Abstract
Background
Higher radiation dose levels have been shown to be associated with improved tumor-control outcomes in localized prostate cancer (PCa) patients.
Objective
Identify predictors of biochemical tumor control and distant metastases–free survival (DMFS) outcomes for patients with clinically localized PCa treated with conformal external-beam radiotherapy (RT) as well as present an updated nomogram predicting long-term biochemical tumor control after RT.
Design, setting, and participants
This retrospective analysis comprised 2551 patients with clinical stages T1–T3 PCa. Median follow-up was 8 yr, extending >20 yr.
Intervention
Prescription doses ranged from 64.8 to 86.4 Gy. A total of 1249 patients (49%) were treated with neoadjuvant and concurrent androgen-deprivation therapy (ADT); median duration of ADT was 6 mo.
Measurements
A proportional hazards regression model predicting the probability of biochemical relapse and distant metastases after RT included pretreatment prostate-specific antigen (PSA) level, clinical stage, biopsy Gleason sum, ADT use, and radiation dose. A nomogram predicting the probability of biochemical relapse after RT was developed.
Results and limitations
Radiation dose was one of the important predictors of long-term biochemical tumor control. Dose levels <70.2 Gy and 70.2–79.2 Gy were associated with 2.3-and 1.3-fold increased risks of PSA relapse compared with higher doses. Improved PSA relapse– free survival (PSA-RFS) outcomes with higher doses were observed for all risk groups. Use of ADT, especially for intermediate- and high-risk patients, was associated with significantly improved biochemical tumor-control outcomes. A nomogram predicting PSA-RFS was generated and was associated with a concordance index of 0.67. T stage, Gleason score, pretreatment PSA, ADT use, and higher radiation doses were also noted to be significant predictors of improved DMFS outcomes.
Conclusions
Higher radiation dose levels were consistently associated with improved biochemical control outcomes and reduction in distant metastases. The use of short-course ADT in conjunction with RT improved long-term PSA-RFS and DMFS in intermediate- and high-risk patients; however, an overall survival advantage was not observed.
1. Introduction
The need for escalated doses of external-beam radiotherapy (EBRT) is well recognized as critical and is considered the standard of care in the treatment of clinically localized prostate cancer (PCa). Randomized trials [1–4] have shown improved biochemical relapse–free survival outcomes with higher radiation doses, and in one of these studies [1] a distant metastases–free survival (DMFS) improvement was observed. We previously reported our dose-escalation experience using three-dimensional conformal radiotherapy (3D-CRT) and intensity-modulated radiotherapy (IMRT) for PCa, which showed that during a 10-yr period, the radiation dose levels were gradually escalated from 68 to 86.4 Gy [5].
In prior reports we identified the use of higher radiation dose levels in addition to other variables as a significant predictor for improved prostate-specific antigen relapse–free survival (PSA-RFS) after radiotherapy (RT) [6]. Based on those results, we developed a nomogram for predicting biochemical outcomes in patients treated with 3D-CRT and IMRT for PCa [7]. A limitation of prior studies was the less-than-optimal long-term follow-up for patients who received higher doses of RT and patients treated with IMRT. In this report we present our updated long-term biochemical tumor-control and survival outcomes after 3D-CRT and IMRT for patients with clinical stages T1–T3 PCa with a follow-up >20 yr. We also present an updated nomogram for predictions of biochemical tumor control at 5 and 10 yr after RT using conformal techniques.
2. Materials and methods
Between 1988 and 2004, 2551 patients were treated with 3D-CRT or IMRT for clinically staged T1– T3 node-negative PCa at Memorial Sloan-Kettering Cancer Center. The clinical characteristics of this patient population are shown in Table 1. Patients were staged according to the 2005 American Joint Committee on Cancer staging classification system. All patients had biopsy-proven adenocarcinoma that was classified according to the Gleason grading system with pathology re-review at our institution. Low, intermediate, and high risk were defined according to the National Comprehensive Cancer Network prognostic risk group classification (www.nccn.org), and the respective breakdowns in these groups were 571 (22.4%), 1074 (42.1%), and 906 (35.5%). All patients underwent pretreatment computed tomography scanning or magnetic resonance imaging of the prostate and pelvis prior to treatment to assess for lymphadenopathy.
Table 1. Patient demographics.
| Characteristics | No | % |
|---|---|---|
| Age, yr | ||
| <65 | 667 | 26 |
| ≥65 | 1884 | 74 |
| Pretreatment PSA, ng/ml | ||
| <10 | 1470 | 58 |
| 10–20 | 643 | 25 |
| >20 | 438 | 17 |
| Total Gleason score | ||
| <7 | 1184 | 46 |
| 7 | 943 | 37 |
| >7 | 424 | 17 |
| T stage | ||
| T1–T2a | 1589 | 62 |
| T2b–T2c | 580 | 23 |
| T3a–T3c | 382 | 15 |
| Androgen-deprivation therapy | ||
| No | 1302 | 51 |
| Yes | 1249 | 49 |
PSA = prostate-specific antigen.
The treatment technique used in this patient population has been previously described in detail [6,8]. Prescription doses ranged from 64.8 to 86.4 Gy. These dose ranges were used as part of a prospective dose escalation study in which radiation dose levels were gradually escalated from 70.2 Gy at 5.4-Gy increments up to 86.4 Gy, as previously described in detail [9]. Radiation dose levels were prescribed to the maximum isodose level that completely encompassed the planning target volume (PTV). In these patients, the PTV included the prostate and seminal vesicles with a 1-cm margin except at the prostate–rectum interface, where a 0.6-cm margin was used. For patients who received neoadjuvant androgen-deprivation therapy (ADT; n = 1249; 49%), therapy consisted of a luteinizing hormone–releasing hormone agonist combined with an antitestosterone. ADT treatment generally was initiated 3 mo prior to 3D-CRT/IMRT and discontinued at the completion of RT. The percentage breakdown of ADT use according to the prognostic risk group was as follows: low risk, 170 (30%); intermediate risk, 456 (42%); and high risk, 623 (69%).
While ADT was administered at the discretion of the treating physician, most high-risk patients in this cohort (69%) were treated with ADT. ADT was used for low-risk patients only for pretreatment target volume reduction of larger prostates. Elective pelvic nodal irradiation was not routinely used in these patients.
Follow-up evaluations after RT were performed at intervals of 3–6 mo for 5 yr and yearly thereafter. The median follow-up time was 8 yr (range: 2–21 yr). Prostate-specific antigen (PSA) relapse was defined according to the Phoenix definition (absolute nadir plus 2 ng/ml dated at the call). None of the patients received postirradiation ADT or other anticancer therapy before documentation of a PSA relapse. Patients with relapsing disease were routinely referred for medical oncology evaluation, and depending on the PSA doubling time and presence of metastatic disease, such patients were considered for ADT. Salvage prostatectomy was offered to <1% of patients with relapsing disease.
Parameters used for the nomogram were similar to those used in prior reports [7] and included pretreatment PSA level, clinical stage, and biopsy Gleason score. The nomogram was developed using a proportional hazards regression model predicting the probability of biochemical relapse after EBRT. Nomogram validation was quantified by the concordance index [10]. Similar to the area under the receiver operating characteristic curve but appropriate for censored data, the concordance index provides the probability that in a randomly selected pair of patients in which one patient develops a relapse before the other, the patient with disease relapse first will have the worse predicted outcome from the nomogram.
The Kaplan-Meier method was used to determine the actuarial likelihood of developing a PSA relapse or distant metastasis. In addition to the Cox model, we also used the log-rank test for comparing different arms univariately. When analyzing various dose levels used, the cut-off values were predetermined based on the dose level increments delivered as part of our prospective dose escalation [9] and before the statistical analysis. Risk of mortality related to PCa and accounting for other causes of death was analyzed by competing-risks analysis. In this analysis, the competing risks were death due to PCa (PCa-related death) and death due to other causes. All p values were derived from the use of two-sided statistical tests. All analyses were performed using SAS software (SAS Institute, Cary, NC, USA) or R library cmprsk.
3. Results
3.1. Prostate-specific antigen relapse–free survival
The 10-yr PSA-RFS outcomes for low-, intermediate-, and high-risk patients were 82%, 68%, and 48%, respectively. For low-risk patients, the use of doses ≥75.6 Gy was associated with improved PSA-RFS, and for intermediate- and high-risk patients, dose levels of ≥81 Gy were required to significantly affect biochemical control. The 10-yr PSA-RFS for low-risk patients was 84% and 70% for patients treated with ≥75.6 Gy and with lower doses, respectively (p = 0.04; Fig. 1). For intermediate-risk patients, the 10-yr PSA-RFS was 76% and 57% for patients treated with ≥81 Gy and with lower doses, respectively (p < 0.0001; Fig. 2). Similarly, the 10-yr PSA-RFS for high-risk patients was 55% and 41% for patients treated with ≥81 Gy and with lower doses, respectively (p < 0.0001; Fig. 3).
Fig. 1.

Ten-year prostate-specific antigen (PSA) relapse-free survival for low-risk patients was 84% and 70% for patients treated with ≥75.6 Gy and with lower doses, respectively (p = 0.04).
RT = radiotherapy.
Fig. 2.

Prostate-specific antigen (PSA) relapse-free survival for intermediate-risk patients according to dose. The 10-yr outcomes were 76% and 57% for patients treated with >81 Gy and with lower doses, respectively (p < 0.0001).
RT = radiotherapy.
Fig. 3.

Prostate-specific antigen (PSA) relapse-free survival for high-risk patients according to dose. The 10-yr outcomes were 55% and 41% for patients treated with >81 Gy and with lower doses, respectively (p < 0.0001).
RT = radiotherapy.
When ADT was used, its median duration was 6 mo; nevertheless, patients benefited from improved PSA-RFS outcomes. As shown in Figure 4 and 5, among intermediate- and high-risk patients, improved outcomes were observed for patients treated with ADT compared with patients treated with RT alone. The 10-yr PSA-RFS rates for high-risk patients treated with and without ADT were 55% and 36%, respectively (p < 0.0001). These results were further confirmed by multivariate analysis, as shown in Table 2. Cox regression analysis identified the radiation dose as an independent variable predicting long-term biochemical tumor control. Dose levels of <70.2 and 70.2–79.2 Gy were associated with 2.3- and 1.3-fold increased risks of PSA relapse compared with higher dose levels. In addition to the Gleason score, T stage, and pretreatment value, the use of ADT was noted to be an independent variable predicting for improved PSA-RFS (p < 0.0001).
Fig. 4.

The 10-yr prostate-specific antigen (PSA) relapse-free survival for high-risk patients treated with and without androgen-deprivation therapy was 55% and 36%, respectively (p < 0.0001).
NeoHT = neoadjuvant hormone therapy.
Fig. 5.

Prostate-specific antigen (PSA) relapse–free survival according to the use of androgen-deprivation therapy (ADT). Use of a 6-mo course of ADT is significantly associated with improved biochemical tumor control.
NeoHT = neoadjuvant hormone therapy.
Table 2. Univariate and multivariate analysis of predictors for prostate-specific antigen relapse–free survival.
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | |
| Androgen-deprivation therapy (yes vs no) | 0.919 | 0.792–1.065 | 0.261 | 0.7284 | 0.6173–0.8595 | 0.0001 |
| T stage | <0.0001 | <0.0001 | ||||
| T1c/T2a (reference) | 1.000 | 1.000 | ||||
| T2b/T2c | 2.049 | 1.722–1.596 | 1.7940 | 1.4903–2.1595 | ||
| T3a/T3b/T3c | 2.006 | 1.591–2.529 | 2.3630 | 1.9255–2.8999 | ||
| Gleason (continuous variable) | 1.266 | 1.187–1.351 | <0.0001 | 1.2331 | 1.1554–1.3160 | <0.0001 |
| Pre-PSA (continuous variable) | 1.017 | 1.017–1.020 | <0.0001 | 1.0118 | 1.0085–1.0152 | <0.0001 |
| RT dose, Gy | <0.0001 | <0.0001 | ||||
| ≥81 (reference) | 1.000 | 1.000 | ||||
| 70.2–75.6 | 1.914 | 1.637–2.438 | <0.0001 | 1.4172 | 1.1946–1.6814 | <0.0001 |
| <70.2 | 3.247 | 2.703–3.900 | <0.0001 | 2.5963 | 1.9142–3.5214 | <0.0001 |
HR = hazard ratio; CI = confidence interval; PSA = prostate-specific antigen; RT = radiotherapy.
Using the significant variables from a Cox regression analysis with methods as previously reported [7], an updated nomogram for predicting biochemical tumor control at 5 and 10 yr after EBRT was produced (Fig. 6). The concordance index of the nomogram was 0.67.
Fig. 6.
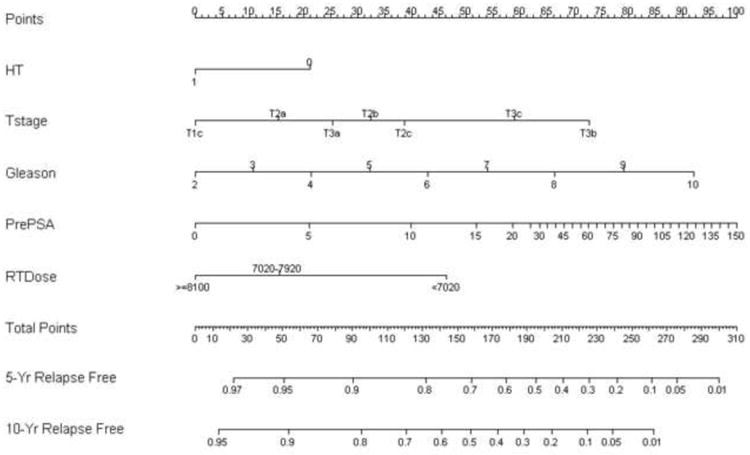
Nomogram for predicting biochemical tumor control at 5 and 10 yr after external-beam radiotherapy (RT) (concordance index: 0.67).
HT = 6 mo neoadjuvant and concurrent hormone therapy; PSA = prostate-specific antigen.
3.2. Distant metastases-free, cause-specific, and overall survival outcomes
The 10-yr DMFS outcomes for low-, intermediate-, and high-risk patients were 98%, 90%, and 73%, respectively (p < 0.0001). The use of higher doses was associated with improved DMFS outcomes. The 10-yr DMFS for patients who were treated with dose levels of ≥81 Gy was 87%, compared with 81% for patients treated with lower dose levels (p < 0.001). As shown in Table 3, a Cox regression analysis revealed that T stage, Gleason score, pretreatment PSA level, the use of ADT, and higher RT dose (≥81 Gy) were significant predictors of DMFS.
Table 3. Univariate and multivariate analysis of predictors for time to distant metastases.
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | |
| HT (yes vs no) | 1.224 | 0.9796–1.529 | 0.075 | 0.7817 | 0.610–1.002 | 0.052 |
| T stage | <0.0001 | <0.0001 | ||||
| T1c/T2a | 1.00 | 1.00 | ||||
| T2b/T2c | 1.93 | 1.459–2.575 | <0.0001 | 1.6279 | 1.207–2.196 | |
| T3a/T3b/T3c | 5.06 | 3.895–6.575 | <0.0001 | 3.2095 | 2.395–4.302 | |
| Gleason | 1.673 | 1.522–1.838 | <0.0001 | 1.5069 | 1.367–1.661 | <0.001 |
| Pre-PSA | 1.017 | 1.013–1.021 | <0.0001 | 1.0107 | 1.006–1.015 | <0.001 |
| RT dose, Gy | <.0001 | 0.027 | ||||
| ≥81 (reference) | 1.00 | 1.00 | ||||
| 70.2–75.6 | 1.839 | 1.449–2.334 | <0.0001 | 1.3528 | 1.044–1.752 | 0.022 |
| <70.2 | 1.909 | 1.223–2.982 | 0.0044 | 1.6737 | 1.045–2.680 | 0.032 |
HR = hazard ratio; CI = confidence interval; HT = hormone therapy; PSA = prostate-specific antigen; RT = radiotherapy.
A competing-risks analysis was performed to describe the PCa mortality rates and associated reduction stratified by various variables [11]. The likelihood of PCa mortality for low-, intermediate-, and high-risk patients at 10 yr was 1.2%, 3.6%, and 15.3%, respectively (p < 0.0001; Fig. 7). The 10-yr overall survival for low-, intermediate-, and high-risk patients was 80%, 76%, and 62%, respectively. Radiation dose level and use of ADT in this population of patients did not influence PCa mortality or overall survival outcomes (results not shown).
Fig. 7.

Competing-risks analysis demonstrating the likelihood of prostate cancer (PCa) mortality for low-, intermediate-, and high-risk patients (p < 0.0001).
4. Discussion
Our long-term results continue to demonstrate the importance of dose escalation in the treatment of patients with clinically localized PCa. In this report, dose levels of ≥75.6 Gy for low-risk patients were associated with improved long-term PSA-RFS outcomes, and for higher-risk patients we observed improved biochemical control with ≥81 Gy. These data suggest that given the larger volume of disease and the possibly increased percentage of more resistant clonogens in higher-risk patients, further escalation of the radiation dose is critical to achieve improved outcomes. Published randomized trials [1-4] have also demonstrated significant improvements in biochemical tumor control for low-risk patients [2] and intermediate- and high-risk patients [1,3,4]. It is quite plausible that additional escalation of the radiation dose for intermediate-risk patients and, especially, high-risk patients would be associated with further improvements in tumor-control outcomes. While significant dose escalation of >86 Gy is not feasible with IMRT or image-guided RT alone, given concerns for normal tissue dose constraints, it is nevertheless achievable with combined brachytherapy-IMRT treatment interventions. Biochemical tumor control with such combined modality approaches for intermediate- and high-risk patients appears to be associated with higher control rates than what we report here for conventionally fractionated EBRT used as monotherapy [12-15].
Our observations of improved outcomes with ADT and RT for intermediate-risk patients are consistent with the phase 3 trial results that demonstrated similar improvements with short-course ADT for this cohort of patients [16,17]. However, we did not find a survival advantage among our patients treated with a 6-mo course of ADT, as those studies have shown. It is possible that the survival advantage may have been more easily realized in such trials because the EBRT dose levels were low (≤70 Gy) and the addition of ADT achieved a relative survival advantage due to the expected inferior outcomes with low-dose EBRT alone. In our treatment cohort, secondary to the escalated radiation dose levels used, a survival advantage may have not been seen with the addition of only short-course ADT given to these patients. For high-risk patients we observed a significant benefit in PSA-RFS and a reduction in distant metastases with the addition of ADT. Yet despite the use of ADT in this cohort, 45% developed biochemical relapse, and 24% were found to have distant metastases 10 yr after treatment. We have previously postulated that further improvements in outcome for this high-risk cohort could have been observed if an ADT course of 2–3 yr had been used. Results of the European Organization for Research and Treatment of Cancer trial indicate that longer durations of ADT were associated with improved long-term control rates compared with 6-mo courses [18]. While this latter study compared short-course ADT with longer courses in the setting of low-dose RT, we believe that even further enhancement in outcomes would have observed in the setting of escalated doses of RT. It is our current practice to recommend longer courses of ADT in conjunction with high-dose RT for high-risk patients treated at our institution. An ongoing randomized trial (Radiation Therapy Oncology Group Study 0815) will shed light on the role of hormone therapy for intermediate-risk patients in the setting of escalated doses of EBRT.
We also present our updated nomogram for predicting biochemical control outcomes in patients treated with 3D-CRT and IMRT. We have previously noted the potential benefit of nomogram-based prediction models compared with risk-group prognostic classification models [7]. The nomogram takes into account combinations of various prognostic variables for predicting outcomes, while a risk-group system is a broader form of classification. For instance, it would be expected that an intermediate-risk patient with a PSA of 10 ng/ml and a Gleason 6 with clinical stage T1c would have a different prognosis than another intermediate-risk patient with T2b disease, Gleason 7, and a baseline PSA value of 19.1 ng/ml. Both patients would be considered intermediate risk, yet each has very different associated prognostic outcomes based on the nomogram prediction.
However, there are limitations to the nomogram of which the oncologist or general practitioner should be aware. The nomogram predicts outcomes based on the individual experience, treatment technique, and staging practices used in the institution from which the data emerge. The results or predictions obtained from the nomogram may not be applicable to patients treated at another center. As mentioned, shorter or longer courses of ADT and the doses and accuracy of delivery of RT would likely affect the outcomes after treatment. In addition, the nomogram results are not reliable for comparison of one modality with another. Given the selection biases associated with cohorts of patients who have chosen surgery compared with RT, other biologic variables that are not included in a nomogram could influence outcomes. Such comparison of the absolute number from one nomogram prediction for a particular treatment with the absolute number extracted from another nomogram prediction for a different treatment is not meaningful or valid. The nomogram results ideally should be used to give a general sense of the probability of tumor control, which could help further guide the treatment plan and strategy. Patients with relatively poor predicted outcomes may do better with longer courses of ADT or consideration of an experimental treatment protocol.
5. Conclusions
Higher radiation dose levels are essential for improved long-term biochemical control outcomes, and this advantage was observed for all risk groups. For low-risk patients, it would appear that doses of 75.6 Gy are required as a minimum, and for higher-risk patients, ≥81 Gy was associated with a superior PSA-RFS outcome. High-dose irradiation can be performed safely with IMRT, and with current image-guided approaches, meticulous treatment planning techniques, and attention to normal tissue dose constraints, long-term toxicities in our treated patients have been low, as we have previously reported [19]. The use of short-course ADT in conjunction with RT improved long-term PSA-RFS in patients with intermediate- and high-risk disease. We recognize that based on randomized trials, longer courses of ADT in higher-risk patients are important, and in the setting of dose escalation, even better results than what have been published in these trials with low-dose irradiation may be observed. We are encouraged that in our study, higher radiation doses were also associated with a reduction in distant metastases, highlighting the recognized association of local metastatic outcome and distant metastatic outcome, an observation we had previously noted [20].
Acknowledgments
Funding/Support and role of the sponsor: None.
Footnotes
Author contributions: Michael J. Zelefsky had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Zelefsky.
Acquisition of data: Pei, Schechter, Fidaleo, Sperling.
Analysis and interpretation of data: Pei, Chou, Zhang.
Drafting of the manuscript: Zelefsky, Pei, Chou.
Critical revision of the manuscript for important intellectual content: Zelefsky, Yamada, Kollmeier, Cox.
Statistical analysis: Chou, Zhang.
Obtaining funding: None.
Administrative, technical, or material support: Zelefsky, Chou.
Supervision: Zelefsky.
Other (specify): None.
Financial disclosures: I certify that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/ affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pollack A, Zagars GK, Starkschall G, et al. Prostate cancer radiation dose response: results of the M. D. Anderson phase III randomized trial. Int J Radiat Oncol Biol Phys. 2002;53:1097–105. doi: 10.1016/s0360-3016(02)02829-8. [DOI] [PubMed] [Google Scholar]
- 2.Zietman AL, Bae K, Slater JD, et al. Randomized trial comparing conventional-dose with high-dose conformal radiation therapy in early-stage adenocarcinoma of the prostate: long-term results from Proton Radiation Oncology Group/American College of Radiology 95–09. J Clin Oncol. 2010;28:1106–11. doi: 10.1200/JCO.2009.25.8475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peeters ST, Heemsbergen WD, Koper PC, et al. Dose-response in radiotherapy for localized prostate cancer: results of the Dutch multicenter randomized phase III trial comparing 68 Gy of radiotherapy with 78 Gy. J Clin Oncol. 2006;24:1990–6. doi: 10.1200/JCO.2005.05.2530. [DOI] [PubMed] [Google Scholar]
- 4.Dearnaley DP, Sydes MR, Graham JD, et al. Escalated-dose versus standard-dose conformal radiotherapy in prostate cancer: first results from the MRC RT01 randomised controlled trial. Lancet Oncol. 2007;8:475–87. doi: 10.1016/S1470-2045(07)70143-2. [DOI] [PubMed] [Google Scholar]
- 5.Kuban DA, Levy LB, Cheung MR, et al. Long-term failure patterns and survival in a randomized dose-escalation trial for prostate cancer: who dies of disease? Int J Radiat Oncol Biol Phys. 2011;79:1310–7. doi: 10.1016/j.ijrobp.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 6.Zelefsky MJ, Chan H, Hunt M, Yamada Y, Shippy AM, Amols H. Long-term outcome of high dose intensity modulated radiation therapy for patients with clinically localized prostate cancer. J Urol. 2006;176:1415–9. doi: 10.1016/j.juro.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 7.Zelefsky MJ, Kattan MW, Fearn P, et al. Pretreatment nomogram predicting ten-year biochemical outcome for three-dimeniosnal conformal radiotherapy and intensity modulated radiotherapy for prostate cancer. Urology. 2007;70:283–7. doi: 10.1016/j.urology.2007.03.060. [DOI] [PubMed] [Google Scholar]
- 8.Alickus ZA, Yamada Y, Zhang Z, et al. Ten year outcomes of high dose, intensity modulated radiotherapy for localized prostate cancer. Cancer. 2011;117:1429–37. doi: 10.1002/cncr.25467. [DOI] [PubMed] [Google Scholar]
- 9.Zelefsky MJ, Leibel SA, Gaudin PB, et al. Dose escalation with three-dimensional conformal radiation therapy affects the outcome in prostate cancer. Int J Radiat Oncol Biol Phys. 1998;41:491–500. doi: 10.1016/s0360-3016(98)00091-1. [DOI] [PubMed] [Google Scholar]
- 10.Gönen M, Heller G. Concordance probability and discriminatory power in proportional hazards regression. Biometrika. 2005;92:965–70. [Google Scholar]
- 11.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Statistical Assoc. 1999;94:496–509. [Google Scholar]
- 12.Stock RG, Cesaretti JA, Hall SJ, Stone NN. Outcomes for patients with high-grade prostate cancer treated with a combination of brachytherapy, external beam radiotherapy and hormonal therapy. BJU Int. 2009;104:1631–6. doi: 10.1111/j.1464-410X.2009.08661.x. [DOI] [PubMed] [Google Scholar]
- 13.Fang LC, Merrick GS, Butler WM, et al. High-risk prostate cancer with Gleason score 8–10 and PSA level <15 ng/ml treated with permanent interstitial brachytherapy. Int J Radiat Oncol Biol Phys. doi: 10.1016/j.ijrobp.2010.07.006. In press. [DOI] [PubMed] [Google Scholar]
- 14.Deutsch I, Zelefsky MJ, Zhang Z, et al. Comparison of PSA relapse-free survival in patients treated with ultra-high dose IMRT versus combination HDR brachytherapy and IMRT. Brachytherapy. 2010;9:313–8. doi: 10.1016/j.brachy.2010.02.196. [DOI] [PubMed] [Google Scholar]
- 15.Martinez AA, Gonzalez J, Ye H, et al. Dose escalation improves cancer-related events at 10 years for intermediate and high risk prostate cancer patients treated with hypofractionated high dose rate boost and external beam radiotherapy. Int J Radiat Oncol Biol Phys. 2011;79:363–70. doi: 10.1016/j.ijrobp.2009.10.035. [DOI] [PubMed] [Google Scholar]
- 16.D'Amico AV, Manola J, Loffredo M, Renshaw AA, DellaCroce A, Kantoff PW. 6-month androgen suppression plus radiation therapy vs radiation therapy alone for patients with clinically localized prostate cancer: a randomized controlled trial. JAMA. 2004;292:821–7. doi: 10.1001/jama.292.7.821. [DOI] [PubMed] [Google Scholar]
- 17.Jones CU, Hunt D, McGowan DG, et al. Radiotherapy and short term androgen deprivation for localized prostate cancer. New Engl J Med. 2011;365:107–18. doi: 10.1056/NEJMoa1012348. [DOI] [PubMed] [Google Scholar]
- 18.Bolla M, de Reijke TM, Van Tienhoven G, et al. Duration of androgen suppression in the treatment of prostate cancer. New Engl J Med. 2009;360:2516–27. doi: 10.1056/NEJMoa0810095. [DOI] [PubMed] [Google Scholar]
- 19.Zelefsky MJ, Levin EJ, Hunt M, et al. Incidence of later rectal and urinary toxicities after three dimensional conformal radiotherapy and intensity modulated radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2008;15:1124–9. doi: 10.1016/j.ijrobp.2007.11.044. [DOI] [PubMed] [Google Scholar]
- 20.Zelefsky MJ, Reuter VE, Fuks Z, et al. Influence of local control on distant metastases and cancer related mortality after external beam radiotherapy for prostate cancer. J Urol. 2008;179:1368–73. doi: 10.1016/j.juro.2007.11.063. [DOI] [PMC free article] [PubMed] [Google Scholar]


