Abstract
We tested the hypothesis that capillary exchange of adenosine is influenced by the ability of endothelial cells (ECs) to take up adenosine. Triple-indicator diffusion experiments were performed by injecting [14C]adenosine, [3H]9-β-d-arabinofuranosylhypoxanthine ([3H]araH), and radioiodinated serum albumin (RISA) into the arterial perfusate of isolated nonworking guinea pig hearts. Tracer appearance in venous effluent was observed over time. The early extraction of [14C]adenosine was much higher than that of [3H]araH. Extracted [3H]araH returned to the vascular space, but [14C]adenosine did not. Quantitative analysis of the curves by using a mathematical model indicates that approximately half of the extracted adenosine enters ECs and is metabolized. The remainder enters the interstitium and is taken up by myocytes, ECs, or other cells and is metabolized. We conclude that uptake of adenosine by ECs represents a significant influence on the capillary exchange of adenosine.
Adenosine is an extracellular messenger that inhibits platelet aggregation, reduces norepinephrine (NE) release from sympathetic nerve endings, reduces the cardiac chronotropic and inotropic effects of NE, has cardiac dromotropic effects, and relaxes vascular smooth muscle. All of these effects are dependent on the concentration of adenosine in the vicinity of plasma membrane receptors. Given this range of effects, it is reasonable to suppose that the extracellular fluid concentration of adenosine is highly regulated. In this regard, investigators have focused on the role of parenchymal cells in the production and removal of extracellular adenosine. More recently it has become apparent that endothelial cells (ECs) are also capable of influencing extracellular adenosine concentration. The following is a brief review of this work.
Pearson and Gordon were the first to demonstrate that cultured ECs exhibit carrier-mediated uptake of adenosine (7). They found that porcine aortic ECs have high-affinity (Km = 3 μm) and low-affinity (Km = 250 μm) uptake processes. Nees and Gerlach have studied adenosine uptake by cultured ECs isolated from guinea pig hearts and confirm the above kinetic parameters (6). Once adenosine has entered ECs, it is rapidly converted to AMP, inosine (7), and possibly S-adenosylhomocysteine (5). Carrier-mediated transport and enzymatic removal of cytosolic adenosine by conversion to these products are in series; the above experiments were not designed to determine whether transport or metabolism is rate limiting for uptake. Rapid kinetic studies of other cell lines suggest that enzymatic conversion is probably the limiting step (5).
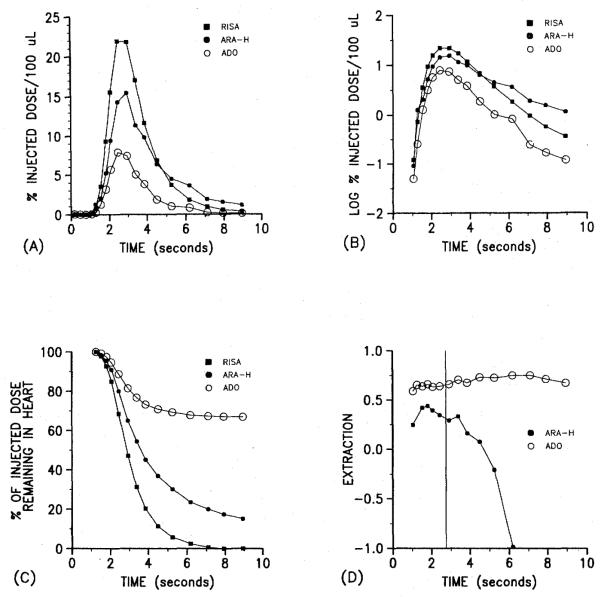
We tested the hypothesis that in situ ECs extract a significant fraction of plasma adenosine by using the multiple-indicator diffusion technique described in the accompanying article by Bassingthwaighte et al. (1). We simultaneously injected three tracers: radioiodinated serum albumin (RISA), an intravascular tracer; [3H]9-β-d-arabinofuranosylhypoxanthine ([3H]araH), an adenosine analog that is not transported by the nucleoside carrier; and [14C]adenosine. After the injection, serial fractions of the effluent were collected so that concentration vs. time curves for each tracer could be obtained. A curve for canine skeletal muscle is given in the above-mentioned article (1). Figure 1 shows an example of the data obtained by using an isolated perfused guinea pig heart. Figure 1A shows the venous time-concentration curves for all three tracers. Less [3H]araH than RISA appears in the venous effluent at early times because [3H]araH but not RISA leaves the capillary. This is reversed later when the back-diffusion of [3H]araH from interstitial fluid to plasma predominates and the RISA and araH curves cross. The crossover is better illustratedand in the semilog plots shown in Fig. 1B. In contrast to [3H]araH, the concentration of [14C]adenosine is lower than [3H]araH in the early samples andnever becomes higher than [3H]araH or RISA in the later samples. This lack of crossover indicates that [14C]adenosine does not return to the vascular compartment as expected if it were confined to the extracellular space. Figure 1C shows that although 100% of the RISA and 80% of the [3H]araH appear in the effluent over the 9 s after injection, only 30% of the injected [14C]adenosine appears. Finally, Fig. 1D shows that the extraction of [14C]adenosine is constant over the entire curve whereas the extraction of [3H]araH rapidly falls as back-diffusion begins to predominate.
Figure 1.
Results of experiments in which [14C]adenosine, [3H]araH, and RISA were simultaneously injected into the perfusate of an isolated guinea pig heart. A) Content of each tracer, expressed as a fraction of the injected dose in 100 μl of serially collected samples of effluent. Note that relative content of araH is higher than that of adenosine. B) Semilog transformation of data in A. Note that araH curve crosses over RISA curve, which indicates significant back-diffusion. C) Retention of tracers in heart over time. Note that adenosine but not araH is retained by heart. D) Extraction of araH and adenosine relative to the intravascular tracer RISA. Note that early extraction before the peak of diffusible tracer curves (vertical line) is higher for adenosine than for araH. After the peak, araH extraction declines but adenosine extraction remains high.
The qualitative characteristics of these curves support the hypothesis that ECs extract a large fraction of arterial adenosine. The early extraction of diffusible indicators reflects on the ease of permeation of the first barrier, the capillary wall. The higher early extraction of [14C]adenosine is evidence that the capillary endothelium is more permeable to it than to its analog [3H]araH. The lack of back-diffusion of [14C]adenosine, indicated by its constant extraction, suggests that it has entered cells and has been sequestered. This is also consistent with our knowledge of the behavior of cultured ECs that rapidly metabolize adenosine (5, 7).
An alternative hypothesis is that the higher extraction of adenosine is the result of uptake by cardiac myocytes. However, the qualitative relationships mentioned above do not support this argument. If ECs did not take up adenosine, the early extraction of [14C]adenosine and [3H]araH would be identical. This is because at very early times after the injection, the concentration of any diffusible tracer must necessarily be zero in the interstitial fluid. At these times, the extraction is determined only by the ability of the indicator to permeate the capillary barrier, because no back-diffusion of indicator is possible. Given the almost identical molecular weights and structures of [14C]adenosine and [3H]araH, their ability to permeate the capillary barrier by simple diffusion should be identical. If cardiac myocytes were responsible for the higher extraction of adenosine, we should have observed identical early extractions of [14C]adenosine and [3H]araH. Because we did not see this, and instead observed a higher extraction of adenosine even in the earliest samples, we can reject this alternative hypothesis.
These qualitative arguments do not allow us to state the quantitative importance of endothelial cell (EC) uptake of adenosine as compared to its diffusion through water-filled clefts. This requires the computation described by Bassingthwaighte et al. (1). When such calculations are done, we find that the adenosine permeabilitysurface area product for EC luminal surface is 6.0 ml/(min·g) whereas that for water-filled channels is 2.3 ml/(min·g). From this we conclude that transport into ECs is a more important route for extraction of plasma adenosine than is diffusion via water-filled clefts. This observation implies that in situ ECs are a significant metabolic barrier between interstitial fluid and plasma. Because of this, large changes is plasma adenosine would not necessarily be expected to lead to quantitatively similar changes in interstitial fluid adenosine concentration. The converse is also true. Venous plasma concentration of adenosine must be far lower than that of the interstitial fluid because adenosine that diffuses into the plasma compartment must be largely taken up by ECs before it can escape via the microvascular bed. This means that the appearance of adenosine in venous effluent can be used as an index of interstitial fluid adenosine only if appropriate correction for EC uptake is possible. Such a correction requires more knowledge of the capillary transport of adenosine, under a variety of conditions, than is now available.
Although we have not discussed it here, there is also reason to believe that ECs can serve as a source of plasma adenosine by release of either adenosine itself or a precursor nucleotide (5, 8).
There are a number of implications of these studies. First, if ECs take up most of the infused adenosine, we must question the meaning of adenosine dose-response curves derived from intact organs. When adenosine is infused, it is likely that interstitial concentration is changed far less than plasma adenosine. This may mean that vascular smooth muscle is much more sensitive to adenosine than would be deduced from dose-response curves. An alternative possibility is that plasma adenosine influences vascular smooth muscle indirectly via an action on ECs. There are data in favor (4) and against (2, 3) such an idea.
A second implication is that the use of adenosine release as an index of interstitial fluid adenosine must be reevaluated. If either release or venous adenosine concentration is to be used as an index of interstitial fluid adenosine, a much more thorough understanding of ECs as a sink as well as a source for plasma adenosine is needed.
Third, an EC metabolic barrier for adenosine means that interstitial fluid and plasma adenosine can be regulated independently. For example, if plasma adenosine were increased to prevent platelet aggregation, it would not necessarily follow that interstitial fluid adenosine would be elevated.
Finally, ECs could serve as a trap to salvage adenosine that would otherwise escape from all organ and be lost to the adenine nucleotide pool. The purine base could be returned to the organ via a metabolite with a favorable concentration gradient, e.g., hypoxanthine.
In summary, ECs appear to be a significant metabolic barrier for the capillary transport of adenosine. This fact may have profound implications related to the uptake and release of purines by the heart and to the biological actions of adenosine.
Footnotes
From the Symposium Capillary Endothelium: A Metabolic Barrier for Solute Transport presented by The American Physiological Society at the 68th Annual Meeting of the Federation of American Societies for Experimental Biology, St. Louis, Missouri, April 3, 1984. Accepted for publication September 13, 1984.
Supported by U.S. Public Health Service grants HL 24232 and HL 19139.
REFERENCES
- 1.Bassingthwaighte JB, Sparks HV, Jr., Chan IS, DeWitt DF, Gorman MW. Modeling of transendothelial transport. Federation Proc. 1985;44:2623–2626. [PMC free article] [PubMed] [Google Scholar]
- 2.De Mey JG, Vanhoutte PM. Role of the intima in cholinergic and purinergic relaxation of isolated canine femoral arteries. J. Physiol. (London) 1981;316:347–355. doi: 10.1113/jphysiol.1981.sp013792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Furchgott RF, Zawadski JV. The obligatory role of endothelial cells ill the relaxation of arterial smooth muscle by acetylcholine. Nature (London) 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 4.Gordon JL, Martin W. Endothelium-dependent relaxation of the pig aorta: relationship to stimulation of 86Rb efflux from isolated endothelial cells. Br. J. Pharmacal. 1983;79:531–541. doi: 10.1111/j.1476-5381.1983.tb11028.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lum CT, Marz R, Plagemann PGW, Wohlhueter RM. Adenosine transport and metabolism in mouse leukemia cells and in canine thymocytes and peripheral blood leukocytes. J. Cell. Physial. 1979;101:173–200. doi: 10.1002/jcp.1041010202. [DOI] [PubMed] [Google Scholar]
- 6.Nees S, Gerlach E. Adenosine nucleotide and adenosine metabolism in cultured coronary endothelial cells: formation and release of adenine compounds and possible functional implications. In: Berne RM, Rail TW, Rubio R, editors. Regulatory function of adenosine. Nijhoff; The Hague: 1983. pp. 347–359. [Google Scholar]
- 7.Pearson JD, Carleton JS, Hutchings A, Gordon JL. Uptake and metabolism of adenosine by pig aortic endothelial and smooth muscle cells im culture. Biochem. J. 1978;170:265–271. doi: 10.1042/bj1700265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pearson JD, Gordon JL. Vascular endothelial and smooth muscle cells in culture selectively release adenine nucleotides. Nature (London) 1979;281:382–384. doi: 10.1038/281384a0. [DOI] [PubMed] [Google Scholar]