-specific antagonists (Oppenheim et al., 2008) as well as the GABA-A agonist muscimol (Oppenheim et al., 2003). Thus, in spinal cord motor neurons, enhanced branching after neuromuscular blockade is likely to be a mechanism for promoting enhanced survival, while in parasympathetic neurons, other factors may influence survival independently of branching. For example, blocking α7 containing receptors with αbtx or MLA rescues ciliary ganglion neurons from cell death (Bunker and Nishi, 2002; Hruska et al., 2007), while non-specific blockers of nicotinic signaling such as chlorisondamine (Wright, 1981) and pempidine (Maderdrut et al., 1988) in addition to dTC exacerbate cell death.
TRKB is also multifunctional during development. Our results show that expression of dnTRKB in vivo enhances axonal branching to the same degree as seen after dTC treatment, suggesting that dTC may not only act by upregulating a GDNF family member in the muscle, but also by downregulating activation of TRKB in ciliary neurons, perhaps by decreasing TRKB receptor number. This finding together with our observation that BDNF can directly inhibit GDNF-induced neurite branching of ciliary neurons in vitro implicate neurotrophins as factors responsible for inhibiting branching in the developing iris. In the chicken hindlimb, application of BDNF after dTC treatment restores synaptic architecture (Loeb et al., 2002). BDNF is also instrumental in activity driven synaptic remodeling during development (Hu et al., 2005). In addition, inhibition through BDNF is vital in the stabilization and maturation of dendrites in Xenopus retinal ganglion cells (Lom et al., 2002). Furthermore, the dependence of older neuronal survival on TRKB is consistent with in vitro results obtained by Pugh et al. (2006) and suggests that trophic dependence of ciliary ganglion neurons transitions from GDNF to BDNF after E14 in vivo.
The sum of our results suggests a homeostatic model of target innervation for the striated muscle of the avian iris that is coordinated by RET and TRKB. At E8, ciliary axons enter the iris and branch. Subsequently, developmental cell death (Landmesser and Pilar, 1974; Lee et al., 2001) and axonal pruning (Role and Fischbach, 1987) reduce the number of axons in the iris between E11 and E17. When the normal number of ciliary neurons is decreased by exogenous manipulations such as dTC and dnRET, axonal outgrowth increases to fill synaptic space. However, when neuromuscular transmission is blocked, the lack of activity causes the muscle to attract more axons through retrograde signaling mediated by RET, leading to a higher than normal axonal density. In addition to retrograde signaling that stimulates branching and axonal outgrowth, RET also is necessary for supporting survival of early embryonic ciliary ganglion neurons (between E8). Finally, as neurons mature, TRKB becomes responsible for maintenance of ciliary neuron survival. Thus, neurotrophic molecules serve diverse functions in coordinating target innervation by differentially regulating both neuronal survival and axonal outgrowth.
Acknowledgments
Contract grant numbers: NS25767, DA17784.
The authors thank Drs. Rodney Parsons and Felix Eckenstein for their helpful comments on this manuscript and John Dewitt for the western blot shown in Supporting Information Figure 2. They also thank the Molecular Cellular Core and the Imaging Core of the UVM Center of Biomedical Research Excellence in Neuroscience, funded by 5P20RR016435, for the use of their facilities, as well as the UVM DNA Analysis Facility.
Footnotes
Additional Supporting Information may be found in the online version of this article.
References
- Airaksinen MS, Saarma M. The GDNF family: Signalling, biological functions and therapeutic value. Nat Rev Neurosci. 2002;3:383–394. doi: 10.1038/nrn812. [DOI] [PubMed] [Google Scholar]
- Brandon EP, Lin W, D’Amour KA, Pizzo DP, Dominguez B, Sugiura Y, Thode S, et al. Aberrant patterning of neuromuscular synapses in choline acetyltransferase-deficient mice. J Neurosci. 2003;23:539–549. doi: 10.1523/JNEUROSCI.23-02-00539.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buj-Bello A, Buchman VL, Horton A, Rosenthal A, Davies AM. GDNF is an age-specific survival factor for sensory and autonomic neurons. Neuron. 1995;15:821–828. doi: 10.1016/0896-6273(95)90173-6. [DOI] [PubMed] [Google Scholar]
- Bunker GL, Nishi R. Developmental cell death in vivo: Rescue of neurons independently of changes at target tissues. J Comp Neurol. 2002;452:80–92. doi: 10.1002/cne.10363. [DOI] [PubMed] [Google Scholar]
- Coulombe JN, Nishi R. Stimulation of somatostatin expression in developing ciliary ganglion neurons by cells of the choroid layer. J Neurosci. 1991;11:553–562. doi: 10.1523/JNEUROSCI.11-02-00553.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahm LM, Landmesser LT. The regulation of intramuscular nerve branching during normal development and following activity blockade. Dev Biol. 1988;130:621–644. doi: 10.1016/0012-1606(88)90357-0. [DOI] [PubMed] [Google Scholar]
- Danzer SC, Crooks KR, Lo DC, McNamara JO. Increased expression of brain-derived neurotrophic factor induces formation of basal dendrites and axonal branching in dentate granule cells in hippocampal explant cultures. J Neurosci. 2002;22:9754–9763. doi: 10.1523/JNEUROSCI.22-22-09754.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeChiara TM, Bowen DC, Valenzuela DM, Simmons MV, Poueymirou WT, Thomas S, Kinetz E, et al. The receptor tyrosine kinase MuSK is required for neuromuscular junction formation in vivo. Cell. 1996;85:501–512. doi: 10.1016/s0092-8674(00)81251-9. [DOI] [PubMed] [Google Scholar]
- DeStefano M, Ciofi LA, Mugnaini E. Neuronal ultra-structure and somatostatin immunolocalization in the ciliary ganglion of chicken and quail. J Neurocytol. 1993;22:868–892. doi: 10.1007/BF01186358. [DOI] [PubMed] [Google Scholar]
- Epstein ML, Davis JP, Gellman LE, Lamb JR, Dahl JL. Cholinergic neurons of the chicken ciliary ganglion contain somatostatin. Neuroscience. 1988;25:1053–1060. doi: 10.1016/0306-4522(88)90058-9. [DOI] [PubMed] [Google Scholar]
- Ernsberger U. The role of GDNF family ligand signalling in the differentiation of sympathetic and dorsal root ganglion neurons. Cell Tissue Res. 2008;333:353–371. doi: 10.1007/s00441-008-0634-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn TP, Kim S, Nishi R. Overexpression of ciliary neurotrophic factor in vivo rescues chick ciliary ganglion neurons from cell death. J Neurobiol. 1998;34:283–293. [PubMed] [Google Scholar]
- Finn TP, Nishi R. Expression of a chicken ciliary neurotrophic factor in targets of ciliary ganglion neurons during and after the cell-death phase. J Comp Neurol. 1996;366:559–571. doi: 10.1002/(SICI)1096-9861(19960318)366:4<559::AID-CNE1>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Gautam M, Noakes PG, Mudd J, Nichol M, Chu GC, Sanes JR, Merlie JP. Failure of postsynaptic specialization to develop at neuromuscular junctions of rapsyn-deficient mice. Nature. 1995;377:232–236. doi: 10.1038/377232a0. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J Morphol. 1951;88:49–92. [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. 1951. Dev Dyn. 1992;195:231–272. doi: 10.1002/aja.1001950404. [DOI] [PubMed] [Google Scholar]
- Hanover JL, Huang ZJ, Tonegawa S, Stryker MP. Brain-derived neurotrophic factor overexpression induces precocious critical period in mouse visual cortex. J Neurosci. 1999;19:RC40. doi: 10.1523/JNEUROSCI.19-22-j0003.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashino E, Shero M, Junghans D, Rohrer H, Milbrandt J, Johnson EM., Jr GDNF and neurturin are target-derived factors essential for cranial parasympathetic neuron development. Development. 2001;128:3773–3782. doi: 10.1242/dev.128.19.3773. [DOI] [PubMed] [Google Scholar]
- Hruska M, Ibanez-Tallon I, Nishi R. Cell-autonomous inhibition of alpha 7-containing nicotinic acetylcholine receptors prevents death of parasympathetic neurons during development. J Neurosci. 2007;27:11501–11509. doi: 10.1523/JNEUROSCI.3057-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B, Nikolakopoulou AM, Cohen-Cory S. BDNF stabilizes synapses and maintains the structural complexity of optic axons in vivo. Development. 2005;132:4285–4298. doi: 10.1242/dev.02017. [DOI] [PubMed] [Google Scholar]
- Huang ZJ, Kirkwood A, Pizzorusso T, Porciatti V, Morales B, Bear MF, Maffei L, et al. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell. 1999;98:739–755. doi: 10.1016/s0092-8674(00)81509-3. [DOI] [PubMed] [Google Scholar]
- Keller-Peck CR, Feng G, Sanes JR, Yan Q, Lichtman JW, Snider WD. Glial cell line-derived neurotrophic factor administration in postnatal life results in motor unit enlargement and continuous synaptic remodeling at the neuromuscular junction. J Neurosci. 2001;21:6136–6146. doi: 10.1523/JNEUROSCI.21-16-06136.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landmesser L. The relationship of intramuscular nerve branching and synaptogenesis to motoneuron survival. J Neurobiol. 1992;23:1131–1139. doi: 10.1002/neu.480230906. [DOI] [PubMed] [Google Scholar]
- Landmesser L, Pilar G. Synaptic transmission and cell death during normal ganglionic development. J Physiol. 1974;241:737–749. doi: 10.1113/jphysiol.1974.sp010681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee VM, Smiley GG, Nishi R. Cell death and neuronal replacement during formation of the avian ciliary ganglion. Dev Biol. 2001;233:437–448. doi: 10.1006/dbio.2001.0236. [DOI] [PubMed] [Google Scholar]
- Link BA, Nishi R. Development of the avian iris and ciliary body: The role of activin and follistatin in coordination of the smooth-to-striated muscle transition. Dev Biol. 1998a;199:226–234. doi: 10.1006/dbio.1998.8918. [DOI] [PubMed] [Google Scholar]
- Link BA, Nishi R. Development of the avian iris and ciliary body: mechanisms of cellular differentiation during the smooth-to-striated muscle transition. Dev Biol. 1998b;203:163–176. doi: 10.1006/dbio.1998.9019. [DOI] [PubMed] [Google Scholar]
- Liu QS, Berg DK. Extracellular calcium regulates responses of both alpha3- and alpha7- containing nicotinic receptors on chick ciliary ganglion neurons. J Neurophysiol. 1999;82:1124–1132. doi: 10.1152/jn.1999.82.3.1124. [DOI] [PubMed] [Google Scholar]
- Liu Z, Neff RA, Berg DK. Sequential interplay of nicotinic and GABAergic signaling guides neuronal development. Science. 2006;314:1610–1613. doi: 10.1126/science.1134246. [DOI] [PubMed] [Google Scholar]
- Loeb JA, Hmadcha A, Fischbach GD, Land SJ, Zakarian VL. Neuregulin expression at neuromuscular synapses is modulated by synaptic activity and neurotrophic factors. J Neurosci. 2002;22:2206–2214. doi: 10.1523/JNEUROSCI.22-06-02206.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lom B, Cogen J, Sanchez AL, Vu T, Cohen-Cory S. Local and target-derived brain-derived neurotrophic factor exert opposing effects on the dendritic arborization of retinal ganglion cells in vivo. J Neurosci. 2002;22:7639–7649. doi: 10.1523/JNEUROSCI.22-17-07639.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maderdrut JL, Oppenheim RW, Prevette D. Enhancement of naturally occurring cell death in the sympathetic and parasympathetic ganglia of the chicken embryo following blockade of ganglionic transmission. Brain Res. 1988;444:189–194. doi: 10.1016/0006-8993(88)90928-6. [DOI] [PubMed] [Google Scholar]
- Mains RE, Patterson PH. Primary cultures of dissociated sympathetic neurons. I. Establishment of long-term growth in culture and studies of differentiated properties. J Cell Biol. 1973;59:329–345. doi: 10.1083/jcb.59.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marwitt R, Pilar G, Weakly JN. Characterization of two ganglion cell populations in avian ciliary ganglia. Brain Res. 1971;25:317–334. doi: 10.1016/0006-8993(71)90441-0. [DOI] [PubMed] [Google Scholar]
- Morgan BA, Fekete DM. Manipulating gene expression with replication-competent retroviruses. Methods Cell Biol. 1996;51:185–218. doi: 10.1016/s0091-679x(08)60629-9. [DOI] [PubMed] [Google Scholar]
- Nguyen QT, Parsadanian AS, Snider WD, Lichtman JW. Hyperinnervation of neuromuscular junctions caused by GDNF overexpression in muscle. Science. 1998;279:1725–1729. doi: 10.1126/science.279.5357.1725. [DOI] [PubMed] [Google Scholar]
- Nishi R. Autonomic and sensory neuron cultures. Methods Cell Biol. 1996;51:249–263. doi: 10.1016/s0091-679x(08)60632-9. [DOI] [PubMed] [Google Scholar]
- Oppenheim RW, Caldero J, Cuitat D, Esquerda J, Ayala V, Prevette D, Wang S. Rescue of developing spinal motoneurons from programmed cell death by the GABA(A) agonist muscimol acts by blockade of neuromuscular activity and increased intramuscular nerve branching. Mol Cell Neurosci. 2003;22:331–343. doi: 10.1016/s1044-7431(02)00020-9. [DOI] [PubMed] [Google Scholar]
- Oppenheim RW, Caldero J, Cuitat D, Esquerda J, McArdle JJ, Olivera BM, Prevette D, Teichert RW. The rescue of developing avian motoneurons from programmed cell death by a selective inhibitor of the fetal muscle-specific nicotinic acetylcholine receptor. Dev Neurobiol. 2008;68:972–980. doi: 10.1002/dneu.20636. [DOI] [PubMed] [Google Scholar]
- Oppenheim RW, Prevette D, D’Costa A, Wang S, Houenou LJ, McIntosh JM. Reduction of neuromuscular activity is required for the rescue of motoneurons from naturally occurring cell death by nicotinic-blocking agents. J Neurosci. 2000;20:6117–6124. doi: 10.1523/JNEUROSCI.20-16-06117.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozdinler PH, Macklis JD. IGF-I specifically enhances axon outgrowth of corticospinal motor neurons. Nat Neurosci. 2006;9:1371–1381. doi: 10.1038/nn1789. [DOI] [PubMed] [Google Scholar]
- Paratcha G, Ledda F. GDNF and GFRalpha: a versatile molecular complex for developing neurons. Trends Neurosci. 2008;31:384–391. doi: 10.1016/j.tins.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Pilar G, Landmesser L, Burstein L. Competition for survival among developing ciliary ganglion cells. J Neurophysiol. 1980;43:233–254. doi: 10.1152/jn.1980.43.1.233. [DOI] [PubMed] [Google Scholar]
- Pilar G, Nunez R, McLennan IS, Meriney SD. Muscarinic and nicotinic synaptic activation of the developing chicken iris. J Neurosci. 1987;7:3813–3826. doi: 10.1523/JNEUROSCI.07-12-03813.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittman R, Oppenheim RW. Cell death of motoneurons in the chick embryo spinal cord. IV. Evidence that a functional neuromuscular interaction is involved in the regulation of naturally occurring cell death and the stabilization of synapses. J Comp Neurol. 1979;187:425–446. doi: 10.1002/cne.901870210. [DOI] [PubMed] [Google Scholar]
- Pugh PC, Zhou X, Jayakar SS, Margiotta JF. Depolarization promotes survival of ciliary ganglion neurons by BDNF-dependent and -independent mechanisms. Dev Biol. 2006;291:182–191. doi: 10.1016/j.ydbio.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Rohrer H, Thoenen H. Relationship between differentiation and terminal mitosis: Chick sensory and ciliary neurons differentiate after terminal mitosis of precursor cells, whereas sympathetic neurons continue to divide after differentiation. J Neurosci. 1987;7:3739–3748. doi: 10.1523/JNEUROSCI.07-11-03739.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Role LW, Fischbach GD. Changes in the number of chick ciliary ganglion neuron processes with time in cell culture. J Cell Biol. 1987;104:363–370. doi: 10.1083/jcb.104.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streppel M, Azzolin N, Dohm S, Guntinas-Lichius O, Haas C, Grothe C, Wevers A, et al. Focal application of neutralizing antibodies to soluble neurotrophic factors reduces collateral axonal branching after peripheral nerve lesion. Eur J Neurosci. 2002;15:1327–1342. doi: 10.1046/j.1460-9568.2002.01971.x. [DOI] [PubMed] [Google Scholar]
- Turner BA, Sparrow J, Cai B, Monroe J, Mikawa T, Hemp-stead BL. TrkB/BDNF signaling regulates photoreceptor progenitor cell fate decisions. Dev Biol. 2006;299:455–465. doi: 10.1016/j.ydbio.2006.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usiak MF, Landmesser LT. Neuromuscular activity blockade induced by muscimol and d-tubocurarine differentially affects the survival of embryonic chick motoneurons. J Neurosci. 1999;19:7925–7939. doi: 10.1523/JNEUROSCI.19-18-07925.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright L. Cell survival in chick embryo ciliary ganglion is reduced by chronic ganglionic blockade. Brain Res. 1981;227:283–286. doi: 10.1016/0165-3806(81)90114-0. [DOI] [PubMed] [Google Scholar]