Abstract
Cartilage degeneration with osteoarthritis (OA) is believed to involve the activities of interleukin-1 (IL-1), which exists as alpha and beta isoforms. The goal of this study was to measure the concentrations of both isoforms of IL-1 in the synovial fluid of normal and spontaneously osteoarthritic porcine knees, and to test the hypothesis that physiologic concentrations of IL-1α and IL-1β exhibit different potencies in activating calcium signaling, the production of matrix metalloproteinases and nitric oxide, and the loss of proteoglycans and tissue mechanical properties in cartilage and meniscus. Median concentrations of IL-1α were 0.043 ng/mL with mild OA and 0.288 ng/mL with moderate OA, whereas IL-1β concentrations were 0.109 ng/mL with mild OA and 0.122ng/mL with moderate OA. Both isoforms induced calcium signaling in chondrocytes and meniscal cells at all concentrations. Overall, cartilage and meniscus catabolism was significantly more sensitive to IL-1α than IL-1β at concentrations of 1 ng/mL or less, while few differences were observed between the two forms at 10 ng/mL. These data provide a range of physiologic IL-1 concentrations that can serve as a framework for the comparison of various in vitro studies, as well as providing further insight for the development of anti-cytokine therapies for OA.
Keywords: post-traumatic arthritis, inflammation, fibrochondrocyte, MMP, glycosaminoglycan
INTRODUCTION
Cartilage degeneration following joint injury or due to osteoarthritis (OA) involves the anti-anabolic and pro-catabolic activities of inflammatory cytokines, particularly interleukin-1 (IL-1). IL-1 is upregulated in injured and degenerated joints and in turn induces many degradative and pro-inflammatory pathways. The IL-1 family consists of two different isoforms, IL-1α and IL-1β, which have only 26% amino acid homology,1 yet elicit similar biological activities. Both proteins bind to the signaling IL-1 type I receptor (IL-1RI) and the soluble IL-1 receptor type II (IL-1RII) that acts as a decoy receptor and thus prevents IL-1 mediated signaling.1 Interestingly, IL-1α has a higher affinity for IL-1RI, while IL-1RII preferentially and almost irreversibly binds IL-1β.1 Upon binding of IL-1 to IL-1RI, complex signaling cascades are activated that induce many degradative processes in the joint.2,3 In particular, IL-1 promotes calcium influx,4,5 enhances the production of the pro-inflammatory mediator nitric oxide (NO), upregulates matrix metalloproteinases (MMPs), alters collagen synthesis and enhances collagen and proteoglycan breakdown and release in cartilage2,6–8 and meniscus.9–11
Few studies have reported physiologic concentrations of IL-1 in synovial fluid. In patients without rheumatic disease or joint effusion, up to 0.020 ng/mL IL-1β has been measured in the synovial fluid.12,13 On the other hand, IL-1β levels range from 0.115 – 0.130 ng/mL in rheumatoid arthritis (RA) patients and 0.021 – 0.146 ng/mL in OA patients.12–14 In people without RA, or with non-erosive RA, synovial fluid IL-1α concentrations are approximately 0.250 ng/mL, whereas IL-1α concentrations are elevated in erosive RA to 0.524 ng/mL.15 IL-1β concentrations range from 0.011 – 0.025 ng/mL in people with an anterior cruciate ligament (ACL) tear or combined ACL and meniscal tears.16 However, there is a paucity of data on the synovial fluid concentrations of both IL-1α and IL-1β in non-OA joints or those with spontaneous OA.
Nonetheless, IL-1 is believed to play a critical role in the pathogenesis of OA.17 It has been proposed that IL-1α is early acting, while IL-1β is more dominant in advanced disease.3,18 The neutralization of IL-1β alone is sufficient to suppress arthritic damage in established murine collagen-induced arthritis (CIA)19 but the combined inhibition of both IL-1α and β is most effective at blocking cartilage and bone destruction in mouse CIA.20 On the other hand, in a murine post-traumatic arthritis (PTA) model, systemic IL-1α levels positively correlated with cartilage degeneration.21 In addition, MRL/MpJ mice, which are protected from the development of PTA, have significantly lower levels of IL-1α and IL-1β in synovial fluid and joint tissues, as compared to mice that are not protected from PTA.22 However, the relative contributions of IL-1α and β to the breakdown of the cartilage and meniscus extracellular matrix are not fully understood but could provide new insights into the development of anti-cytokine therapies. The goal of this study was to determine the physiologic concentrations of IL-1α and IL-1β in the synovial fluid, and to test the hypothesis that IL-1α and IL-1β show different potencies for inducing catabolic and pro-inflammatory responses in cartilage and meniscus. We measured the concentrations of IL-1α and IL-1β in porcine synovial fluid from knee joints with various degrees of spontaneous OA degeneration. We treated chondrocytes and meniscal cells with a range of physiologic concentrations of either IL-1α or IL-1β and measured peak increases in cytosolic calcium concentrations (peak Ca++). Furthermore, we incubated cartilage and meniscal explants with physiologic concentrations of IL-1α or IL-1β and assessed MMP activity, NO production, S-GAG release, aggregate modulus, and hydraulic permeability.
MATERIALS AND METHODS
Synovial Fluid Measurement of IL-1
Synovial fluid was aspirated from the knee joints of 2–3 year old skeletally mature female pigs (n = 22) obtained from a local abattoir. The synovial fluid was spun down to remove cell debris and then aliquoted and stored at −80°C. Photographs were also taken of the cartilage, meniscus, and synovium of each joint for macroscopic tissue grading. Commercially available ELISA kits were used to measure porcine IL-1α and porcine IL-1β in the synovial fluid (MyBiosource, San Diego, CA) and assays were performed per the manufacturer’s instructions. For statistical analysis, the concentration of IL-1 was set to one-half the lower limit of detection for samples that had undetectable concentrations of IL-1α or IL-1β.
Macroscopic Joint Grading
OA degeneration in the cartilage was assessed using the Collins grading scale23,24 and similar scales were developed for the meniscus and synovium. In summary for cartilage, grade 0 had normal surface morphology, grade 1 had slight fibrillation, grade 2 showed deeper fibrillation, and grade 3 had serious fibrillation or degeneration.24 For the meniscus, grade 0 were intact and had normal morphology, grade 1 had mild degradation, grade 2 showed moderate degradation, and grade 3 had severe degradation. For the synovium, grade 0 showed a normal morphology, grade 1 had mild inflammation, grade 2 showed moderate inflammation, and grade 3 had severe inflammation, characterized by gross thickening of the synovial lining and increased vascularization. The macroscopic OA score for each tissue was determined by averaging the scores of three blinded graders and then a total macroscopic OA score for the joint was determined by summing the individual tissue grades. The total joint scores were divided into mild (total score 0 – 4.5) and moderate (total score 4.6 – 9) OA groups.
Calcium Imaging
Chondrocytes and meniscal cells were isolated from the femoral condyles and middle 1/3 of the medial meniscus of skeletally mature female pigs (n = 6) using a sequential pronase (Calbiochem, San Diego, CA) and collagenase (Worthington, Lakewood, NJ) digestion.25,26 Cells were plated on a 96-well plate at 100,000 cells/well and cultured overnight in Dulbecco’s Modified Eagle’s Medium (DMEM; Invitrogen, Carlsbad, CA), containing 10% fetal bovine serum (FBS; Invitrogen), 15 mM HEPES (Invitrogen), 0.1 mM non-essential amino acids (Invitrogen) and adjusted to 380 mOsm for chondrocytes and 310 mOsm for meniscal cells with sucrose. The baseline osmolarites were based on the estimated in situ osmolarity of cartilage (380 mOsm)27,28 and the much lower proteoglycan concentration in meniscus,29 suggests that the in situ osmolarity should be closer to other tissues and blood (300 mOsm).30 Wells were rinsed once with Hank’s balanced salt solution (HBSS, Invitrogen) and then loaded with the Ca++-sensitive dye Fluo-4 No Wash (Sigma-Aldrich, St. Louis, MO) according to the manufacturer’s instructions. All experiments were carried out in HBSS at 380 mOsm for chondrocytes and 310 mOsm for meniscal cells. A Fluorometric Imaging Plate Reader (FLIPR; Molecular Devices, Sunnyvale, CA) was used to add solutions [0, 0.01, 0.1, 1, or 10 ng/mL recombinant porcine IL-1α or IL-1β (R & D Systems, Minneapolis, MN)] to each well and measure the change in fluorescence in each well for 10 min. Each treatment was run in quadruplicate and replicate wells were averaged to obtain a single value for peak Ca++. The activity of the IL-1α and IL-1β used in these studies was determined by the manufacturer, using an assay to assess cell proliferation of a mouse helper T cell line in response to IL-1 treatment.
Explant Culture
Femoral condyles and medial menisci were isolated from 2–3 year old skeletally mature female pig knees obtained from a local abattoir. Using a 5 mm biopsy punch (Miltex, Inc, York, PA) oriented perpendicular to the meniscal surface, explants were harvested from the outer 1/3 of the femoral surface of the medial meniscus. Full thickness cartilage explants (5 mm diameter) from the opposing medial femoral condyle that articulates with the meniscus were also harvested. Explants were incubated in DMEM, containing 1000 U/mL penicillin/streptomycin (Invitrogen) for 1 hour at 37°C. Explants were then cultured for 72 hours in DMEM containing 10% heat inactivated FBS (HyClone, Logan, UT), 0.1 mM non-essential amino acids, 10 mM HEPES, 100 U/mL penicillin/streptomycin, and 37.5 μg/mL L-ascorbic acid 2-phosphate (Sigma-Aldrich, St. Louis, MO) to allow the tissue to equilibrate at 37°C and 5% CO2. Explants (n ≥ 7 per group) were then incubated for 24 hours with 0, 0.01, 0.1, 1, or 10 ng/mL IL-1α or IL-1β. Media were collected after 24 hours and measured for MMP activity, NO production, and S-GAG release. Mechanical properties of the explants were also determined.
MMP Activity Assay
Latent MMPs were activated using a stock solution of 10 mM p-aminophenylmercuric acetate (APMA; Sigma, St. Louis, MO) in 0.1 M NaOH, as previously described.11 Media samples were incubated with 2.5 mM APMA in assay buffer (200 mM NaCl, 50 mM Tris, 5 mM CaCl2, 10 μM ZnSO4, 0.01% Brij 35, pH 7.5) or assay buffer alone for 5 hours at 37°C. Total specific MMP activity (including both the pro-MMPs and active MMPs secreted into the media) was then measured using the quenched fluorogenic substrate Dab-Gly-Pro-Leu-Gly-Met-Arg-Gly-Lys-Flu (New England Peptide; Gardner, MA) as described previously.11,31–34
NO Production
Total NO production was determined by measuring total nitrate and nitrite concentrations, as detailed previously.35,36 All media samples and standards were filtered using Micron Ultracel YM-10 10,000 molecular weight cut-off filters (Millipore, Bedford, MA) and diluted 1:2 in dH2O. Nitrate reductase (Roche Diagnostics; Mannheim, Germany) was used to convert nitrate to nitrite. Total nitrite concentrations were determined using the Greiss reagent and absorbance was read at 540 nm. Total μmol NO were normalized to the wet weight of the tissue.
S-GAG Release
Total S-GAG release was determined using the 1,9-dimethylmethylene blue (DMB) assay.37 Standards of bovine trachea chondroitin-4-sulfate type A (Sigma, St. Louis, MO) and samples (20μl) were added to a 96 well plate in duplicate and 125 μl/well of DMB reagent was also added to each well. Absorbance was read within 5 min of DMB addition at 540 nm. Total μg of S-GAG released from each sample was corrected for the wet weight of the tissue.
Biomechanical Testing
Creep experiments were performed in a confined-compression configuration, using an ELF 3200 materials testing system (Bose, Minnetonka, Minnesota). Explants were placed in a confining chamber in a phosphate buffered saline bath and compressive loads were applied with a rigid porous platen. Following equilibration of a 2 gf tare load for cartilage or 5 gf tare load for meniscus, a step compressive load of 6 – 25 gf was applied to the explants and allowed to equilibrate for 10800 s. The step compressive loads were chosen to result in approximately 10 – 20% strain. The compressive modulus (HA) and hydraulic permeability (k) were determined using a three-parameter, nonlinear least-squares regression procedure38 assuming intrinsic incompressibility of the tissue.39 The third-parameter was an “instantaneous deformation parameter”38 that was not used to directly calculate the aggregate modulus or permeability.
Statistical Analyses
Statistical analyses were performed using Statistica 7.0 (StatSoft Inc., Tulsa, OK). The synovial fluid measurements were not normally distributed, thus the non-parametric Mann-Whitney U test was performed to determine differences in IL-1 concentration between mild and moderate OA joints, and Spearman correlations were performed to determine the relationship between IL-1α and β, and between IL-1 and tissue degeneration scores. For the cell and explant assays, a two-factor ANOVA and Newman-Keuls post hoc test were performed to determine significant differences (α=0.05) and the interactive effect of IL-1 isoform and concentration.
RESULTS
IL-1 Concentrations in Synovial Fluid
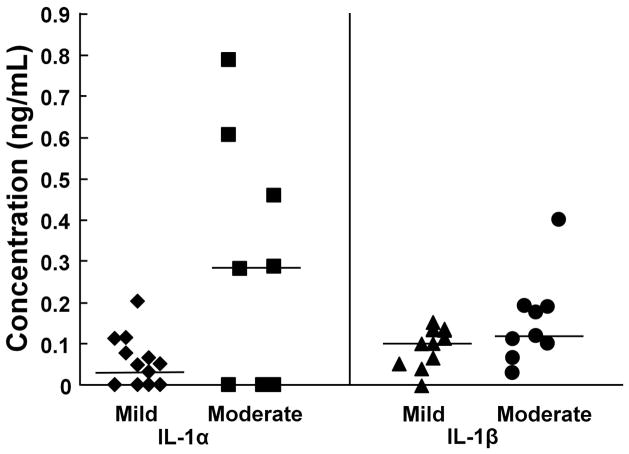
Both IL-1α and IL-1β were detectable in most synovial fluid samples and there was a strong correlation between IL-1α and IL-1β concentrations in individual synovial fluid samples (r2 = 0.60, p < 0.0005). IL-1α was detectable in 13 of 22 samples, and the median concentration was 0.043 ng/mL in mild OA joints and 0.288 ng/mL in moderate OA joints (Fig. 1; p = 0.13). In addition, there was a positive correlation between increasing IL-1α concentrations and increased degradation grade of the meniscus (r2 = 0.26, p < 0.05). On the other hand, IL-1β was measurable in all but 1 sample and the median concentration was 0.109 ng/mL in mild OA joints and 0.122 ng/mL in moderate OA joints (Fig. 1; p = 0.22). There was also a positive correlation between increasing IL-1β levels and meniscus degeneration (r2 = 0.25, p < 0.05) and total IL-1 concentration in the synovial fluid and meniscus grade (r2 = 0.42, p < 0.005).
Figure 1.
Synovial fluid concentrations of IL-1. The synovial fluid concentration of IL-1α in porcine knee joints with mild (n = 14; diamonds) or moderate (n = 8; squares) osteoarthritis (OA) is indicated on the left panel. The synovial fluid concentration of IL-1β in porcine knee joints with mild (n = 12; triangles) or moderate (n = 9; circles) OA is indicated on the right. Each point indicates the concentration for an individual sample and the median for each group is indicated by the line.
IL-1 effects on the peak Ca++ signal in chondrocytes and meniscal cells
The peak Ca++ signal in chondrocytes was increased by all concentrations of IL-1 (p < 0.05); however, there was no difference between the IL-1 isoforms (Fig. 2a). In the meniscal cells, all concentrations of IL-1 increased the peak Ca++ signal, as compared to the control (Fig. 2b; p < 0.05). IL-1α elevated the meniscal cell peak Ca++ signal, as compared to IL-1β (p < 0.05).
Figure 2.
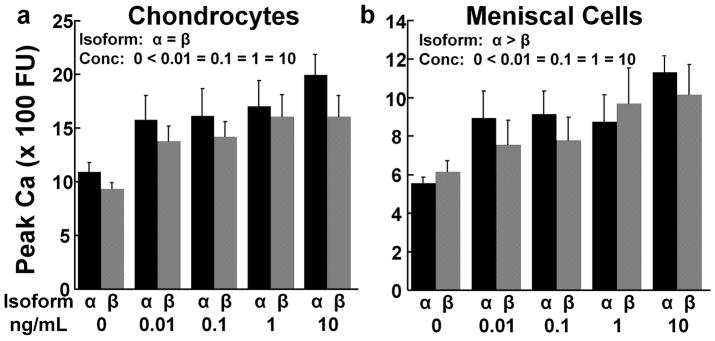
IL-1 effects on the peak Ca++ signal. (a) Peak Ca++ signal of chondrocytes in response to various concentrations of IL-1α (black bars) or IL-1β (gray bars). The data is expressed as fluorescence units (FU) + standard error. There was a significant effect of IL-1 concentration (conc) with 0 < all IL-1 concentrations. (b) Peak Ca++ signal of meniscal cells in response to various concentrations of IL-1α (black bars) or IL-1β (gray bars). There are significant effects of isoform with α > β and concentration (conc) with 0 < all IL-1 concentrations.
IL-1 effects on MMP activity in cartilage and meniscus explants
In both cartilage (Fig. 3a) and meniscus explants (Fig. 3b), total MMP activity was increased in a dose-dependent manner by both IL-1α and IL-1β, resulting in increased production of MMPs with increasing IL-1 concentrations (p < 0.05). Maximal MMP activity was obtained by treating the cartilage with 1 ng/mL IL-1α and the meniscus with only 0.01 ng/mL IL-1α. Total MMP activity in both tissues was increased by IL-1α at lower concentrations than IL-1β (p < 0.05). However, at 10 ng/mL in cartilage and 1 and 10 ng/mL in meniscus, there was no difference between the effects of IL-1α and β on MMP activity.
Figure 3.
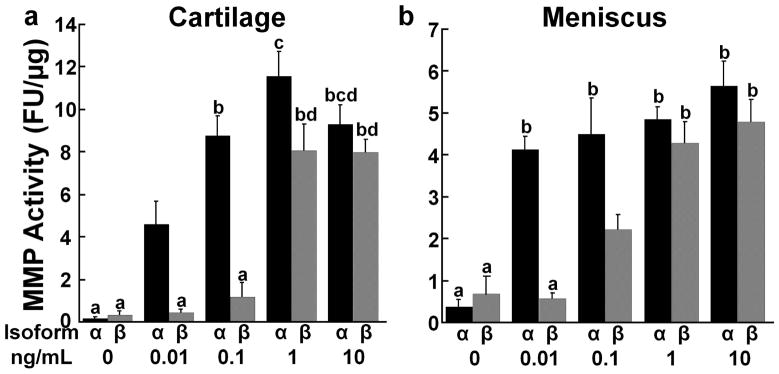
IL-1 effects on matrix metalloproteinase (MMP) activity. (a) Total MMP activity was measured in the media of cartilage explants exposed to various concentrations of IL-1α (black bars) or IL-1β (gray bars). The data is expressed as fluorescence units (FU)/μg wet weight + standard error. There are significant effects of isoform with α > β and concentration with 0 < 0.01 < 0.1 < 1 = 10. Groups not showing the same letter are statistically different from each other. (b) Total MMP activity was measured in the media of meniscus explants exposed to various concentrations of IL-1α (black bars) or IL-1β (gray bars). There are significant effects of isoform with α > β and concentration with 0 < 0.01 < 0.1 < 1 < 10.
IL-1 effects on NO production by cartilage and meniscus explants
NO production was increased in a dose-dependent manner by both the α- and β-isoforms of IL-1 in cartilage (Fig. 4a) and meniscus explants (Fig. 4b). Maximal NO production was obtained by treating the cartilage with 1 ng/mL IL-1α and the meniscus with 10 ng/mL IL-1α or β. NO levels were increased by IL-1α at lower concentrations than IL-1β in both cartilage and meniscus explants (p < 0.0005). However, at 10 ng/mL IL-1 there was no difference between the effects of IL-1α and β on NO production by cartilage and meniscus explants.
Figure 4.
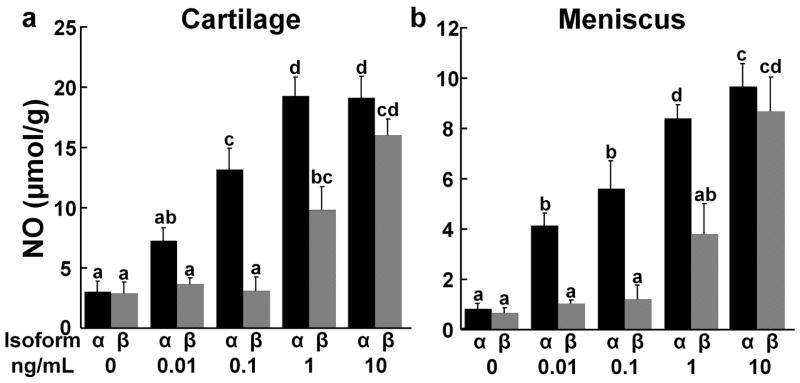
IL-1 effects on nitric oxide (NO) release. (a) NO release was measured in the media of cartilage explants exposed to various concentrations of IL-1α (black bars) or IL-1β (gray bars). The data is expressed as μmol NO/g wet weight + standard error. There are significant effects of isoform with α > β and concentration with 0 = 0.01 < 0.1 < 1 < 10. Groups not showing the same letter are statistically different from each other. (b) NO release was measured in the media of meniscus explants exposed to various concentrations of IL-1α (black bars) or IL-1β (gray bars). There are significant effects of isoform with α > β and concentration with 0 < 0.01 = 0.1 < 1 < 10.
IL-1 effects on S-GAG release from cartilage and meniscus explants
In cartilage explants there was a dose-dependent effect of both IL-1 isoforms on S-GAG release, resulting in elevated release of S-GAGs with increasing IL-1 concentrations (Fig. 5a, p < 0.001). The α isoform of IL-1 increased the release of cartilage S-GAGs, as compared to the β isoform (p < 0.001). On the other hand, there were no significant IL-1 isoform or concentration effects on meniscus S-GAG release (Fig. 5b).
Figure 5.
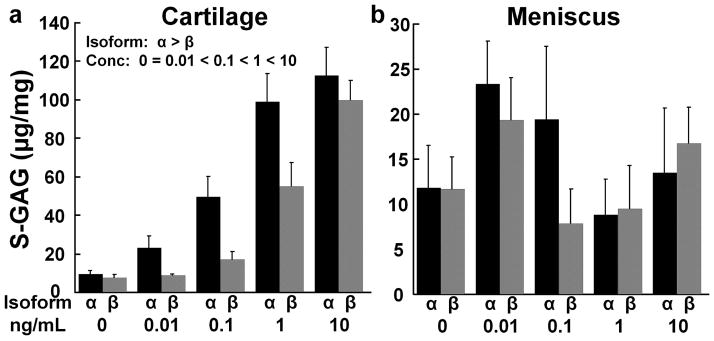
IL-1 effects on sulfated glycosaminoglycan (S-GAG) release. (a) S-GAG release was measured in the media of cartilage explants exposed to various concentrations of IL-1α (black bars) or IL-1β (gray bars). The data is expressed as μg S-GAG/mg wet weight + standard error. There are significant effects of isoform with α > β and concentration with 0 = 0.01 < 0.1 < 1 < 10. (b) S-GAG release was measured in the media of meniscus explants exposed to various concentrations of IL-1α (black bars) or IL-1β (gray bars).
IL-1 effects on the aggregate modulus of cartilage and meniscal explants
The curve-fits of the creep results were excellent with an average r2 value of 0.986 for cartilage and 0.996 for meniscus. IL-1 treatment of cartilage (Fig. 6a) and meniscal explants (Fig. 6b) decreased the aggregate modulus, as compared to control (p < 0.05) except in the cartilage 0.1 ng/mL IL-1 treatment group. Overall, there was a dose-dependent decrease in the aggregate modulus of both cartilage and meniscus tissue with increasing concentrations of IL-1. The cartilage aggregate modulus was decreased by IL-1α, as compared to the β isoform (p < 0.005). However, there was no significant IL-1 isoform effect on the meniscus aggregate modulus.
Figure 6.
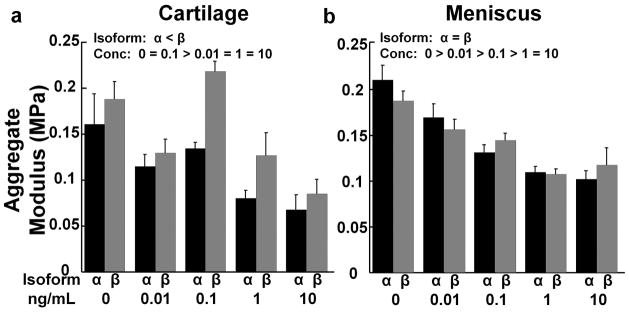
IL-1 effects on aggregate modulus. (a) Aggregate modulus of the cartilage explants treated with various concentrations of IL-1α (black bars) or IL-1β (gray bars). The data is expressed as MPa + standard error. There are significant effects of isoform with α < β and concentration (conc) with 0 = 0.1 > 0.01 = 1 = 10. (b) Aggregate modulus of the meniscal explants treated with various concentrations of IL-1α (black bars) or IL-1β (gray bars). There is a significant effect of concentration (conc) with 0 > 0.01 > 0.1 > 1 = 10.
IL-1 effects on the hydraulic permeability of cartilage and meniscus explants
In cartilage explants hydraulic permeability was highest in explants treated with 10 ng/mL IL-1, as compared to all other concentrations (Fig. 7a, p < 0.05). However, there were no significant IL-1 isoform effects on hydraulic permeability of cartilage explants. In addition, there were no significant IL-1 isoform or concentration effects on meniscus hydraulic permeability (Fig. 7b).
Figure 7.

IL-1 effects on hydraulic permeability. (a) Hydraulic permeability of the cartilage explants treated with various concentrations of IL-1α (black bars) or IL-1β (gray bars). The data is expressed as m4/Ns × 10−15 + standard error. There is a significant effect of concentration (conc) with 10 ng/mL > all other treatment groups. (b) Hydraulic permeability of the meniscal explants treated with various concentrations of IL-1α (black bars) or IL-1β (gray bars).
DISCUSSION
IL-1α synovial fluid concentrations were approximately 0.050 ng/mL in mild OA joints and increased to nearly 0.300 ng/mL in moderate OA porcine knee joints. On the other hand, IL-1β concentrations in these same joints were 0.110 – 0.120 ng/mL. At these physiologic concentrations of IL-1, the α-isoform significantly increased cartilage and meniscus catabolism, as evidenced by the upregulation of pro-inflammatory mediators and degradative enzymes, concomitant with a loss of tissue mechanical properties. While IL-1β also promoted tissue degradation, higher concentrations were required to elicit comparable responses to IL-1α. At the highest concentration of IL-1, there were no differences between the α- and β-isoforms.
The differences in the response to IL-1α and β may be due to differences in binding affinity to IL-1RI40 and/or expression of the decoy receptor IL-1RII.1 Porcine chondrocytes have been shown to highly express IL-1 receptors and it has been suggested that both isoforms of IL-1 bind with approximately equal affinity.41 However, the affinity of binding to the IL-1 receptor is rather controversial and it has also been suggested in rabbit chondrocytes that IL-1α binds to the receptor more strongly than IL-1β.42 IL-1 has been shown to increase the surface expression of IL-1RII, possibly altering the biological effects of IL-1α and β.1 Additionally, the signaling IL-1RI is upregulated43 and the decoy IL-1RII is downregulated44 in OA, resulting in a 4-fold reduction in the IL-1 concentration needed for half maximal activity in OA chondrocytes.43 Our data is consistent with a higher binding affinity for IL-1α to IL-1RI and/or IL-1 mediated upregulation of IL-1RII, resulting in suppression of IL-1β signaling.
In addition to being in the same gene family, both IL-1α and IL-1β share a strong interdependence that was evidenced by the correlation between the IL-1α and IL-1β levels in the synovial fluid samples. This correlation suggests that either both genes are regulated by the same cell signaling pathways, or that the production of one of these isoforms regulates the synthesis of the other gene.1 IL-1α was undetectable in more synovial fluid samples than IL-1β but was elevated nearly 7-fold in the moderate OA joints, as compared to a 1.1-fold increase in IL-1β levels in these joints. In this naturally occurring porcine OA model, the IL-1 levels correlated with degenerative changes to the meniscus, which has been well established using in vitro tissue explants.9,11
One of the earliest signaling events in the chondrocyte response to IL-1 appears to be an increase in cytosolic concentrations of Ca++.4,5 However, the mechanisms by which IL-1-induced Ca++ signaling influences chondrocyte or meniscal cell physiology are not fully understood. In another study, activation of Ca++ signaling through the transient receptor potential vanilloid 4 (TRPV4) ion channel was found to reverse the deleterious effects of 10 ng/ml of IL-1α on chondrocyte volume regulation.45 An important advance of the current study was to demonstrate the range of sensitivity of Ca++ signaling to IL-1α or β in both chondrocytes and meniscal cells. Interestingly, no differences were observed between the two isoforms of IL-1 on chondrocytes, whereas in the meniscal cells, the peak Ca++ signal was elevated more strongly by IL-1α than IL-1β. The peak Ca++ response in meniscal cells was also lower overall than that of chondrocytes. While additional studies are needed to further elucidate these pathways and their role in downstream IL-1 signaling, our findings suggest that the IL-1 responsive machinery is differentially regulated in chondrocytes and meniscal cells.
Overall, MMP activity, NO production, and S-GAG release were more potently increased by IL-1α than IL-1β in cartilage explants. The literature is currently divided on the potencies of IL-1α versus IL-1β on chondrocytes. IL-1α has been shown to be 23-fold more biologically active in rabbit chondrocytes based on stimulation of the proinflammatory mediator prostaglandin E2 (PGE2).46,47 In bovine cartilage explants, IL-1α was 5-fold more potent at S-GAG release than IL-1β.42 In contrast, IL-1β caused more synthesis of the proinflammatory mediators arachidonic acid and PGE in human chondrocytes.48 It is possible that there are species-specific differences in the chondrocyte responsiveness to IL-1α and IL-1β.
MMP activity and NO production were more potently increased by IL-1α than IL-1β in meniscal explants. However, MMP activity was also elevated in the meniscus by physiologic concentrations of IL-1β. There was no effect of either isoform of IL-1 on S-GAG release from the meniscal explants, likely due to the low proteoglycan content of the outer 1/3 of the meniscus,49 as well as the spontaneous loss of S-GAG from meniscus during normal tissue culture conditions.50
The IL-1 mediated catabolism of the extracellular matrix corresponded to a lower aggregate modulus for both cartilage and meniscus explants and increased permeability of the cartilage. Our cartilage and meniscus mechanical properties were consistent with previously measured values.51–53 In the cartilage but not the meniscus, the elevated catabolism in the IL-1α treated tissues resulted in a decreased aggregate modulus. A more potent decrease in equilibrium Young’s modulus and dynamic modulus has also been measured in IL-1α treated tissue engineered constructs containing young bovine chondrocytes, as compared to IL-1β treated constructs.54 In this study, the increase in tissue permeability corresponded well to the increase in S-GAG release from cartilage, while this trend was not observed in meniscus, likely due to differences in the proteoglycan content of the tissues. In the cartilage explants, breakdown of the proteoglycan rich extracellular matrix with IL-1α treatment was likely coincident with MMP mediated breakdown of the collagen network, resulting in decreased stiffness of the tissue. However, the differential effects of IL-1α and IL-1β on the meniscus, which contains approximately 10% the proteoglycan content of cartilage,55 were not strong enough over 24 hours to result in detectable differences in the mechanical properties or permeability of the tissue.
Based on the IL-1 synovial fluid concentrations in arthritic patients and our results in this study, IL-1α is expected to be the more dominant acting cytokine than IL-1β on cartilage and meniscus catabolism. However, in post-traumatic arthritis (PTA) both IL-1α and IL-1β may contribute to joint damage, as IL-1α synovial fluid levels peak at nearly 10 ng/mL and IL-1β levels peak at approximately 5 ng/mL following intra-articular fracture in a mouse model of PTA.22 At these concentrations, IL-1α and IL-1β were equally potent in the breakdown of the cartilage and meniscus. Thus the contribution of the two isoforms of IL-1 to breakdown of the joint tissues is likely to depend significantly on the timecourse, the concentrations of IL-1α and IL-1β that occur in various joint pathologies, and whether the simultaneous exposure to both isoforms results in additive or synergistic effects. These data also provide a framework for the comparison of results from in vitro studies that use either the α or β isoform of IL-1 and provide further insight for the development of anti-cytokine therapies for arthritis.
Acknowledgments
We thank Jonathan Brunger for programming assistance. This work was supported by grants from the Arthritis Foundation and National Institutes of Health grants AR50245, AG15768, AR48182, AR48852, and AR55434.
References
- 1.Dinarello CA. The interleukin-1 family: 10 years of discovery. FASEB J. 1994;8(15):1314–1325. [PubMed] [Google Scholar]
- 2.Lotz M, Blanco FJ, von Kempis J, et al. Cytokine regulation of chondrocyte functions. J Rheumatol Suppl. 1995;43:104–108. [PubMed] [Google Scholar]
- 3.van den Berg WB, Joosten LA, van de Loo FA. TNF alpha and IL-1 beta are separate targets in chronic arthritis. Clin Exp Rheumatol. 1999;17(6 Suppl 18):S105–114. [PubMed] [Google Scholar]
- 4.Luo L, Cruz T, McCulloch C. Interleukin 1-induced calcium signalling in chondrocytes requires focal adhesions. The Biochemical Journal. 1997;324 (Pt 2):653–658. doi: 10.1042/bj3240653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pritchard S, Guilak F. Effects of interleukin-1 on calcium signaling and the increase of filamentous actin in isolated and in situ articular chondrocytes. Arthritis Rheum. 2006;54(7):2164–2174. doi: 10.1002/art.21941. [DOI] [PubMed] [Google Scholar]
- 6.Hubbard JR, Steinberg JJ, Bednar MS, Sledge CB. Effect of purified human interleukin-1 on cartilage degradation. J Orthop Res. 1988;6(2):180–187. doi: 10.1002/jor.1100060204. [DOI] [PubMed] [Google Scholar]
- 7.Pelletier JP, DiBattista JA, Roughley P, McCollum R, Martel-Pelletier J. Cytokines and inflammation in cartilage degradation. Rheum Dis Clin North Am. 1993;19(3):545–568. [PubMed] [Google Scholar]
- 8.Stefanovic-Racic M, Mollers MO, Miller LA, Evans CH. Nitric oxide and proteoglycan turnover in rabbit articular cartilage. J Orthop Res. 1997;15(3):442–449. doi: 10.1002/jor.1100150318. [DOI] [PubMed] [Google Scholar]
- 9.Shin SJ, Fermor B, Weinberg JB, Pisetsky DS, Guilak F. Regulation of matrix turnover in meniscal explants: role of mechanical stress, interleukin-1, and nitric oxide. J Appl Physiol. 2003;95(1):308–313. doi: 10.1152/japplphysiol.00131.2003. [DOI] [PubMed] [Google Scholar]
- 10.Cao M, Stefanovic-Racic M, Georgescu HI, Miller LA, Evans CH. Generation of nitric oxide by lapine meniscal cells and its effect on matrix metabolism: stimulation of collagen production by arginine. J Orthop Res. 1998;16(1):104–111. doi: 10.1002/jor.1100160118. [DOI] [PubMed] [Google Scholar]
- 11.McNulty AL, Miller MR, O’Connor SK, Guilak F. The Effects of Adipokines on Cartilage and Meniscus Catabolism. Connect Tissue Res. 2011;52(6):523–533. doi: 10.3109/03008207.2011.597902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kahle P, Saal JG, Schaudt K, Zacher J, Fritz P, Pawelec G. Determination of cytokines in synovial fluids: correlation with diagnosis and histomorphological characteristics of synovial tissue. Annals of the Rheumatic Diseases. 1992;51(6):731–734. doi: 10.1136/ard.51.6.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Westacott CI, Whicher JT, Barnes IC, Thompson D, Swan AJ, Dieppe PA. Synovial fluid concentration of five different cytokines in rheumatic diseases. Ann Rheum Dis. 1990;49(9):676–681. doi: 10.1136/ard.49.9.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hopkins SJ, Humphreys M, Jayson MI. Cytokines in synovial fluid. I. The presence of biologically active and immunoreactive IL-1. Clinical & Experimental Immunology. 1988;72(3):422–427. [PMC free article] [PubMed] [Google Scholar]
- 15.Fong KY, Boey ML, Koh WH, Feng PH. Cytokine concentrations in the synovial fluid and plasma of rheumatoid arthritis patients: correlation with bony erosions. Clin Exp Rheumatol. 1994;12(1):55–58. [PubMed] [Google Scholar]
- 16.Bigoni M, Sacerdote P, Turati M, et al. Acute and late changes in intraarticular cytokine levels following anterior cruciate ligament injury. J Orthop Res. 2012 doi: 10.1002/jor.22208. [DOI] [PubMed] [Google Scholar]
- 17.Goldring MB. Osteoarthritis and cartilage: the role of cytokines. Curr Rheumatol Rep. 2000;2(6):459–465. doi: 10.1007/s11926-000-0021-y. [DOI] [PubMed] [Google Scholar]
- 18.Towle CA, Hung HH, Bonassar LJ, Treadwell BV, Mangham DC. Detection of interleukin-1 in the cartilage of patients with osteoarthritis: a possible autocrine/paracrine role in pathogenesis. Osteoarthritis Cartilage. 1997;5(5):293–300. doi: 10.1016/s1063-4584(97)80008-8. [DOI] [PubMed] [Google Scholar]
- 19.Joosten LA, Helsen MM, van de Loo FA, van den Berg WB. Anticytokine treatment of established type II collagen-induced arthritis in DBA/1 mice: a comparative study using anti-TNFalpha, anti-IL-1alpha/beta and IL-1Ra. Arthritis Rheum. 1996;58(2 Suppl):S110–122. doi: 10.1002/art.23363. [DOI] [PubMed] [Google Scholar]
- 20.Joosten LA, Helsen MM, Saxne T, van De Loo FA, Heinegard D, van Den Berg WB. IL-1 alpha beta blockade prevents cartilage and bone destruction in murine type II collagen-induced arthritis, whereas TNF-alpha blockade only ameliorates joint inflammation. J Immunol. 1999;163(9):5049–5055. [PubMed] [Google Scholar]
- 21.Ward BD, Furman BD, Huebner JL, Kraus VB, Guilak F, Olson SA. Absence of posttraumatic arthritis following intraarticular fracture in the MRL/MpJ mouse. Arthritis Rheum. 2008;58(3):744–753. doi: 10.1002/art.23288. [DOI] [PubMed] [Google Scholar]
- 22.Lewis JS, Jr, Furman BD, Zeitler E, et al. Genetic and cellular evidence of decreased inflammation associated with reduced post-traumatic arthritis in MRL/MpJ mice. In Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Collins DH, Mc ET. Sulphate (35SO4) uptake by chondrocytes in relation to histological changes in osteoarthritic human articular cartilage. Ann Rheum Dis. 1960;19:318–330. doi: 10.1136/ard.19.4.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li G, Thomson M, Dicarlo E, et al. A chemometric analysis for evaluation of early-stage cartilage degradation by infrared fiber-optic probe spectroscopy. Appl Spectrosc. 2005;59(12):1527–1533. doi: 10.1366/000370205775142593. [DOI] [PubMed] [Google Scholar]
- 25.Fermor B, Jeffcoat D, Hennerbichler A, Pisetsky DS, Weinberg JB, Guilak F. The effects of cyclic mechanical strain and tumor necrosis factor alpha on the response of cells of the meniscus. Osteoarthritis Cartilage. 2004;12(12):956–962. doi: 10.1016/j.joca.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 26.Kuettner KE, Pauli BU, Gall G, Memoli VA, Schenk RK. Synthesis of cartilage matrix by mammalian chondrocytes in vitro. I. Isolation, culture characteristics, and morphology. J Cell Biol. 1982;93(3):743–750. doi: 10.1083/jcb.93.3.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maroudas A, Evans H. A Study of Ionic Equilibria in Cartilage. Connect Tissue Res. 1972;1(1):69–77. [Google Scholar]
- 28.Urban JPG, Hall AC, Gehl KA. Regulation of Matrix Synthesis Rates by the Ionic and Osmotic Environment of Articular Chondrocytes. J Cell Physiol. 1993;154(2):262–270. doi: 10.1002/jcp.1041540208. [DOI] [PubMed] [Google Scholar]
- 29.Mcnicol D, Roughley PJ. Extraction and Characterization of Proteoglycan from Human Meniscus. Biochem J. 1980;185(3):705–713. doi: 10.1042/bj1850705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmidt-Nielsen K. Animal Physiology: Adaptation and Environment. Cambridge: Cambridge University Press; 1997. Water and Osmotic Regulation; pp. 301–354. [Google Scholar]
- 31.Deng S-J, Bickett DM, Mitchell JL, et al. Substrate Specificity of Human Collagenase 3 Assessed Using a Phage-displayed Peptide Library. The Journal of Biological Chemistry. 2000;275:31422–31427. doi: 10.1074/jbc.M004538200. October 6, 2000. [DOI] [PubMed] [Google Scholar]
- 32.McNulty AL, Estes BT, Wilusz RE, Weinberg JB, Guilak F. Dynamic loading enhances integrative meniscal repair in the presence of interleukin-1. Osteoarthritis Cartilage. 2010;18(6):830–838. doi: 10.1016/j.joca.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McNulty AL, Weinberg JB, Guilak F. Inhibition of matrix metalloproteinases enhances in vitro repair of the meniscus. Clin Orthop Relat Res. 2009;467(6):1557–1567. doi: 10.1007/s11999-008-0596-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilusz RE, Weinberg JB, Guilak F, McNulty AL. Inhibition of Integrative Repair of the Meniscus Following Acute Exposure to Interleukin-1 in vitro. Journal of Orthopaedic Research. 2008;26:504–512. doi: 10.1002/jor.20538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fermor B, Weinberg JB, Pisetsky DS, Misukonis MA, Fink C, Guilak F. Induction of cyclooxygenase-2 by mechanical stress through a nitric oxide-regulated pathway. Osteoarthritis Cartilage. 2002;10(10):792–798. doi: 10.1053/joca.2002.0832. [DOI] [PubMed] [Google Scholar]
- 36.Granger DL, Anstey NM, Miller WC, Weinberg JB. Measuring nitric oxide production in human clinical studies. Methods Enzymol. 1999;301:49–61. doi: 10.1016/s0076-6879(99)01068-x. [DOI] [PubMed] [Google Scholar]
- 37.Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochimica et Biophysica Acta. 1986;883(2):173–177. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 38.Mow VC, Kuei SC, Lai WM, Armstrong CG. Biphasic creep and stress relaxation of articular cartilage in compression? Theory and experiments. J Biomech Eng. 1980;102(1):73–84. doi: 10.1115/1.3138202. [DOI] [PubMed] [Google Scholar]
- 39.Bachrach NM, Mow VC, Guilak F. Incompressibility of the solid matrix of articular cartilage under high hydrostatic pressures. J Biomech. 1998;31(5):445–451. doi: 10.1016/s0021-9290(98)00035-9. [DOI] [PubMed] [Google Scholar]
- 40.Dower SK, Kronheim SR, Hopp TP, et al. The cell surface receptors for interleukin-1 alpha and interleukin-1 beta are identical. Nature. 1986;324(6094):266–268. doi: 10.1038/324266a0. [DOI] [PubMed] [Google Scholar]
- 41.Bird TA, Saklatvala J. Identification of a common class of high affinity receptors for both types of porcine interleukin-1 on connective tissue cells. Nature. 1986;324(6094):263–266. doi: 10.1038/324263a0. [DOI] [PubMed] [Google Scholar]
- 42.Smith RJ, Rohloff NA, Sam LM, Justen JM, Deibel MR, Cornette JC. Recombinant human interleukin-1 alpha and recombinant human interleukin-1 beta stimulate cartilage matrix degradation and inhibit glycosaminoglycan synthesis. Inflammation. 1989;13(4):367–382. doi: 10.1007/BF00914921. [DOI] [PubMed] [Google Scholar]
- 43.Martel-Pelletier J, McCollum R, DiBattista J, et al. The interleukin-1 receptor in normal and osteoarthritic human articular chondrocytes. Identification as the type I receptor and analysis of binding kinetics and biologic function. Arthritis Rheum. 1992;35(5):530–540. doi: 10.1002/art.1780350507. [DOI] [PubMed] [Google Scholar]
- 44.Attur MG, Dave M, Cipolletta C, et al. Reversal of autocrine and paracrine effects of interleukin 1 (IL-1) in human arthritis by type II IL-1 decoy receptor. Potential for pharmacological intervention. J Biol Chem. 2000;275(51):40307–40315. doi: 10.1074/jbc.M002721200. [DOI] [PubMed] [Google Scholar]
- 45.Phan MN, Leddy HA, Votta BJ, et al. Functional characterization of TRPV4 as an osmotically sensitive ion channel in porcine articular chondrocytes. Arthritis & Rheumatism. 2009;60(10):3028–3037. doi: 10.1002/art.24799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chin JE, Horuk R. Interleukin 1 receptors on rabbit articular chondrocytes: relationship between biological activity and receptor binding kinetics. FASEB J. 1990;4(5):1481–1487. doi: 10.1096/fasebj.4.5.2137805. [DOI] [PubMed] [Google Scholar]
- 47.Pelletier JP, Roughley PJ, DiBattista JA, McCollum R, Martel-Pelletier J. Are cytokines involved in osteoarthritic pathophysiology? Semin Arthritis Rheum. 1991;20(6 Suppl 2):12–25. doi: 10.1016/0049-0172(91)90024-t. [DOI] [PubMed] [Google Scholar]
- 48.Knott I, Dieu M, Burton M, Lecomte V, Remacle J, Raes M. Differential effects of interleukin-1 alpha and beta on the arachidonic acid cascade in human synovial cells and chondrocytes in culture. Agents Actions. 1993;39(3–4):126–131. doi: 10.1007/BF01998964. [DOI] [PubMed] [Google Scholar]
- 49.Arnoczky SP, Warren RF. Microvasculature of the human meniscus. Am J Sports Med. 1982;10(2):90–95. doi: 10.1177/036354658201000205. [DOI] [PubMed] [Google Scholar]
- 50.McNulty AL, Moutos FT, Weinberg JB, Guilak F. Enhanced Integrative Repair of the Porcine Meniscus In Vitro by Inhibition of Interleukin-1 or Tumor Necrosis Factor-alpha. Arthritis Rheum. 2007;56(9):3033–3042. doi: 10.1002/art.22839. [DOI] [PubMed] [Google Scholar]
- 51.Hennerbichler A, Rosenberger R, Arora R, Hennerbichler D. Biochemical, biomechanical and histological properties of osteoarthritic porcine knee cartilage: implications for osteochondral transplantation. Arch Orthop Trauma Surg. 2008;128(1):61–70. doi: 10.1007/s00402-007-0360-5. [DOI] [PubMed] [Google Scholar]
- 52.Sweigart MA, Zhu CF, Burt DM, et al. Intraspecies and interspecies comparison of the compressive properties of the medial meniscus. Ann Biomed Eng. 2004;32(11):1569–1579. doi: 10.1114/b:abme.0000049040.70767.5c. [DOI] [PubMed] [Google Scholar]
- 53.Hunter SA, Noyes FR, Haridas B, Levy MS, Butler DL. Meniscal material properties are minimally affected by matrix stabilization using glutaraldehyde and glycation with ribose. J Orthop Res. 2005;23(3):555–561. doi: 10.1016/j.orthres.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 54.Lima EG, Tan AR, Tai T, et al. Physiologic deformational loading does not counteract the catabolic effects of interleukin-1 in long-term culture of chondrocyte-seeded agarose constructs. J Biomech. 2008;41(15):3253–3259. doi: 10.1016/j.jbiomech.2008.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Proctor CS, Schmidt MB, Whipple RR, Kelly MA, Mow VC. Material properties of the normal medial bovine meniscus. J Orthop Res. 1989;7(6):771–782. doi: 10.1002/jor.1100070602. [DOI] [PubMed] [Google Scholar]