Abstract
In a facile and potentially general method for protein modification at the C-terminus, aldehyde-modified proteins, obtained from enzymatic protein prenylation, react rapidly with hydrazide and aminooxy surfaces and fluorophores at neutral pH and in micromolar concentration ranges of reagents. This strategy was used for fluorescent labeling of eGFP-CVIA, as a model protein, with aminooxy and hydrazide fluorophores or PEGs, and immobilization onto and subsequent release of the protein from hydrazide-functionalized agarose beads using hydrazone-oxime exchange. This method is described in detail here and provides site-specifically PEGylated or fluorescently labeled proteins starting from crude cellular extract in three steps: prenylation, capture, and release.
Keywords: PFTase, farnesyl diphosphate, site-specific protein modification, protein immobilization, oxime ligation, hydrazine ligation
Introduction
One of the most important challenges in modern chemical biology is the site-specific modification of proteins, a problem that is due to the large number of reactive functional groups typically present in polypeptides. Developing methods for site-specific modification of proteins is of great importance given the broad utility of this procedure in fields such as medicine (Gault et al., 2008), chemistry, and biology (Keppler et al., 2004). Site-specific protein modification experiments have been useful for studies of naturally occurring post-translational modifications, for introduction of fluorophores and other small molecules for biophysical studies (Saraogi et al., 2011), for creating antibody-drug conjugates (Axup et al., 2012), for oriented protein immobilization (Seo et al., 2011), for preparation of protein-polymer conjugates (Deiters et al., 2004), and for examining protein structure, folding, dynamics and protein-protein interactions (Berggård et al., 2007). One of the main advantages of site-specific protein modification is that it can be used to create site-specific covalent linkages between proteins and other biomolecules, surfaces, or materials (Kalia et al., 2007). This ensures accessibility to the active site of the protein and homogeneous coverage of surfaces. These are important factors in structure-function studies, to improve bioavailability and pharmacokinetics of proteinbased drugs (Jevsevar et al., 2010), in studying protein expression and localization, and in the development of biosensors (Monošík et al., 2012). Notably, protein immobilization is an important first step for many biotechnology applications, including immunoassays (Lee et al., 2003) and protein conjugates used for medical therapies, as well as for the construction of protein microarrays and biosensors.
Recently, a number of groups have exploited the high specificity of the enzyme protein farnesyltransferase (PFTase) for site-specifically modifying proteins (Duckworth et al., 2006; Gauchet et al., 2006). In nature, PFTase transfers a farnesyl group from its natural substrate, farnesyl pyrophosphate (FPP), to the cysteine of a “CaaX-box” tetrapeptide sequence (where C is cysteine, “a” is an aliphatic amino acid, and X is one of a variety of amino acids). PFTase is promiscuous in the nature of its substrates, in that it can tolerate a variety of CaaX sequences as well as some modifications to the FPP structure. Moreover, the reaction can also be performed in vitro.
Several different azide-, alkyne- (Duckworth et al., 2007), and diene-functionalized (Nguyen et al., 2007) FPP analogs have been developed, including FPP analogs that incorporate aldehyde functionality. Distefano and coworkers showed that a simple aldehyde-containing analog of FPP could be used to incorporate an aldehyde into eGFP-CVIA (Rashidian et al., 2010). The resulting aldehyde-functionalized protein was then used for immobilization or was labeled with a fluorophore via oxime formation. In a subsequent study, we demonstrated that such aldehyde-modified proteins could be prepared and captured from crude extract by incubation with a hydrazide-containing resin and released by treatment with an alkoxyamine (Rashidian et al., 2012; also see Fig. 1). Using this strategy, fluorescently labeled or PEGylated eGFP-CVIA was prepared without prior purification of the starting protein. A PEGylated form of GIP, a protein with potential as a therapeutic agent for diabetes, was also prepared in an analogous fashion (Rashidian et al., 2012). This approach for protein modification could be particularly useful for large-scale production of protein conjugates for industrial or therapeutic applications. Here, we describe a general protocol for implementing this strategy.
Figure 1.

Schematic representation of (A) site-specific prenylation of a protein and subsequent fluorophore labeling through oxime ligation. (B) Crude prenylation, immobilization, and subsequent labeling and release of proteins.
Basic Protocol 1 describes the synthesis of farnesyl aldehyde diphosphate, an FPP analog that can be enzymatically incorporated into proteins using PFTase. Basic Protocol 2 describes enzymatic incorporation of farnesyl aldehyde diphosphate into proteins and site-specific labeling through oxime ligation chemistry, where eGFP-CVIA is used as a model protein. Basic Protocol 3 describes specific prenylation of eGFP-CVIA in crude cellular extract using the aldehyde FPP analog, immobilization of the aldehyde-labeled eGFP-CVIA onto hydrazide beads through hydrazine ligation, and subsequent release and labeling of the immobilized protein by a transoximization reaction.
Basic Protocol 1
Synthesis of Farnesyl Aldehyde Diphosphate (Compound 1)
In our previous studies, we have reported two aldehyde-containing analogs of farnesyl diphosphate (FPP), compounds 1 and 2 (Fig. 2), that can be enzymatically incorporated into proteins (Fig. 3B; Rashidian et al., 2012). The steady-state kinetic parameters for both substrates are presented in Figure 2. Here we describe the procedure for synthesis of farnesyl aldehyde diphosphate (1), which manifests greater catalytic efficiency. The procedure and the data for synthesis of this FPP analog have been adapted from earlier publications (Rashidian et al., 2010, 2012). The synthesis scheme and the 1H NMR spectrum of compound 1 are presented in Figures 4 and 5, respectively.
Figure 2.
Steady state kinetic parameters of aldehyde-containing PFTase substrates. The subscript “rel” refers to kcat/KM with respect to FPP.
Figure 3.

(A) Structures of farnesyl diphosphate (FPP), and farnesyl aldehyde diphosphate (1). (B) Schematic representation of prenylation of a protein containing a CaaX-box positioned at its C-terminus with an aldehyde-containing analog (FPP-aldehyde) to yield an aldehyde functionalized protein.
Figure 4.
Schematic representation of synthesis of farnesyl aldehyde diphosphate from farnesol.
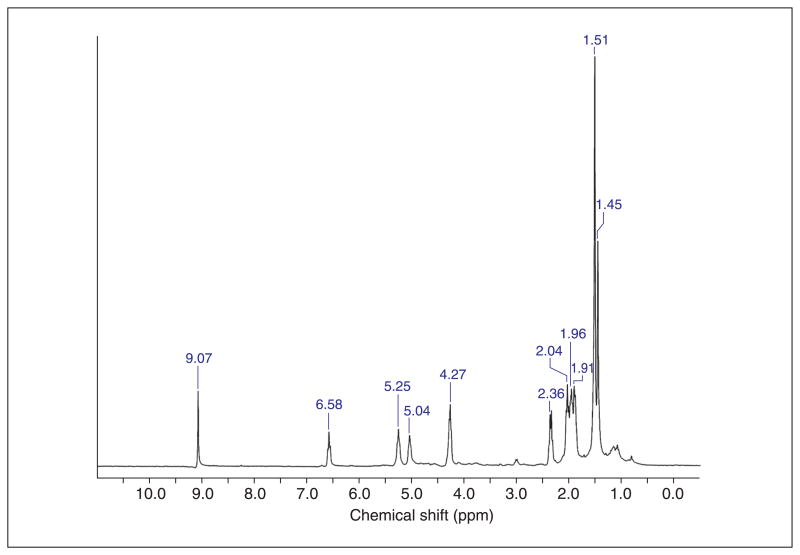
Figure 5.
1H NMR for FPP-aldehyde 1. 1H NMR: (500 MHz, D2O) δ 9.07 (s, 1H), 6.58 (t, J = 7.5 Hz, 1H), 5.25 S9 (t, J=7 Hz, 1H), 5.04 (t, J = 6 Hz, 1H), 4.27 (t, J = 6 Hz, 2H), 2.36 (t, J = 7 Hz, 2H), 2.04 (t, J = 7 Hz, 2H), 1.96 (t, J = 7 Hz, 2H), 1.917 (t, J = 7 Hz, 2H), 1.54 (s, 6H), 1.49 (s, 3H). 31P NMR: (121 MHz, D2O) δ -5.971 (d, J = 22.6, 1P), -10.013 (d, J = 22.6, 1P). HR-ESI-MS calcd for C15H26O8P2 [M-H]- 395.1025, found 395.0907.
Materials
Farnesol (Aldrich)
Dichloromethane (DCM)
3,4-dihydropyran (Aldrich)
Pyridinium p-toluenesulfonate (PPTS; Aldrich)
Diethyl ether (Et2O)
Sodium bicarbonate (NaHCO3)
Sodium sulfate (Na2SO4)
Silica gel 60
tert-butyl hydroperoxide (Aldrich)
Selenous acid (H2SeO3; Aldrich)
Salicylic acid (Aldrich)
Toluene
Ethyl acetate (EtOAc)
Dimethylsulfoxide (DMSO)
Triethylamine (TEA; Aldrich)
Sulfur trioxide pyridine complex (SO3·Py; Aldrich)
Hexanes
5 M HCl (aqueous)
Brine (saturated aqueous NaCl)
Isopropyl alcohol
Trichloroacetonitrile (CCl3CN)
Acetonitrile (ACN)
Bis(triethylammonium) hydrogen phosphate (Et3NH)2HPO4
Ammonium bicarbonate (NH4HCO3)
Acetonitrile (CH3CN)
Heavy water (D2O)
Sodium pyrophosphate (Na2H2P2O7)
200-ml and 50-ml round-bottom flasks
Magnetic stirrer and stir bar
Büchi Rotavapor model R-114 or equivalent rotary evaporator
10 × 2–in. (25.4 × 5.0–cm) chromatography column(s)
TLC plates (silica gel 60 F-254; Merck)
Separatory funnel
Oil bath
Büchner funnel
0.45-μm syringe filter
HPLC instrument: Beckman model 127/166 equipped with a UV detector and a Phenomenex C18 column (Luna, 10 μm, 10 × 250 mm) with a 5-cm guard column
Electro spray ionization mass spectrometer (ESI-MS; Bruker BioTOF II)
Lyophilizer
500-MHz 1H NMR instrument (Oxford VI-500 MHz)
Synthesis of compound 3
Dissolve 8.89 g (40 mmol) farnesol in 60 ml dichloromethane in a 200-ml round-bottom flask equipped with a magnetic stir bar.
Add 5.00 g (60 mmol) 3,4-dihydropyran.
Add 1.00 g (4 mmol) pyridinium p-toluenesulfonate.
Stir the resulting solution for 5 hr at room temperature on a magnetic stirrer.
Remove solvent under reduced pressure in a Büchi Rotavapor or equivalent rotary evaporator.
Dissolve the residue in 40 ml Et2O, then wash two times, each time with 20 ml of saturated aqueous NaHCO3 solution in a separatory funnel.
Dry the organic solution over Na2SO4.
Evaporate the solvent under reduced pressure in a rotary evaporator, to yield 11.0 g (90% yield) of compound 3 as a colorless oil (90% yield).
Synthesis of compound 4
-
9
Dissolve 11.3 g (37 mmol) compound 3 in 50 ml of DCM.
-
10
Add 14 ml t-butyl hydroperoxide.
-
11
Add 480 mg (3.4 mmol) H2SeO3.
-
12
Add 480 mg (3.4 mmol) salicylic acid.
-
13
Stir the mixture for 10 hr at room temperature.
-
14
Evaporate off the DCM under reduced pressure in a rotary evaporator.
-
15
Add toluene to the remaining mixture followed by evaporation in a rotary evaporator. Repeat this step three more times to remove all remaining t-butyl hydroperoxide.
Toluene forms a low-boiling point azeotrope with t-butyl hydroperoxide, thus making it easier to evaporate the excess peroxide under reduced pressure.
-
16
Dilute the residue in 30 ml Et2O, then wash it two times with 20 ml saturated NaHCO3 solution.
-
17
Dry the resulting solution over Na2SO4 and filter off the drying agent.
-
18
Evaporate the solvent using a rotary evaporator.
-
19
Load 150 g silica gel 60 in a 10 × 2–in. (25.4 × 5.0–cm) column equilibrated with 5:2 (v/v) toluene/EtOAc (mobile phase).
-
20
Dissolve the crude sample in a small amount of the mobile phase and load it onto the column. Elute with 6 column volumes of the mobile phase. Analyze the eluate as it emerges from the column by TLC (use mobile phase as solvent, Rf = ∼ 0.25). Pool fractions that contain the product.
-
21
Evaporate the solvents using reduced pressure in a rotary evaporator to yield 2.98 g (25%) of 4 as a pale yellow oil.
Synthesis of compound 5
-
22
Dissolve 1.00 g of 5 in 20 ml anhydrous DCM in a 50-ml round bottom flask.
-
23
Cool the solution to 0°C using an ice bath.
-
24
Add 3.10 ml (43 mmol) DMSO dropwise over 3 min.
-
25
Add 2.75 g (197 mmol) TEA to the solution.
-
26
Add 2.52 g (15.8 mmol) sulfur trioxide pyridine complex (SO3·Py) dropwise over 10 min.
-
27
Stir the reaction for 1 hr at 0°C, until TLC analysis (Hex: EtOAc, 1:1) indicates complete conversion to the product.
-
28
Dilute the reaction mixture with 100 ml DCM.
-
29
To remove TEA and SO3·Py, wash the solution at least two times, each time with 10 ml 5 M HCl, until the aqueous layer remains acidic. Check the pH with pH paper.
-
30
Wash the resulting solution with 15 ml saturated NaHCO3 in a separatory funnel, then wash it two times, each time with 10 ml brine (saturated aqueous NaCl).
-
31
Dry the resulting solution over Na2SO4 and remove drying agent by filtration.
-
32
Evaporate solvents under reduced pressure in a rotary evaporator to obtain a pale yellow oil.
-
33
Load 150 g silica gel into a 10 × 2–in. (25.4 × 5.0–cm) column pre-equilibrated with hexanes.
-
34
Dissolve the oil from step 32 in small amount of hexanes and load it onto the column. Elute with a solvent gradient starting from pure hexanes going to 3:1 Hex:EtOAc in 5 column volumes. Analyze the eluate as it emerges from the column by TLC in 1:1 Hex/EtOAc and collect the fractions that contain the desired product. (Rf = ∼0.45; use same solvent as mobile phase).
-
35
Evaporate solvents under reduced pressure using a rotary evaporator to give 0.79 g of compound 5 as a pale yellow oil (80% yield).
Synthesis of compound 6
-
36
Dissolve 1.30 g (4.05 mmol) of 5 in 20 ml of isopropyl alcohol in 50-ml round bottom flask.
-
37
Add 30 mg PPTS as the catalyst.
-
38
Reflux the reaction for 4 hr at 85°C in an oil bath, until TLC analysis shows almost complete conversion to the product (Rf = ∼0.20).
-
39
Add 10 ml EtOAc to quench the reaction, and then add an additional 100 ml EtOAc.
-
40
Separate the organic layer using a separatory funnel, then dry over Na2SO4.
-
41
Remove the solvent under reduced pressure using a rotary evaporator to obtain 0.87 g of compound 6 as a pale yellow oil (91% yield; Rf of ∼0.20 in 1:1 hexanes/EtOAc).
Synthesis of compound 1
-
42
Dissolve 0.18 g (0.76 mmol) of 6 in 460 μl CCl3CN (4.6 mmol).
-
43
Add 0.57 g (1.9 mmol) of (Et3NH)2HPO4 to 3.6 ml dry ACN in a separate flask.
-
44
Place the mixture from step 43 in a 30°C oil bath for 5 min to dissolve all the salts.
-
45
Add the resulting solution dropwise over 3 hr to the mixture obtained from step 43 while it is stirring at room temperature.
Slow addition is essential for obtaining higher yields.
-
46
After the addition of (Et3NH)2HPO4, stir the solution for an additional 15 min at room temperature.
-
47
Remove the solvent under reduced pressure in a rotary evaporator.
-
48
Add 15 ml 25 mM aqueous NH4HCO3 solution to the residue, resulting in the formation of a white precipitate.
-
49
Filter the solution by vacuum filtration using a Büchner funnel and collect the filtrate.
Purification of compound 1
-
50
Filter the resulting solution through a syringe filter and then purify by RP-HPLC using a semi-preparative column under following conditions:
detection at 214 nm
flow rate 5.0 ml/min
5-ml injection loop
solvent A, 25 mM NH4HCO3 in water
solvent B, CH3CN
Elution time program:
Gradient 0% to 30% solvent B in 30 min
-
30% to 100% solvent B in 5 min.
Compound 1 elutes from 20% to 25% solvent B. As soon as a peak appears in that range, collect fractions every 2 min in a new test tube.
-
51
Analyze each collected fraction by ESI-MS in negative mode looking for a calculated mass of C15H26O8P2 [M-H]- 395.1025.
-
52
Combine all product-containing fractions in a lyophilization jar, freeze in liquid nitrogen, and lyophilize for 24 hr to obtain a white powder.
Measure the concentration of aldehyde-FPP 1
-
53
Dissolve the resulting powder in 2 ml D2O.
This is a purified solution of final product, but the concentration has to be measured.
-
54
Prepare a 10 mM solution of Na2H2P2O7 in water.
This will be used as internal standard for measuring the phosphorus concentration in the solution.
-
55
Combine equal volumes of the solutions from steps 53 and 54 (0.5 ml of each solution). Add the resulting mixture to an NMR tube and acquire a phosphorus NMR with relaxation time set to 45 sec for each pulse (Fig. 6). Use the integration ratio between the two peaks to calculate the concentration of 1 in the solution.
For the integration calculation, note that the singlet peak corresponds to the two phosphorus atoms from Na2H2P2O7 and each doublet corresponds to one phosphorus atom from compound 1.
Figure 6.
31P NMR of FPP-aldehyde 1 and Na2H2P2O7 added as an internal standard.
Basic Protocol 2
Enzymatic Incorporation of Farnesylaldehyde Diphosphate into Protein, and Subsequent Site-Specific Labeling and PEGylation
Once the FPP-aldehyde (compound 1) has been synthesized and purified, it can be appended to any desired protein that contains a C-terminal CaaX box sequence using PFTase. The aldehyde-labeled protein can then be site-specifically coupled to aminooxyor hydrazide-functionalized molecules through ligation reactions. In this protocol, using yeast PFTase, compound 1 is incorporated into eGFP-CVIA as a model protein. Then, the aldehyde-labeled eGFP-CVIA (8) is conjugated to aminooxy-AlexaFluor 488 (a fluorescent dye) and aminooxy polyethylene glycol (aminooxy-PEG) using aniline or m-phenylenediamine as a catalyst (Fig. 7A,B).
Figure 7.

(A) Site-specific PEGylation of eGFP-CVIA from reaction between pure aldehyde-eGFP-CVIA (8) and aminooxy-PEG (9). (B) MALDI analysis of PEGylated eGFP-CVIA (11). The lower panel is the MALDI spectrum of pure 8 and the top panel is the MALDI spectrum of the oxime PEGylated eGFP-CVIA (10), which shows a complete oxime ligation. The reaction was performed using 8 (10 μM) and 9 (100 μM) for 2 hr. Excess of 10 was removed via a ZipTip protocol prior to MALDI analysis.
Materials
Tris hydrochloride Tris base
MgCl2
KCl
ZnCl2
Dithiothreitol (DTT)
6×His–tagged eGFP-CVIA stock solution (see Support Protocol 2)
Compound 1 stock solution (see Basic Protocol 1)
PFTase stock solution (see Support Protocol 1)
Aminooxy-AlexaFluor 488 (Anaspec)
Dimethylsulfoxide (DMSO)
Aniline or m-phenylenediamine (Aldrich)
1.0 M sodium phosphate buffer, pH 7
Aminooxy-PEG (9; mol. wt. 5,000; NOF Corp., http://nofamerica.net/)
30°C incubator
Amicon Centriprep centrifugal filter (MWCO 10,000; Millipore)
Centrifuge (Beckman-Coulter)
UV-Vis spectrophotometer (50 Bio; Varian)
600-μl microcentrifuge tubes
NAP-5 column (Amersham)
LC-MS instrument (Waters Synapt G2 Quadropole TOF mass spectrometer instrument)
MALDI-MS instrument (Bruker MALDI TOF)
Additional reagents and equipment for analyzing the efficiency of prenylation (Support Protocol 3), SDS-PAGE (e.g., Gallagher, 2006), and MALDI-MS (e.g., Carr and Annan, 1997)
Incorporate FPP-aldehyde 1 into eGFP-CVIA
-
In a 15-ml plastic centrifuge tube, prepare 10 ml of solution containing:
50 mM Tris·Cl, pH 7.5
10 mM MgCl2
30 mM KCl
10 μM ZnCl2
5 mM DTT
2.4 μM 6×His–tagged eGFP-CVIA
30 to 50 μM FPP-aldehyde 1
80 to 200 nM PFTase.
Add the PFTase last, after everything else is prepared, to avoid losing any enzyme activity before initiating the reaction.
-
Incubate the solution for 2 hr in a 30°C incubator. To concentrate the aldehyde-modified protein (7), load the solution in a centrifugal filter (10,000 mol. wt. cut off) and centrifuge at 5300 × g to reduce the volume to about 500 μl.
Yeast PFTase has maximal activity at 30 C.
-
Apply the resulting solution onto a NAP-5 column pre-equilibrated with 50 mM Tris·Cl, pH 7.5. Elute the column with 1 ml of 50 mM Tris·Cl, pH 7.5, and collect the eluate.
This step removes salts and remaining aldehyde-FPP 1.
To determine the final concentration of the aldehyde-modified protein (8), measure the UV absorbance of the solution at 488 nm using a UV-Vis spectrophotometer(ε488 = 55,000 M−1cm−1).
Analyze the efficiency of prenylation by LC-MS analysis (see Support Protocol 3).
Coupling reaction between aldehyde-modified eGFP-CVIA (8) with aminooxy AlexaFluor488
-
6
Prepare a 5 mM stock solution of aminooxy-AlexaFluor 488 in DMSO.
-
7
Use the solution from step 6 and the stock solution of 8 obtained in step 3 to prepare a 100 μl solution containing 60 μM of 8, 300 μM of aminooxy-AlexaFluor 488, and 100 mM of aniline or 50 mM of m-phenylenediamine in 1.0 M phosphate buffer, pH 7, in a 600 μl microcentrifuge tube.
-
8
Vortex the solution and allow the reaction to proceed for 2 to 4 hr.
-
9
Load the solution onto a NAP-5 column pre-equilibrated with 50 mM Tris·Cl, pH 7.5. Elute the column with 1 ml of 50mM Tris·Cl, pH 7.5, and then collect the eluate in a new microcentrifuge tube.
This removes excess aminooxy-AlexaFluor 488 and aniline from the solution.
-
10
Analyze the reaction by either MALDI-MS or LC-MS to confirm the formation of the desired AlexaFluor 488–modified eGFP-CVIA.
Calculated mass = 27,559.
Coupling reaction between aldehyde-modified eGFP-CVIA (8) and aminooxy-PEG (9)
-
11
In a 600-μl microcentrifuge tube, dissolve 2 mg aminooxy PEG (9) in 100 μl of 0.1 phosphate buffer.
-
12
Add 8 to above solution to final concentration of 10 μM.
-
13
Add aniline or m-phenylenediamine to final concentration of 100 mM or 50 mM respectively, to initiate the reaction.
-
14
Vortex the tube and leave it for 2 hr at room temperature.
-
15
Confirm the formation of product by SDS-PAGE analysis (e.g., Gallagher, 2006).
Load 3 to 5 μg of protein in each lane of the gel. The PEGylated protein appears as a lower-mobility band.
-
16
Confirm the formation of product by MALDI-MS analysis (Fig. 7; also see Carr and Annan, 1997).
Since the PEG used in this case is not a monodisperse PEG, ESI-MS cannot be used to analyze the reaction. Thus, MALDI-MS is preferred over ESI-MS for analysis of PEGylated proteins and polypeptides.
Basic Protocol 3
Crude Prenylation, Immobilization, and Subsequent Labeling and Release of Proteins
An important advantage of aldehyde labeling of proteins through prenylation is the high specificity resulting from the enzymatic nature of this process. Since only proteins that contain a CaaX box at their C-terminus can be prenylated, it is possible to label a specific protein of interest in complex biological media, such as crude cellular extracts. The aldehyde-labeled protein can then be selectively immobilized onto hydrazide beads, thus purifying it from the many other proteins present in the crude mixture. Additionally, the immobilized protein can be released into a solution by addition of excess of an alkoxyamine that is linked to a variety of molecules such as a fluorophore or a PEG-based compound as illustrated here (Fig. 8).
Figure 8.

Chemoenzymatic site-specific tagging of proteins with FPP-aldehyde analogs by PFTase followed by capture of the aldehyde-functionalized protein in the crude cell lysate with hydrazide functionalized beads. Prenylation in the crude extract is confirmed by LC-MS analysis. The immobilized protein is then released into the solution or fluorescently labeled by addition of hydroxylamine or an aminooxy-fluorophore, using aniline or m-phenylenediamine as the catalyst. SDS-PAGE analysis: lane 1, crude E. colilysate containing the protein-CaaX visualized by Coomassie blue staining; lane 2, fluorescently labeled protein of interest released from hydrazide beads after treatment with an aminooxy-fluorophore and visualized by Coomassie blue staining; lane 3, fluorescently labeled protein of interest released from hydrazide beads after treatment with the aminooxy fluorophore and visualized by in-gel fluorescence analysis.
In this protocol, eGFP-CVIA, which is expressed in E. coli, is labeled with aldehyde-FPP (1) in crude cell lysate. The resulting eGFP-CVIA-aldehyde is then immobilized on hydrazide-functionalized agarose beads. The immobilized protein is then released back into solution and simultaneously labeled by the addition of aminooxy-PEG or aminooxy-AlexaFluor 488.
Materials
6×His–tagged eGFP-CVIA cell extracts (see Support Protocol 2)
Tris base
Tris hydrochloride
MgCl2
KCl
ZnCl2
Dithiothreitol (DTT)
Compound 1 (Basic Protocol 1)
PFTase stock solution (see Support Protocol 1)
50 mM Tris·Cl, pH 7.5
Hydrazide-functionalized agarose beads (Thermo Scientific)
Purified 6×His–tagged eGFP-CVIA solution (see Support Protocol 2)
1.0 M sodium phosphate buffer, pH 7
Aniline or m-phenylenediamine (Aldrich)
1 M KCl in 50 mM Tris·Cl, pH 7.5
50 mM Tris·Cl, pH 7.5
Aminooxy-PEG (9; mol. wt. 5,000; NOF Corp., http://nofamerica.net/) or aminooxy-AlexaFluor 488 (Anaspec)
UV-Vis spectrophotometer (50 Bio; Varian)
0.45 μm syringe filter
Centrifuge (Beckman-Coulter)
Amicon Centriprep centrifugal filter (MWCO 10,000; Millipore)
Additional reagents and equipment for SDS-PAGE (Gallagher, 2006) and MALDI-MS (Carr and Annan, 1997)
Prenylation of eGFP-CVIA in crude extracts
Quantify the amount of eGFP-CVIA present in crude E. coli extract by UV absorbance (see Basic Protocol 2, step 4).
-
Prepare a 30-ml solution containing:
50 mM Tris·Cl, pH 7.5
10 mM MgCl2
30 mM KCl
10 μM ZnCl2
5 mM DTT
30 to 50 μM compound 1
200 nM PFTase.
Lastly, add eGFP-CVIA (present in crude extract) from step 1 to a final concentration of ∼2.0 μM.
Incubate the reaction at 30°C for 4 hr in an incubator.
Filter the mixture using a 0.45-μm syringe filter.
Load the solution into a centrifugal filter (MWCO 10,000) and centrifuge for 45 min at 5300 × g to a final volume of ∼500 μl.
To remove excess aldehyde-FPP (1), apply the solution onto a NAP-5 column pre-equilibrated with 50 mM Tris·Cl, pH 7.5. Elute the column with 1 ml of 50 mM Tris·Cl, pH 7.5, and collect the eluate.
Determine the concentration of aldehyde labeled eGFP-CVIA in the resulting solution by UV absorbance (see Basic Protocol 2, step 4).
Immobilize aldehyde-eGFP-CVIA on hydrazide functionalized beads
Based on the loading of the resin, the amount of hydrazide-functionalized agarose beads used for immobilization experiment has to be at least 10 times more on a molar basis than the aldehyde-modified eGFP-CVIA.
-
8
Shake the bottle of beads to create a homogenous suspension, then quickly pipet the desired volume of the suspended beads into two microcentrifuge tubes.
One tube is for the experiment and the other is for the control.
-
9
Centrifuge the suspension for 30 sec at 1000 × g, then discard the supernatant.
-
10
Add 300 μl of 0.1 M phosphate buffer, pH 7.0, vortex the mixture, and centrifuge for 30 sec at 1000 × g. Again, discard the supernatant.
-
11
Repeat steps 9 and 10 two more times.
-
12
Add a solution of aldehyde-eGFP-CVIA (8) prepared in step 6 to the experimental tube containing the beads, and add the same volume of 2 μM pure eGFP-CVIA (prepared as described in Support Protocol 2) to the control tube.
-
13
Add aniline to a final concentration of 100 mM.
-
14
Tightly tape the cap of the microcentrifuge tube in such a way that it does not leak while shaking, and then use tape to attach the tube to a vortexer.
-
15
Turn on the vortexer (gentle shaking) and let the reaction proceed for 2 hr.
Shaking is necessary for completion of this reaction. Since reaction occurs on the surface of resin, if shaking is not performed, the resin will quickly settle.
-
16
Centrifuge the mixture 30 sec at 1000 × g, and remove and discard the supernatant.
-
17
Wash the beads three times with 0.1 M phosphate buffer, pH 7.0, and then three times with 1 M KCl/50 mM Tris·Cl, pH 7.5, using the volumes and centrifugation conditions described in step 9. Store the immobilized protein at 4°C in 50 mM Tris·Cl, pH 7.5 for the next steps.
Washing with KCl helps in removing nonspecifically bound proteins.
At this step, beads in the experimental tube should be green and beads in the control tube should be completely colorless. If both remain green, more washing is needed. If none of them remain green, it means immobilization was not successful and should be repeated.
The immobilized protein can be frozen and stored at −80°C for at least 6 months.
For simultaneous PEGylation and release of immobilized eGFP-CVIA
The amount of aminooxy-PEG used for the following experiment has to be at least ten times more on a molar basis than the amount of immobilized eGFP-CVIA.
-
18a
Add a 1 mM solution of aminooxy-PEG (9) to the immobilized protein sample, then add aniline or m-phenylenediamine to final concentration of 100 mM. Add 1.0 M sodium phosphate buffer to final concentration of 0.1 M (pH 7.0).
-
19a
Tightly tape the cap of the microcentrifuge tube in such a way that it does not leak while shaking, then use tape to attach the tube to a vortexer.
-
20a
Turn on the vortexer and let the reaction proceed for 6 to 8 hr.
-
21a
Centrifuge the mixture for 30 sec at 1000 × g.
-
22a
Collect the supernatant, which contains the eGFP-CVIA-PEG conjugate.
-
23a
Analyze the supernatant by SDS-PAGE (Gallagher, 2006) and MALDI-MS (Carr and Annan, 1997) to verify PEGylation.
For simultaneous release and fluorophore labeling of immobilized eGFP-CVIA
-
18b
Add a 1 mM solution of aminooxy-AlexaFluor 488 to the immobilized protein sample, then add aniline or m-phenylenediamine to final concentration of 100 mM. Add 1.0 M sodium phosphate buffer to final concentration of 0.1 M (pH 7.0).
-
19b
Tightly tape the cap of the microcentrifuge tube in such a way that it does not leak while shaking, then use tape to attach the tube to a vortexer.
-
20b
Turn on the vortexer and let the reaction proceed for 6 to 8 hr.
-
21b
Centrifuge the mixture for 30 sec at 1000 × g.
-
22b
Collect the supernatant, which contains the eGFP-CVIA-PEG conjugate.
-
23b
Analyze the supernatant by LC-MS to verify fluorescent labeling.
Support Protocol 1
Expression and Purification of Pftase
A previously described 6 × His–tagged PFTase construct is expressed in BL21(DE3)pLyS E. coli and purified by metal-affinity chromatography (Dozier and Distefano, 2012).
Materials
-
Stock of BL21(DE3)pLysS E. coli cells containing PFTase on a CDF-Duet1 vector; available from Distefano laboratory (diste001@umn.edu)
LB medium (see recipe) containing 50 mg/liter streptomycin
IPTG
ZnSO4
Lysis buffer A (see recipe)
Protease inhibitor (for use with His-tagged proteins; Sigma-Aldrich, cat. no. P8849)
Nickel affinity resin (Ni-NTA resin; Gold Biotechnology, https://www.goldbio.com/)
Elution buffer A (see recipe)
Storage buffer A (see recipe)
Glycerol
Shaking incubator (Excella E-24; Brunswick Scientific)
Spectrophotometer for determining OD600
Centrifuge (Beckman Coulter)
Probe sonicator
30-ml chromatography column
Amicon Centriprep centrifugal filter (MWCO 10,000; Millipore)
Additional reagents and equipment for MALDI-MS (Support Protocol 3)
Bacterial growth
Use a frozen stock of cells to inoculate 50 mL of LB medium containing 50 mg/liter streptomycin.
Grow cultures overnight at 37°C with shaking at 240 rpm.
Inoculate 1 liter LB medium containing 50 mg/liter streptomycin with 10 ml of the overnight culture.
Grow cultures at 37°C with shaking at 240 rpm to an OD600 of ∼0.8.
Add IPTG and ZnSO4to the cell cultures to final concentrations of 1 mM and 500 μM, respectively.
Incubate the cultures at 15°C with shaking at 240 rpm overnight.
-
Harvest the cells by centrifugation for 5 min at 5400 × g, room temperature, and use the resulting cell pellet directly for the next step.
The cell pellet can be stored at −80°C if it is not used immediately.
Preparation of crude cell lysate
-
8
Resuspend the cell pellet from 1 liter of cells in 50 ml of lysis buffer A.
-
9
Add 1 ml of protease inhibitor (developed for His-tagged proteins, from Sigma-Aldrich).
-
10
Lyse the cells by pulse sonication in a probe sonicator for 10 min (10 sec on, 10 sec off) at 50 W on ice.
-
11
Centrifuge cell lysate 30 min at 13,000 × g, 4°C, to remove insoluble cellular debris.
Protein purification
-
12
Load the supernatant onto a 30-ml Ni-NTA column equilibrated with lysis buffer A.
-
13
Wash the column with 200 ml of lysis buffer A at a rate of 2 ml/min.
-
14
Elute the PFTase protein using 20 ml of elution buffer A.
-
15
Collect eluate as it emerges from the column in 5-ml fractions. Check the presence of protein by monitoring UV absorbance at 280 nm.
Absorbance decreases and remains constant while all of the proteins elute from the column.
-
16
Load the solution into a centrifugal filter (MWCO 10,000) and centrifuge for 45 min at 5300 × g, 4°C, down to a final volume of ∼2 ml.
-
17
Dilute the resulting solution three times with 20 ml of storage buffer A and concentrate again.
This step is for removing excess imidazole left in solution from the elution.
-
18
Add glycerol to the resulting PFTase solution to a final concentration of 40% by volume and store up to 1 year at −80°C.
Divide the enzyme solution into 10- to 20-μl aliquots in microcentrifuge tubes and store at −80°C, to avoid multiple freezing and thawing of enzyme stocks for each use.
Support Protocol 2
Expression and Purification of Egfp-Cvia
A 6×His–tagged eGFP-CVIA is expressed in BL21(DE3)pLyS E. coli and purified by metal-affinity chromatography All the materials and steps are similar to those mentioned in Support Protocol 1, except those specified in the materials list of this protocol.
The protein sequence for 6×His–tagged eGFP-CVIA is given below:
1 MASHHHHHHVSKGEELFTGV
21 VPILVELDGDVNGHKFSVSG
41 EGEGDATYGKLTLKFICTTG
61 KLPVPWPTLVTTLTYGVQCF
81 SRYPDHMKQHDFFKSAMPEG
101 YVQERTIFFKDDGNYKTRAE
121 VKFEGDTLVNRIELKGIDFK
141 EDGNILGHKLEYNYNSHNVY
161 IMADKQKNGIKVNFKIRHNI
181 EDGSVQLADHYQQNTPIGDG
201 PVLLPDNHYLSTQSALSKDP
221 NEKRDHMVLLEFVTAAGITL
241 GMDELYKCVIA
Materials
pJexpress414 plasmid containing the His-tagged eGFP-CVIA (eGFP with a 6xHisitidine tag at the N-terminus and the prenylation sequence CVIA at the C-terminus; DNA 2.0, https://www.dna20.com/)
One Shot BL21(DE3) chemically competent E. coli (Invitrogen)
LB agar plates and liquid medium (see recipe) containing 100 μg/ml ampicillin (Aldrich)
1 M IPTG
Lysis buffer B (see recipe)
Protease inhibitor (for use with His-tagged proteins; Sigma-Aldrich, cat. no. P8849) Nickel affinity resin (Ni-NTA resin; Gold Biotechnology, https://www.goldbio.com/)
Elution buffer B (see recipe)
Storage buffer B (see recipe)
Glycerol
Shaking incubator (Excella E-24; Brunswick Scientific)
UV-Vis spectrophotometer (50 Bio; Varian)
Centrifuge (Beckman-Coulter)
Probe sonicator
25-ml chromatography column
NOTE: BL21(DE3)pLysS E. coli cells containing 6 × His–tagged eGFP-CVIA are available from Distefano laboratory (diste001@umn.edu). Instructions for creating this cell line are given below.
Grow bacteria
Transform pJexpress414 plasmid into BL21(DE3) cells following using One Shot BL21(DE3) chemically competent E. coli.
Plate cells on LB agar plates containing 100 μg/ml ampicillin.
Pick one colony on plate to inoculate 50 ml of LB medium containing 100 μg/ml ampicillin.
Grow colony overnight shaking 250 rpm at 37°C.
Inoculate 1 liter of LB medium containing 100 μg/ml ampicillin with 10 ml of the overnight cell growth.
Grow cells at 37°C with shaking at 250 rpm to an OD600 of 0.7.
Add 500 μl of 1 M IPTG to induce protein expression.
Grow cells at 25°C for 16 hr with shaking at 250 rpm.
Pellet cells by centrifuging them 10 min at 5400 × g, 4°C.
Store cell pellet at −80°C for long-term storage.
Prepare crude cell lysate
-
11
Resuspend cell pellet in 50 ml of lysis buffer B at 4°C.
-
12
Add 1 ml of protease inhibitor (developed for His-tagged proteins, from Sigma-Aldrich).
-
13
Sonicate at 50 W for 5 min at 0°C with a probe sonicator using a pulse sequence of 10 sec on 10 sec off (total time, 10 min).
-
14
Centrifuge lysate for 30 min at 13,000 × g, 4°C.
Purify protein
-
15
Load supernatant onto a 25-ml Ni-NTA column pre-equilibrated with lysis buffer B.
-
16
Wash column with lysis buffer B until absorbance of the buffer at 280 nm is stable.
-
17
Elute the protein from the column by washing with 50 ml elution buffer B.
Collect eluate as it emerges from the column in 5-ml fractions. Check the presence of protein by monitoring UV absorbance at 280 nm. Absorbance decreases and remains constant while all of the proteins elute from the column.
-
18
Load the solution into a centrifugal filter (MWCO 10,000) and centrifuge 45 min at 5300 × g, 4°C, down to a final volume of ∼2 ml.
-
19
Dilute the resulting solution with 20 ml of storage buffer B and concentrate again. Repeat two more times.
This step is for removing excess imidazole left in solution from the elution.
-
20
Add 2 ml of 80% (v/v) glycerol in water.
-
21
Store protein at −80°C up to 2 years.
Support Protocol 3
Procedure for Maldi-Ms Analysis of Protein Samples
This protocol describes the procedure for analyzing the molecular mass of proteins using MALDI-MS.
Materials
0.1% TFA in ACN (Solvent A)
0.1 % TFA in H2O (Solvent B)
10 μl of protein sample
Freshly made saturated solution of sinapinic acid (Aldrich) in H2O (MALDI matrix)
C4 ZipTip micro columns (Millipore)
MALDI plate (Bruker)
MALDI-MS instrument (Bruker MALDI-TOF)
Additional reagents and equipment for MALDI-TOF (Carr and Annan, 1997)
Using a pipettor, wash the C4 ZipTip micro column with 20 μl of solvent A.
Equilibrate ZipTip with 20 μl of solvent B.
-
Adsorb the sample onto the column by repeated aspiration and ejection (5 to 10 times each time with 20 μl) into the ZipTip.
Make sure not to aspirate air through the column, which can cause less adsorption of protein onto the column.
-
Wash the ZipTip five times with 20 μl of solvent B to remove salts and small molecules.
Since PEGylated proteins are more soluble in water, you can reduce the number of washes to three, to avoid eluting some of the adsorbed protein into the water.
Elute the protein adsorbed on the column by aspirating 2 μl of solvent B and ejecting into a small microcentrifuge tube.
Pipet 0.7 μl of eluted material on the MALDI plate.
-
Pipet 0.7 μl of the matrix solution on top of the sample plate and mix the solutions by shaking the pipet tip and performing several aspiration-ejection cycles.
Mixing is important for obtaining good MALDI data. It is also essential to add the matrix quickly after adding protein solution on the plate, otherwise the protein solution will evaporate, which makes it difficult to mix it with matrix on the plate.
-
Wait for 5 min until the sample becomes completely dry on the plate and form crystals.
At that point, the sample is ready for MALDI-MS analysis.
Reagents and Solutions
Use deionized, distilled water for all solutions and protocol steps.
Elution buffer A
50 mM Tris·Cl, pH 7.5
20 mM NaCl
5 μM ZnCl2
5 mM MgCl2
250 mM imidazole
Store up to 1 year at room temperature
Elution buffer B
20 mM NaH2PO4, pH 8.0
500 mM NaCl
250 mM imidazole
Store up to 1 year at room temperature
LB liquid medium and agar plates
20 g LB medium, Lennox (BD Difco)
15 g agar (for plates; otherwise omit)
Add H2O to 1 liter and autoclave
Cool to ∼50°C
Add antibiotic (if called for in protocol)
If preparing agar plates, pour 90-mm plates
Store at room temperature until use
Lysis buffer A
50 mM Tris·Cl, pH 7.5
200 mM NaCl
5 μM ZnCl2
5 mM MgCl2
20 mM imidazole
Store with the above ingredients up to 1 year at room temperature
Just before use add:
1 mM 2-mercaptoethanol
Lysis buffer B
20 mM NaH2PO4, pH 8.0
500 mM NaCl
10 mM imidazole
Store up to 1 year at room temperature
Storage buffer A
50 mM Tris·Cl, pH 7.5
200 mM NaCl
5 μM ZnCl2
5 mM MgCl2
Store with the above ingredients up to 1 year at room temperature
Just before use add:
1 mM 2-mercaptoethanol
Storage buffer B
50 mM Tris·Cl, pH 7.3
Store up to 1 year at room temperature
Commentary
Background Information
A challenge in current chemical biology is the development of new methods for site-specific modification of proteins that involve mild conditions. This is due to the complex cellular environment of proteins and intrinsic structural sensitivity of biomolecules. In fact, chemical transformations of proteins need to proceed under mild conditions that are compatible with all functional groups present therein; this includes such diverse molecules as nucleic acids, carbohydrates, proteins, and various metabolites. The reaction should occur in water or aqueous solution at or near-physiological pH, have rapid kinetics even with sub-millimolar concentrations of reactants, and occur at ambient temperatures.
After a few decades of research, there are only a limited number of reactions that satisfy the aforementioned requirements. These include the Huisgen [3 + 2] cycloaddition, which is commonly referred to as the “click” reaction (Kolb et al., 2001), the Staudinger ligation (Saxon et al., 2000), a photoinducible reaction of an alkene with a tetrazole (Song et al., 2008), the Diels-Alder reaction (Holder et al., 1975), an inverse-electron-demand Diels-Alder reaction (Lang et al., 2012), and oxime or hydrazone ligations (Dirksen et al., 2010). Currently, the Cu(I) catalyzed click reaction is the most widely used bioorthogonal reaction. While useful, this reaction employs Cu(I) which is toxic to cells and can erode enzymatic activity.
Ligation reactions between aldehydes or ketones with hydrazine derivatives or alkoxyamines are attractive alternatives, but these reactions suffer from slow kinetics. However, the reaction can be catalyzed using aromatic amines, such as aniline, p-methoxy aniline, or m-phenylendiamine, which have fewer adverse effects in biological media in comparison with Cu(I). The amine-catalyzed oxime ligation has the advantage of rapid kinetics and complete conversion even with sub-millimolar reagent concentrations. Since oximes are more stable than hydrazones, addition of an alkoxyamine compound can reverse the hydrazone to form an oxime. Using this feature, we capture the aldehyde-functionalized protein from crude cellular extract via hydrazone formation and subsequently release the protein while simultaneously site-specifically PEGylating or fluoroescently labeling the protein using oxime ligation. Thus, this strategy can provide a highly pure, site-specifically PEGylated or fluoroescently labeled protein starting from crude cellular extract in three steps: prenylation, capture, and release. No additional purification steps are required.
Critical Parameters
Reagent preparation and storage
All protein stock solutions must be stored at −80°C for long-term storage. Avoid multiple freezing and thawing cycles of the enzyme stock solutions, as this may decrease enzyme activity.
Aldehyde-analogs or aldehyde-proteins must be stored at −80°C for long-term storage. The aldehyde oxidizes to the corresponding carboxylic acid over time. Aminooxy PEG, and aminooxy AlexaFluor 488 stock solutions should be stored at −20°C. Since DTT oxidizes over time, solutions that contain DTT should be prepared fresh on the day of use.
Performing the experiment
The crude cell lysates must be centrifuged to completely remove insoluble materials prior to the prenylation reaction. Prenylation using PFTase works poorly if performed in crude cellular extract that contains insoluble components.
m-Phenylenediamine is a somewhat more efficient catalyst than aniline. However, if m-phenylenediamine is used as the catalyst, the ratio of [aminooxy compound]/[m-phenylenediamine] should be higher than 0.01 to ensure complete oxime ligation.
After performing the immobilization, it is critical to wash the beads with KCl solution to ensure that nonspecifically bound proteins are washed off the beads.
When performing the prenylation reaction, the [protein-CaaX] should not exceed 5 μM. Empirically, we have found that the optimal concentration for the [protein-CaaX] is 1 to 2 μM.
Experimental controls
Two immobilization reactions should be run for each set of samples. One reaction with the aldehyde-functionalized protein and one with the unprenylated protein should be performed. This makes it possible to confirm that site-specific labeling has occurred.
Troubleshooting
While the protocols described herein detail a robust method for selective labeling of proteins from crude cell extract, occasionally reactions may not proceed as desired; below are described some recommended troubleshooting procedures.
Reagent quality and concentration
Aldehydes are prone to oxidation. Therefore, the FPP-aldehyde analogs should be prepared fresh and stored at −80°C. Every time after thawing the aldehyde, it should be returned quickly to −80°C to preserve the quality of the reagent. PFTase activity will decrease at room temperature, as well. Therefore, for each use, it should be thawed on ice and returned to −80°C for subsequent applications.
Prenylation of a protein in crude cell extract
Prenylation of a protein in crude cell extract will not work unless insoluble material is removed via centrifugation. Thus, it is important to make sure that prenylation is performed in the soluble crude extract.
Also, the concentration of the target protein that is being prenylated should not exceed 5 μM, to avoid substrate inhibition. The optimal concentration for the protein undergoing prenylation is in the range of 1 to 2 μM.
Protein immobilization
The beads should be extensively washed, first three times with 50 mM Tris·Cl, pH 7.5, and then with 0.5 M KCl in 50 mM Tris·Cl, pH 7.5. The volume of the washes depends on the scale of immobilization reaction. The recommended volume is 5× initial bead volume.
Oxime-hydrazone exchange: Release of the immobilized protein from surface
The hydrazone-oxime exchange reaction is very slow. Thus, it is important that the reaction is allowed to proceed for a sufficient amount of time (usually more than 10 hr for substantial conversion). Care should be taken to agitate the beads during the reaction so that they do not spread out along the wall of the reaction vessel.
Ratio of catalyst over aminooxy reagent
The catalysis of the oxime formation reaction involves initial formation of a Schiff base complex between the catalyst and the aldehyde followed by subsequent reaction with the aminooxy reagent. If the ratio of the catalyst over aminooxy compound is too large (>1000 for aniline and >250 for m-phenylenediamine), the Schiff base with the catalyst will form competitively with the desired oxime product. Thus, it is important that the ratio of catalyst to aminooxy reagent be within the ranges noted.
Anticipated Results
Basic Protocol 1 will generate FPP-aldehyde analog (Fig. 2). This enables investigators to site-specifically label proteins with aldehyde functionality for subsequent oxime or hydrazone reactions. In Basic Protocol 2, first, a protein of interest is site-specifically functionalized with an aldehyde moiety, and subsequently it is fluorescently labeled or PEGylated using oxime or hydrazine ligation. The labeling method described here is very robust, and the experimental results are typically consistent and reproducible across multiple runs. The oxime ligation reaction proceeds to completion even when the protein is present in low micromolar concentrations. In Basic Protocol 3, the protein of interest is aldehyde-functionalized within the (soluble) crude cellular extract, captured with hydrazide beads, and then released back into solution and simultaneously fluorescently labeled or PEGylated using aminooxy-containing reagents. The hydrazine-oxime exchange is a relatively slow reaction which typically takes about 5 to 10 hr (depending to the aminooxy concentration); however, the oxime reaction is relatively robust resulting in reproducible and consistent protein modification (Kalia and Raines, 2008). This procedure, using proteins in crude extract, has been successfully employed to incorporate fluorescent labels and PEG groups into eGFP-CVIA, as well as PEG groups into glucose-dependent insulinotropic protein (Rashidian et al., 2012).
Time Considerations
Starting from farnesol, it takes approximately 1 week to obtain purified FPP-aldehyde stock solution. Enzyme expression and purification can be done in 3 days. Once the proteins, FPP-aldehyde, and all the necessary reagents are in hand, each of the labeling or immobilization procedures described above can be completed within 2 to 3 days.
Acknowledgments
We thank Dr. Joseph Dalluge for helpful discussions regarding mass spectrometry. This work was supported by the National Institutes of Health (GM058842 and GM084152), and the University of Minnesota.
Literature Cited
- Axup JY, Bajjuri KM, Ritland M, Hutchins BM, Kim CH, Kazane SA, Halder R, Forsyth JS, Santidrian AF, Stafin K, Lu Y, Tran H, Seller AJ, Biroc SL, Szydlik A, Pinkstaff JK, Tian F, Sinha SC, Felding-Habermann B, Smider VV, Schultz PG. Synthesis of site-specific antibody-drug conjugates using unnatural amino acids. Proc Nat Acad Sci USA. 2012;109:16101–16106. doi: 10.1073/pnas.1211023109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berggård T, Linse S, James P. Methods for the detection and analysis of protein-protein interactions. Proteomics. 2007;7:2833–2842. doi: 10.1002/pmic.200700131. [DOI] [PubMed] [Google Scholar]
- Carr SA, Annan RS. Overview of peptide and protein analysis by mass spectrometry. Curr Protoc Mol Biol. 1997;38:10.21.1–10.21.27. doi: 10.1002/0471142727.mb1021s38. [DOI] [PubMed] [Google Scholar]
- Deiters A, Cropp TA, Summerer D, Mukherji M, Schultz PG. Site-specific PEGylation of proteins containing unnatural amino acids. Bioorg Med Chem Lett. 2004;14:5743–5745. doi: 10.1016/j.bmcl.2004.09.059. [DOI] [PubMed] [Google Scholar]
- Dirksen A, Yegneswaran S, Dawson PE. Bisaryl hydrazones as exchangeable biocompatible linkers. Angew Chem Int Ed Engl. 2010;49:2023–2027. doi: 10.1002/anie.200906756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dozier JK, Distefano MD. An enzyme-coupled continuous fluorescence assay for farnesyl diphosphate synthases. Anal Biochem. 2012;421:158–163. doi: 10.1016/j.ab.2011.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckworth BP, Xu J, Taton TA, Guo A, Distefano MD. Site-specific, covalent attachment of proteins to a solid surface. Bioconjugate Chem. 2006;17:967–974. doi: 10.1021/bc060125e. [DOI] [PubMed] [Google Scholar]
- Duckworth BP, Zhang Z, Hosokawa A, Distefano MD. Selective labeling of proteins by using protein farnesyltransferase. ChemBioChem. 2007;8:98–105. doi: 10.1002/cbic.200600340. [DOI] [PubMed] [Google Scholar]
- Gallagher S. One-dimensional SDS gel electrophoresis of proteins. Curr Protoc Mol Biol. 2006;75:10.2A.1–10.2A.37. doi: 10.1002/0471142727.mb1002as75. [DOI] [PubMed] [Google Scholar]
- Gauchet C, Labadie GR, Poulter CD. Regio- and chemoselective covalent immobilization of proteins through unnatural amino acids. J Am Chem Soc. 2006;128:9274–9275. doi: 10.1021/ja061131o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gault VA, Kerr BD, Irwin N, Flatt PR. C-terminal mini-PEGylation of glucose-dependent insulinotropic polypeptide exhibits metabolic stability and improved glucose homeostasis in dietary-induced diabetes. Biochem Pharmacol. 2008;75:2325–2333. doi: 10.1016/j.bcp.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Holder AA, Wootton JC, Baron AJ, Chambers GK, Fincham JR. The amino acid sequence of Neurospora NADP-specific glutamate dehydrogenase: Peptic and chymotryptic peptides and the complete sequence. Biochem J. 1975;149:757–773. doi: 10.1042/bj1490757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jevsevar S, Kunstelj M, Porekar VG. PEGylation of therapeutic proteins. Biotechnol J. 2010;5:113–128. doi: 10.1002/biot.200900218. [DOI] [PubMed] [Google Scholar]
- Kalia J, Abbott NL, Raines RT. General method for site-specific protein immobilization by Staudinger ligation. Bioconjugate Chem. 2007;18:1064–1069. doi: 10.1021/bc0603034. [DOI] [PubMed] [Google Scholar]
- Kalia J, Raines RT. Hydrolytic stability of hydrazones and oximes. Angew Chem. 2008;47:7523–7526. doi: 10.1002/anie.200802651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keppler A, Pick H, Arrivoli C, Vogel H, Johnsson K. Labeling of fusion proteins with synthetic fluorophores in live cells. Proc Nat Acad Sci USA. 2004;101:9955–9959. doi: 10.1073/pnas.0401923101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb HC, Finn MG, Sharpless KB. Click chemistry: Diverse chemical function from a few good reactions. Angew Chem Int Ed Engl. 2001;40:2004–2021. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Lang K, Davis L, Torres-Kolbus J, Chou C, Deiters A, Chin JW. Genetically encoded norbornene directs site-specific cellular protein labelling via a rapid bioorthogonal reaction. Nat Chem. 2012;4:298–304. doi: 10.1038/nchem.1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Lee EK, Cho YW, Matsui T, Kang IC, Kim TS, Han MH. ProteoChip: A highly sensitive protein microarray prepared by a novel method of protein immobilization for application of protein-protein interaction studies. Proteomics. 2003;3:2289–2304. doi: 10.1002/pmic.200300541. [DOI] [PubMed] [Google Scholar]
- Monošík R, Stred'anský M, Šturd'ík E. Biosensors—classification, characterization and new trends. Acta Chim Slov. 2012;5:109–120. [Google Scholar]
- Nguyen UTT, Cramer J, Gomis J, Reents R, Gutierrez-Rodriguez M, Goody RS, Alexandrov K, Waldmann H. Exploiting the substrate tolerance of farnesyltransferase for site-selective protein derivatization. ChemBioChem. 2007;8:408–423. doi: 10.1002/cbic.200600440. [DOI] [PubMed] [Google Scholar]
- Rashidian M, Dozier JK, Lenevich S, Distefano MD. Selective labeling of polypeptides using protein farnesyltransferase via rapid oxime ligation. Chem Commun. 2010;46:8998. doi: 10.1039/c0cc03305g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashidian M, Song JM, Pricer RE, Distefano MD. Chemoenzymatic reversible immobilization and labeling of proteins without prior purification. J Am Chem Soc. 2012;134:8455–8467. doi: 10.1021/ja211308s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraogi I, Zhang D, Chandrasekaran S, Shan S. Site-specific fluorescent labeling of nascent proteins on the translating ribosome. J Am Chem Soc. 2011;133:14936–14939. doi: 10.1021/ja206626g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxon E, Armstrong JI, Bertozzi CR. A “traceless” Staudinger ligation for the chemoselective synthesis of amide bonds. Org Lett. 2000;2:2141–2143. doi: 10.1021/ol006054v. [DOI] [PubMed] [Google Scholar]
- Seo MH, Han J, Jin Z, Lee DW, Park HS, Kim HS. Controlled and oriented immobilization of protein by site-specific incorporation of unnatural amino acid. Anal Chem. 2011;83:2841–2845. doi: 10.1021/ac103334b. [DOI] [PubMed] [Google Scholar]
- Song W, Wang Y, Qu J, Lin Q. Selective Functionalization of a genetically encoded alkene-containing protein via “photoclick chemistry” in bacterial cells. J Am Chem Soc. 2008;130:9654–9655. doi: 10.1021/ja803598e. [DOI] [PubMed] [Google Scholar]