Abstract
Context
Associations have been found between day-to-day particulate air pollution and increased risk of various adverse health outcomes, including cardiopulmonary mortality. However, studies of health effects of long-term particulate air pollution have been less conclusive.
Objective
To assess the relationship between long-term exposure to fine particulate air pollution and all-cause, lung cancer, and cardiopulmonary mortality.
Design, Setting, and Participants
Vital status and cause of death data were collected by the American Cancer Society as part of the Cancer Prevention II study, an ongoing prospective mortality study, which enrolled approximately 1.2 million adults in 1982. Participants completed a questionnaire detailing individual risk factor data (age, sex, race, weight, height, smoking history, education, marital status, diet, alcohol consumption, and occupational exposures). The risk factor data for approximately 500000 adults were linked with air pollution data for metropolitan areas throughout the United States and combined with vital status and cause of death data through December 31, 1998.
Main Outcome Measure
All-cause, lung cancer, and cardiopulmonary mortality.
Results
Fine particulate and sulfur oxide–related pollution were associated with all-cause, lung cancer, and cardiopulmonary mortality. Each 10-μg/m3 elevation in fine particulate air pollution was associated with approximately a 4%, 6%, and 8% increased risk of all-cause, cardiopulmonary, and lung cancer mortality, respectively. Measures of coarse particle fraction and total suspended particles were not consistently associated with mortality.
Conclusion
Long-term exposure to combustion-related fine particulate air pollution is an important environmental risk factor for cardiopulmonary and lung cancer mortality.
Based on several severe air pollution events,1-3 a temporal correlation between extremely high concentrations of particulate and sulfur oxide air pollution and acute increases in mortality was well established by the 1970s. Subsequently, epidemiological studies published between 1989 and 1996 reported health effects at unexpectedly low concentrations of particulate air pollution.4 The convergence of data from these studies, while controversial,5 prompted serious reconsideration of standards and health guidelines6-10 and led to a long-term research program designed to analyze health-related effects due to particulate pollution.11-13 In 1997, the Environmental Protection Agency adopted new ambient air quality standards that would impose regulatory limits on fine particles measuring less than 2.5 μm in diameter (PM2.5). These new standards were challenged by industry groups, blocked by a federal appeals court, but ultimately upheld by the US Supreme Court.14
Although most of the recent epidemiological research has focused on effects of short-term exposures, several studies suggest that long-term exposure may be more important in terms of overall public health.4 The new standards for long-term exposure to PM2.5 were originally based primarily on 2 prospective cohort studies,15,16 which evaluated the effects of long-term pollution exposure on mortality. Both of these studies have been subjected to much scrutiny,5 including an extensive independent audit and reanalysis of the original data.17 The larger of these 2 studies linked individual risk factor and vital status data with national ambient air pollution data.16 Our analysis uses data from the larger study and (1) doubles the follow-up time to more than 16 years and triples the number of deaths; (2) substantially expands exposure data, including gaseous copollutant data and new PM2.5 data, which have been collected since the promulgation of the new air quality standards; (3) improves control of occupational exposures; (4) incorporates dietary variables that account for total fat consumption, and consumption of vegetables, citrus, and high-fiber grains; and (5) uses recent advances in statistical modeling, including the incorporation of random effects and nonpara-metric spatial smoothing components in the Cox proportional hazards model.
METHODS
Study Population
The analysis is based on data collected by the American Cancer Society (ACS) as part of the Cancer Prevention Study II (CPS-II), an ongoing prospective mortality study of approximately 1.2 million adults.18,19 Individual participants were enrolled by ACS volunteers in the fall of 1982. Participants resided in all 50 states, the District of Columbia, and Puerto Rico, and were generally friends, neighbors, or acquaintances of ACS volunteers. Enrollment was restricted to persons who were aged 30 years or older and who were members of households with at least 1 individual aged 45 years or older. Participants completed a confidential questionnaire, which included questions about age, sex, weight, height, smoking history, alcohol use, occupational exposures, diet, education, marital status, and other characteristics.
Vital status of study participants was ascertained by ACS volunteers in September of the following years: 1984, 1986, and 1988. Reported deaths were verified with death certificates. Subsequently, through December 31, 1998, vital status was ascertained through automated linkage of the CPS-II study population with the National Death Index.19 Ascertainment of deaths was more than 98% complete for the period of 1982-1988 and 93% complete after 1988.19 Death certificates or codes for cause of death were obtained for more than 98% of all known deaths. Cause of death was coded according to the International Classification of Diseases, Ninth Revision (ICD-9). Although the CPS-II cohort included approximately 1.2 million participants with adequate questionnaire and cause-of-death data, our analysis was restricted to those participants who resided in US metropolitan areas with available pollution data. The actual size of the analytic cohort varied depending on the number of metropolitan areas for which pollution data were available. TABLE 1 provides the number of metropolitan areas and participants available for each source of pollution data.
Table 1.
Summary of Alternative Pollution Indices*
| Pollutant (Years of Data Collection) | Units | Source of Data | Data Compilation Team† | No. of Metropolitan Areas | No. of Participants, in Thousands | Mean (SD) |
|---|---|---|---|---|---|---|
| PM2.5 | μg/m3 | |||||
| 1979-1983 | IPMN | HEI | 61 | 359 | 21.1 (4.6) | |
| 1999-2000 | AIRS | NYU | 116 | 500 | 14.0 (3.0) | |
| Average | 51 | 319 | 17.7 (3.7) | |||
| PM10 | μg/m3 | |||||
| 1982-1998 | AIRS | NYU | 102 | 415 | 28.8 (5.9) | |
| PM15 | μg/m3 | |||||
| 1979-1983 | IPMN | HEI | 63 | 359 | 40.3 (7.7) | |
| PM15-2.5 | μg/m3 | |||||
| 1979-1983 | IPMN | HEI | 63 | 359 | 19.2 (6.1) | |
| Total suspended particles | μg/m3 | |||||
| 1980-1981 | NAD | HEI | 156 | 590 | 68.0 (16.7) | |
| 1979-1983 | IPMN | HEI | 58 | 351 | 73.7 (14.3) | |
| 1982-1998 | AIRS | NYU | 150 | 573 | 56.7 (13.1) | |
| Sulfate | μg/m3 | |||||
| 1980-1981 | IPMN and NAD, artifact adjusted | HEI | 149 | 572 | 6.5 (2.8) | |
| 1990 | Compilation and analysis of PM10 filters | NYU | 53 | 269 | 6.2 (2.0) | |
| Sulfur dioxide | ppb | AIRS | ||||
| 1980 | HEI | 118 | 520 | 9.7 (4.9) | ||
| 1982-1998 | NYU | 126 | 539 | 6.7 (3.0) | ||
| Nitrogen dioxide | ppb | AIRS | ||||
| 1980 | HEI | 78 | 409 | 27.9 (9.2) | ||
| 1982-1998 | NYU | 101 | 493 | 21.4 (7.1) | ||
| Carbon monoxide | ppm | AIRS | ||||
| 1980 | HEI | 113 | 519 | 1.7 (0.7) | ||
| 1982-1998 | NYU | 122 | 536 | 1.1 (0.4) | ||
| Ozone | ppb | AIRS | ||||
| 1980 | HEI | 134 | 569 | 47.9 (11.0) | ||
| 1982-1998 | NYU | 119 | 525 | 45.5 (7.3) | ||
| 1982-1998‡ | NYU | 134 | 557 | 59.7 (12.8) |
PM2.5 indicates particles measuring less than 2.5 μm in diameter; PM10, particles measuring less than 10 μm in diameter; PM15, particles measuring less than 15 μm in diameter; PM15-2.5, particles measuring between 2.5 and 15 μm in diameter; μg/m3, micrograms per cubic meter; ppb, parts per billion; ppm, parts per million; IPMN, Inhalable Particle Monitoring Network; AIRS, Aerometric Information Retrieval System [Environmental Protection Agency]; and NAD, National Aerometric Database.
HEI indicates data were compiled by the Health Effects Institute reanalysis team, which was previously published.17 NYU indicates data were compiled at the New York University School of Medicine, Nelson Institute of Environmental Medicine (K.I. and G.D.T.).
Daily 1-hour maximums were used. Values were calculated only for the third quarter (ie, July, August, September).
Air Pollution Exposure Estimates
Each participant was assigned a metropolitan area of residence based on address at time of enrollment and 3-digit ZIP code area.20 Mean (SD) concentrations of air pollution for the metropolitan areas were compiled from various primary data sources (Table 1). Many of the particulate pollution indices, including PM2.5, were available from data from the Inhalable Particle Monitoring Network for 1979-1983 and data from the National Aerometric Database for 1980-1981, periods just prior to or at the beginning of the follow-up period. An additional data source was the Environmental Protection Agency Aerometric Information Retrieval System (AIRS). The mean concentration of each pollutant from all available monitoring sites was calculated for each metropolitan area during the 1 to 2 years prior to enrollment.17
Additional information on ambient pollution during the follow-up period was extracted from the AIRS database as quarterly mean values for each routinely monitored pollutant for 1982 through 1998. All quarterly averages met summary criteria imposed by the Environmental Protection Agency and were based on observations made on at least 50% of the scheduled sampling days at each site. The quarterly mean values for all stations in each metro politan area were calculated across the study years using daily average values for each pollutant except ozone. For ozone, daily 1-hour maximums were used and were calculated for the full year and for the third quarter only (ie, July, August, September). While gaseous pollutants generally had recorded data throughout the entire follow-up period of interest, the particulate matter monitoring protocol changed in the late 1980s from total suspended particles to particles measuring less than 10 μm in diameter (PM10), resulting in the majority of total suspended particle data being available in the early to mid-1980s and PM10 data being mostly available in the early to mid-1990s.
As a consequence of the new PM2.5 standard, a large number of sites began collecting PM2.5 data in 1999. Daily PM2.5 data were extracted from the AIRS database for 1999 and the first 3 quarters of 2000. For each site, quarterly averages for each of the 2 years were computed. The 4 quarters were averaged when at least 1 of the 2 corresponding quarters for each year had at least 50% of the sixth-day samples and at least 45 total sampling days available. Measurements were averaged first by site and then by metropolitan area. Although no network of PM2.5 monitoring existed in the United States between the early 1980s and the late 1990s, the integrated average of PM2.5 concentrations during the period was estimated by averaging the PM2.5 concentration for early and later periods.
Mean sulfate concentrations for 1980-1981 were available for many cities based on data from the Inhalable Particle Monitoring Network and the National Aerometric Database. Recognizing that sulfate was artifactually overestimated due to glass fiber filters used at that time, season and region-specific adjustments were made.17 Since few states analyzed particulate samples for sulfates after the early 1980s, individual states were directly contacted for data regarding filter use. Ion chromatography was used to analyze PM10 filters and this data could be obtained from metropolitan areas across the United States. Filters were collected for a single reference year (1990) in the middle of the 1982-1998 study period. The use of quartz filters virtually eliminated the historical overestimation of sulfate. Mean sulfate concentrations for 1990 were estimated using sulfate from AIRS, data reported directly from individual states, and analysis of archived filters.
Statistical Analysis
The basic statistical approach used in this analysis is an extension of the standard Cox proportional hazards survival model,21 which has been used for risk estimates of pollution-related mortality in previous longitudinal cohort studies.15,16 The standard Cox model implicitly assumes that observations are statistically independent after controlling for available risk factors, resulting in 2 concerns with regard to risk estimates of pollution-related mortality.22 First, if the assumption of statistical independence is not valid, the uncertainty in the risk estimates of pollution-related mortality may be misstated. Second, even after controlling for available risk factors, survival times of par ticipants living in communities closer together may be more similar than participants living in communities farther apart, which results in spatial autocorrelation. If this spatial autocorrelation is due to missing or systematically mismeasured risk factors that are spatially correlated with air pollution, then the risk estimates of pollution-related mortality may be biased due to inadequate control of these factors. Therefore, in this analysis, the Cox proportional hazards model was extended by incorporating a spatial random-effects component, which provided accurate estimates of the uncertainty of effect estimates. The model also evaluated spatial autocorrelation and incorporated a nonparametric spatial smooth component (to account for unexplained spatial structure). A more detailed description of this modeling approach is provided elsewhere.22
The baseline analysis in this study estimated adjusted relative risk (RR) ratios for mortality by using a Cox proportional hazards model with inclusion of a metropolitan-based random-effects component. Model fitting involved a 2-stage process. In the first stage, survival data were modeled using the standard Cox proportional hazards model, including individual level covariates and indicator variables for each metropolitan area (without pollution variables). Output from stage 1 provided estimates of the metropolitan-specific logarithm of the RRs of mortality (relative to an arbitrary reference community), which were adjusted for individual risk factors. The correlation between these values, which was induced by using the same reference community, was then removed.23 In the second stage, the estimates of adjusted metropolitan-specific health responses were related to fine particulate air pollution using a linear random-effects regression model.24 The time variable used in the models was survival time from the date of enrollment. Survival times of participants who did not die were censored at the end of the study period. To control for age, sex, and race, all of the models were stratified by 1-year age categories, sex, and race (white vs other), which allowed each category to have its own baseline hazard. Models were estimated for all-cause mortality and for 3 separate mortality categories: cardiopulmonary (ICD-9 401-440 and 460-519), lung cancer (ICD-9 162), and all others.
Models were estimated separately for each of the 3 fine particle variables, PM2.5 (1979-1983), PM2.5 (1999-2000), and PM2.5 (average). Individual level covariates were included in the models to adjust for various important individual risk factors. All of these variables were classified as either indicator (ie, yes/no, binary, dummy) variables or continuous variables. Variables used to control for tobacco smoke, for example, included both indicator and continuous variables. The smoking indicator variables included: current cigarette smoker, former cigarette smoker, and a pipe or cigar smoker only (all vs never smoking) along with indicator variables for starting smoking before or after age 18 years. The continuous smoking variables included: current smoker's years of smoking, current smoker's years of smoking squared, current smoker's cigarettes per day, current smoker's cigarettes per day squared, former smoker's years of smoking, former smoker's years of smoking squared, former smoker's cigarettes per day, former smoker's cigarettes per day squared, and the number of hours per day exposed to passive cigarette smoke.
To control for education, 2 indicator variables, which indicated completion of high school or education beyond high school, were included. Marital status variables included indicator variables for single and other vs married. Both body mass index (BMI) values and BMI values squared were included as continuous variables. Indicator variables for beer, liquor, and wine drinkers and nonresponders vs non-drinkers were included to adjust for alcohol consumption. Occupational exposure was controlled for using various indicator variables: regular occupational exposure to asbestos, chemicals/ acids/solvents, coal or stone dusts, coal tar/pitch/asphalt, diesel engine exhaust, or formaldehyde, and additional indicator variables that indicated 9 different rankings of an occupational dirtiness index that has been developed and described elsewhere.17,25 Two diet indices that accounted for fat consumption and consumption of vegetables, citrus, and high-fiber grains were derived based on information given in the enrollment questionnaire.18 Quintile indicator variables for each of these diet indices were also included in the models.18
In addition to the baseline analysis, several additional sets of analysis were conducted. First, to more fully evaluate the shape of the concentration-response function, a robust locally weighted regression smoother26 (within the generalized additive model framework27) was used to estimate the relationship between particulate air pollution and mortality in the second stage of model fitting. Second, the sensitivity of the fine particle mortality risk estimates compared with alternative modeling approaches and assumptions was evaluated. Standard Cox proportional hazards models were fit to the data including particulate air pollution as a predictor of mortality and sequentially adding (in a controlled forward stepwise process) groups of variables to control for smoking, education, marital status, BMI, alcohol consumption, occupational exposures, and diet.
In addition, to evaluate the sensitivity of the estimated pollution effect while more aggressively controlling for spatial differences in mortality, a 2-dimensional term to account for spatial trends was added to the models and was estimated using a locally weighted regression smoother. The “span” parameter, which controls the complexity of the surface smooth, was set at 3 different settings to allow for increasingly aggressive fitting of the spatial structure. These included a default span of 50%, the span that resulted in the lowest unexplained variance in mortality rate between metropolitan areas, and the span that resulted in the strongest evidence (highest P value) to suggest no residual spatial structure. The risk estimates and SEs (and thus the confidence intervals) were estimated using generalized additive modeling27 with S-Plus statistical software,28 which provides unbiased effect estimates, but may underestimate SEs if there is significant spatial autocorrelation and significant correlations between air pollution and the smoothed surface of mortality. Therefore, evidence of spatial autocorrelation was carefully evaluated and tested using the Bartlett test.29 The correlations of residual mortality with distance between metropolitan areas were graphically examined.
Analyses were also conducted of effect modification by age, sex, smoking status, occupational exposure, and education. Finally, models were fit using a variety of alternative pollution indices, including gaseous pollutants. Specifically, models were estimated separately for each of the pollution variables listed in Table 1, while also including all of the other risk factor variables.
RESULTS
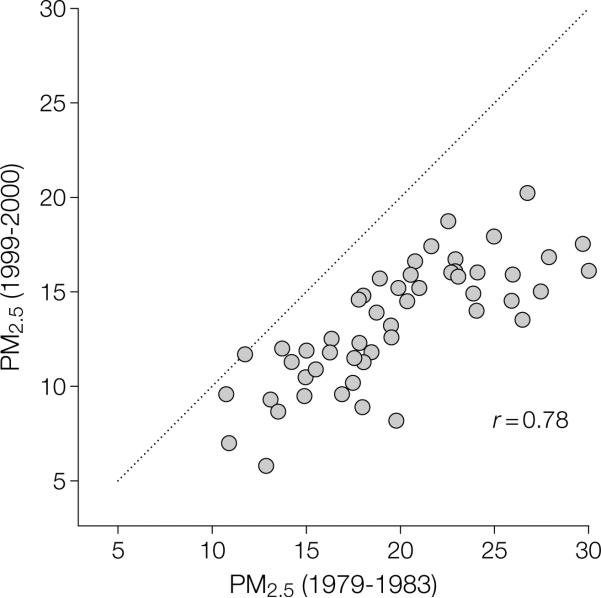
Fine particulate air pollution generally declined in the United States during the follow-up period of this study. FIGURE 1 plots mean PM2.5 concentrations for 1999-2000 over mean PM2.5 concentrations for 1979-1983 for the 51 cities in which paired data were available. The concentrations of PM2.5 were lower in 1999-2000 than in 1979-1983 for most cities, with the largest reduction observed in the cities with the highest concentrations of pollution during 1979-1983. Mean PM2.5 levels in the 2 periods were highly correlated (r=0.78). The rank ordering of cities by relative pollution levels remained nearly the same. Therefore, the relative levels of fine particle concentrations were similar whether based on measurements at the beginning of the study period, shortly following the study period, or an average of the 2.
Figure 1.
Mean Fine Particles Measuring Less Than 2.5 μm in Diameter (PM2.5)
As reported in TABLE 2, all 3 indices of fine particulate air pollution were associated with all-cause, cardiopulmo-nary, and lung cancer mortality, but not mortality from all other causes combined. FIGURE 2 presents the nonpara-metric smoothed exposure response relationships between cause-specific mortality and PM2.5 (average). The log RRs for all-cause, cardiopulmonary, and lung cancer mortality increased across the gradient of fine particulate matter. Goodness-of-fit tests indicated that the associations were not significantly different from linear associations (P>.20).
Table 2.
Adjusted Mortality Relative Risk (RR) Associated With a 10-μg/m3 Change in Fine Particles Measuring Less Than 2.5 μm in Diameter
| Adjusted RR (95% CI)* | |||
|---|---|---|---|
| Cause of Mortality | 1979-1983 | 1999-2000 | Average |
| All-cause | 1.04 (1.01-1.08) | 1.06 (1.02-1.10) | 1.06 (1.02-1.11) |
| Cardiopulmonary | 1.06 (1.02-1.10) | 1.08 (1.02-1.14) | 1.09 (1.03-1.16) |
| Lung cancer | 1.08 (1.01-1.16) | 1.13 (1.04-1.22) | 1.14 (1.04-1.23) |
| All other cause | 1.01 (0.97-1.05) | 1.01 (0.97-1.06) | 1.01 (0.95-1.06) |
Estimated and adjusted based on the baseline random-effects Cox proportional hazards model, controlling for age, sex, race, smoking, education, marital status, body mass, alcohol consumption, occupational exposure, and diet. CI indicates confidence interval.
Figure 2.
Nonparametric Smoothed Exposure Response Relationship
The fine particle mortality RR ratios from various alternative modeling approaches and assumptions are presented in FIGURE 3. After controlling for smoking, education, and marital status, the controlled forward stepwise inclusion of additional covariates had little influence on the estimated associations with fine particulate air pollution on cardiopulmonary and lung cancer mortality. As expected, cigarette smoking was highly significantly associated with el evated risk of all-cause, cardiopulmo-nary, and lung cancer mortality (P<.001). Estimated RRs for an average current smoker (men and women combined, 22 cigarettes/day for 33.5 years, with initiation before age 18 years) were equal to 2.58, 2.89, and 14.80 for all-cause, cardiopulmonary, and lung cancer mortality, respectively. Statistically significant, but substantially smaller and less robust associations, were also observed for education, marital status, BMI, alcohol consumption, occupational exposure, and diet variables. Although many of these covariates were also statistically associated with mortality, the risk estimates of pollution-related mortality were not highly sensitive to the inclusion of these additional covariates.
Figure 3.
Mortality Relative Risk (RR) Ratio Associated With 10-μg/m3 Differences of PM2.5 Concentrations
Figure 3 also demonstrates that the introduction of the random-effects component to the model resulted in larger SEs of the estimates and, therefore, somewhat wider 95% confidence intervals. There was no evidence of statistically significant spatial autocorrelation in the survival data based on the Bartlett test (P[H11022].20) after controlling for fine particulate air pollution and the various individual risk factors. Furthermore, graphical examination of the correlations of the residual mortality with distance between metropolitan areas did not reveal significant spatial autocorrelation (results not shown). Nevertheless, the incorporation of spatial smoothing was included to further investigate the robustness of the estimated particulate pollution effect. Effect estimates were not highly sensitive to the incorporation of spatial smoothing to account for regional clustering or other spatial patterns in the data.
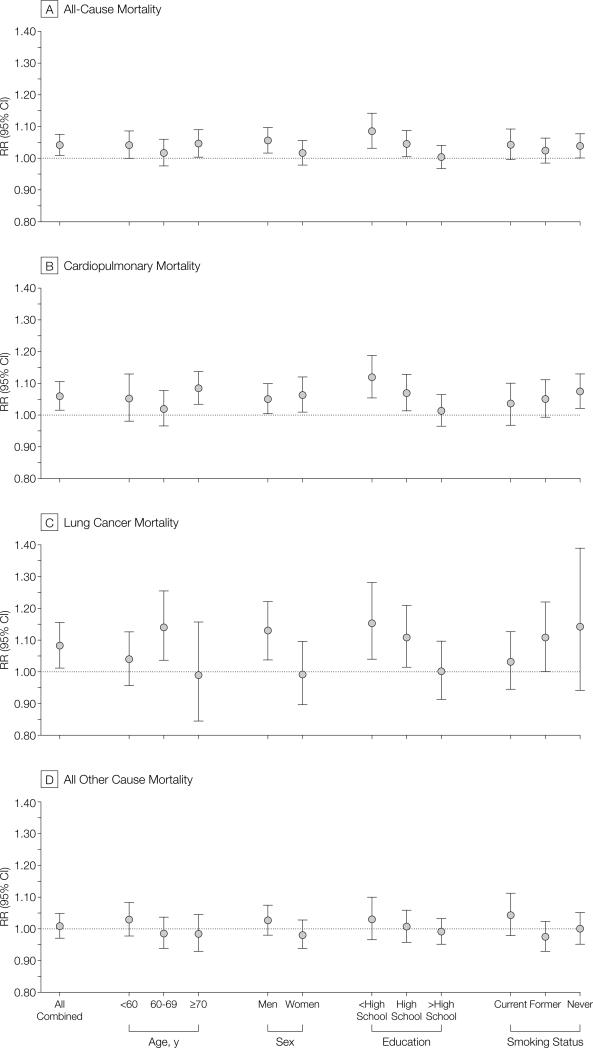
FIGURE 4 presents fine particle air pollution–related mortality RR ratios after stratifying by age, sex, education, and smoking status, and adjusting for all other risk factors. The differences across age and sex strata were not generally consistent or statistically significant. However, a consistent pattern emerged from this stratified analysis: the association with particulate pollution was stronger for both cardiopulmonary and lung cancer mortality for participants with less education. Also, for both cardiopulmonary and lung cancer mortality, the RR estimates were higher for nonsmokers.
Figure 4.
Adjusted Mortality Relative Risk (RR) Ratio Associated With 10-μg/m3 Differences of PM2.5 Concentrations
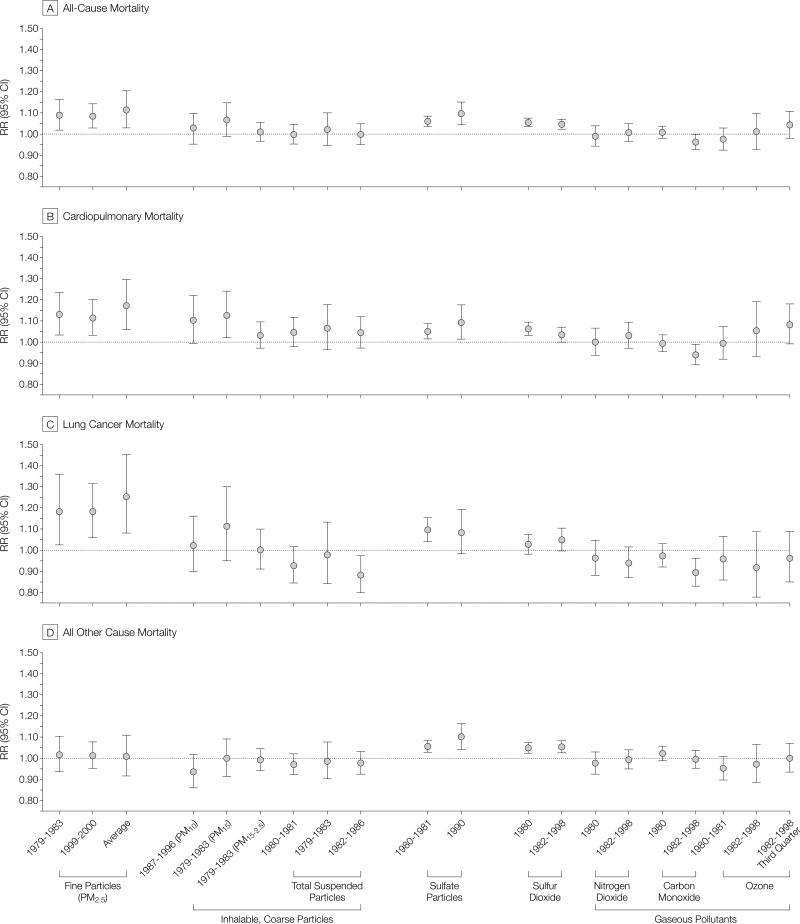
FIGURE 5 summarizes the associations between mortality risk and air pollutant concentrations listed in Table 1. Statistically significant and relatively consistent mortality associations existed for all measures of fine particulate exposure, including PM2.5 and sulfate particles. Weaker less consistent mortality associations were observed with PM10 and PM15. Measures of the coarse particle fraction (PM15-2.5) and total suspended particles were not consistently associated with mortality. Of the gaseous pollutants, only sulfur dioxide was associated with elevated mortality risk. Interestingly, measures of PM2.5 were associated with all-cause cardiopulmonary, and lung cancer mortality, but not with all other mortality. However, sulfur oxide pollution (as measured by sulfate particles and/or sulfur dioxide) was significantly associated with mortality from all other causes in addition to all-cause, cardiopulmo-nary, and lung cancer mortality.
Figure 5.
Adjusted Mortality Relative Risk (RR) Ratio Evaluated at Subject-Weighted Mean Concentrations
COMMENT
Thisstudydemonstratedassociationsbetween ambient fine particulate air pollution and elevated risks of both cardio-pulmonary and lung cancer mortality. Each 10-μg/m3 elevation in long-term average PM2.5 ambient concentrations was associated with approximately a 4%, 6%, and 8% increased risk of all-cause, cardiopulmonary, and lung cancer mortality, respectively, although the magnitude of the effect somewhat depended on the time frame of pollution monitoring. In addition, this analysis addresses many of the important questions concerning the earlier, more limited analysis of the large CPS-II cohort, including the following issues.
First, does the apparent association between pollution and mortality persist with longer follow-up and as the cohort ages and dies? The present analysis more than doubled the follow-up time to more than 16 years, resulting in approximately triple the number of deaths, yet the associations between pollution and mortality persisted.
Second, can the association between fine particulate air pollution and increased cardiopulmonary and lung cancer mortality be due to inadequate control of important individual risk factors? After aggressively controlling for smoking, the estimated fine particulate pollution effect on mortality was remarkably robust. When the analysis was stratified by smoking status, the estimated pollution effect on both cardio-pulmonary and lung cancer mortality was strongest for never smokers vs former or current smokers. This analysis also controlled for education, marital status, BMI, and alcohol consumption. This analysis used improved variables to control for occupational exposures and incorporated diet variables that accounted for total fat consumption, as well as for consumption of vegetables, citrus, and high-fiber grains. The mortality associations with fine particulate air pollution were largely unaffected by the inclusion of these indi vidualriskfactorsinthemodels.Thedata on smoking and other individual risk factors, however, were obtained directly by questionnaire at time of enrollment and do not reflect changes that may have occurred following enrollment. The lack of risk factor follow-up data results in some misclassificationofexposure,reducesthe precision of control for risk factors, and constrains our ability to differentiate time dependency.
Third, are the associations between fine particulate air pollution and mortality due to regional or other spatial differences that are not adequately controlled for in the analysis? If there are unmeasured or inadequately modeled risk factors that are different across locations, then spatial clustering will occur. If this clustering is independent or random across metropolitan areas, then the spatial clustering can be modeled by adding a random-effects component to the Cox proportional hazards model as was done in our analysis. The clustering may not be independent or random across metropolitan areas due to inadequately measured or modeled risk factors (either individual or ecological). If these inadequately measured or modeled risk factors are also spatially correlated with air pollution, then biased pollution effects estimates may occur due to confounding. However, in this analysis, significant spatial autocorrelation was not observed after controlling for fine particulate air pollution and the various individual risk factors. Furthermore, to minimize any potential confounding bias, sensitivity analyses, which directly modeled spatial trends using nonparametric smoothing techniques, were conducted. A contribution of this analysis is that it included the incorporation of both random effects and nonparametric spatial smoothing components to the Cox proportional hazards model. Even after accounting for random effects across metropolitan areas and aggressively modeling a spatial structure that accounts for regional differences, the association between fine particulate air pollution and cardiopulmonary and lung cancer mortality persists.
Fourth, is mortality associated primarily with fine particulate air pollution or is mortality also associated with other measures of particulate air pollution, such as PM10, total suspended particles, or with various gaseous pollutants? Elevated mortality risks were associated primarily with measures of fine particulate and sulfur oxide pollution. Coarse particles and gaseous pollutants, except for sulfur dioxide, were generally not significantly associated with elevated mortality risk.
Fifth, what is the shape of the concentration-responsefunction?Withinthe range of pollution observed in this analysis, the concentration-response function appears to be monotonic and nearly linear. However, this does not preclude a leveling off (or even steepening) at much higher levels of air pollution.
Sixth, how large is the estimated mortality effect of exposure to fine particulate air pollution relative to other risk factors? A detailed description and interpretationofthemanyindividualrisk factors that are controlled for in the analysis goes well beyond the scope of this report. However, the mortality risk associated with cigarette smoking has been well documented using the CPS-II cohort.16 The risk imposed by exposure to fine particulate air pollution is obviously much smaller than the risk of cigarette smoking. Another risk factor that has been well documented using the CPS-II cohort data is body mass as measured by BMI.30 The Word Health Organization has categorized BMI values between 18.5-24.9 kg/m2 as normal; 25- 29.9 kg/m2, grade 1 overweight; 30- 39.9 kg/m2, grade 2 overweight; and 40 kg/m2 or higher, grade 3 overweight.31 In the present analysis, BMI values and BMI values squared were included in the proportional hazards models. Consistent with previous ACS analysis,30 BMI was significantly associated with mortality, optimal BMI was between approximately 23.5 and 24.9 kg/m2, and the RR of mortality for different BMI values relative to the optimal were dependent on sex and smoking status. For example, the RRs associated with BMI values between 30.0 and 31.9 kg/m2 (vs optimal) would be up to approxi mately 1.33 for never smokers. Based on these calculations, mortality risks associated with fine particulate air pollution at levels found in more polluted US metropolitan areas are less than those associated with substantial obesity (grade 3 overweight), but comparable with the estimated effect of being moderately overweight (grade 1 to 2).
In conclusion, the findings of this study provide the strongest evidence to date that long-term exposure to fine particulate air pollution common to many metropolitan areas is an important risk factor for cardiopulmonary mortality. In addition, the large cohort and extended follow-up have provided an unprecedented opportunity to evaluate associations between air pollution and lung cancer mortality. Elevated fine particulate air pollution exposures were associated with significant increases in lung cancer mortality. Although potential effects of other unaccounted for factors cannot be excluded with certainty, the associations between fine particulate air pollution and lung cancer mortality, as well as cardiopulmonary mortality, are observed even after controlling for cigarette smoking, BMI, diet, occupational exposure, other individual risk factors, and after controlling for regional and other spatial differences.
Acknowledgment
We thank Morton Lippmann, PhD, for his help in developing the research grant application and various comments and suggestions and Yuanli Shi, MD, for computer programming and statistical analysis support.
Funding/Support: The research for this article was supported largely by grant ES09560-01A1 from the National Institutes of Health/National Institute of Environmental Health Sciences (NIEHS). It was also supported in part by grant ES00260 from the New York University Center/NIEHS, grant R-827351 from the Environmental Protection Agency PM Health Effects Research Center, and funding from the R. Samuel McLaughlin Centre for Population Health Risk Assessment at the University of Ottawa.
Footnotes
Author Contributions: Study concept and design: Pope, Burnett, Krewski, Thurston.
Acquisition of data: Thun, Calle, Krewski, Ito, Thurston.
Analysis and interpretation of data: Pope, Burnett, Krewski, Thurston.
Drafting of the manuscript: Pope, Burnett, Ito, Thurston.
Critical revision of the manuscript for important intellectual content: Pope, Thun, Calle, Krewski, Thurston.
Statistical expertise: Pope, Burnett, Krewski. Obtained funding: Pope, Thun, Thurston. Administrative, technical, or material support: Pope, Calle, Krewski, Ito, Thurston. Study supervision: Pope, Krewski.
REFERENCES
- 1.Firket J. The cause of the symptoms found in the Meuse Valley during the fog of December, 1930. Bull Acad R Med Belgium. 1931;11:683–741. [Google Scholar]
- 2.Ciocco A, Thompson DJ. A follow-up of Donora ten years after: methodology and findings. Am J Public Health. 1961;51:155–164. doi: 10.2105/ajph.51.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Logan WPD, Glasg MD. Mortality in London fog incident, 1952. Lancet. 1953;1:336–338. doi: 10.1016/s0140-6736(53)91012-5. [DOI] [PubMed] [Google Scholar]
- 4.Pope CA, III, Dockery DW. Epidemiology of particle effects. In: Holgate ST, Koren H, Maynard R, Samet J, editors. Air Pollution and Health. Academic Press; London, England: 1999. pp. 673–705. [Google Scholar]
- 5.Kaiser J. Showdown over clean air science. Science. 1997;277:466–469. doi: 10.1126/science.277.5325.466. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization-European Region . Update and Revision of the Air Quality Guidelines for Europe. World Health Organization-European Region; Copenhagen, Denmark: 1995. Document EUR/ICP/EHAZ 9405/PB01. [Google Scholar]
- 7.CEPA/FPAC Working Group on Air Quality Objectives and Guidelines . National Ambient Air Quality Objectives for Particulate Matter. Public Works and Government Services; Ottawa, Ontario: 1998. Category No. H46-2/98-220. [Google Scholar]
- 8.Committee of the Environmental and Occupational Health Assembly of the American Thoracic Society Health effects of outdoor air pollution. Am J Respir Crit Care Med. 1996;153:3–50. doi: 10.1164/ajrccm.153.1.8542133. [DOI] [PubMed] [Google Scholar]
- 9.Committee on the Medical Effects of Air Pollution . Non-Biological Particles and Health. United Kingdom Dept of Health; London, England: 1995. [Google Scholar]
- 10.Environmental Protection Agency . Air Quality Criteria for Particulate Matter. Environmental Protection Agency; Washington, DC: 1996. Document EPA/600/P-95/001cf. [Google Scholar]
- 11.Samet JM, Dominici F, Curriero FC, Coursac I, Zeger SL. Fine particulate air pollution and mortality in 20 US cities. N Engl J Med. 2000;343:1742–1749. doi: 10.1056/NEJM200012143432401. [DOI] [PubMed] [Google Scholar]
- 12.National Research Council . Research Priorities for Airborne Particulate Matter, I: Immediate Priorities and a Long-Range Research Portfolio. National Academy Press; Washington, DC: 1998. [PubMed] [Google Scholar]
- 13.National Research Council . Research Priorities for Airborne Particulate Matter, III: Early Research Progress. National Academy Press; Washington, DC: 2001. [Google Scholar]
- 14.Whitman v. Vol. 457. American Trucking Associations Inc; 532 US: 2001. [Google Scholar]
- 15.Dockery DW, Pope CA III, Xu X, et al. An association between air pollution and mortality in six US cities. N Engl J Med. 1993;329:1753–1759. doi: 10.1056/NEJM199312093292401. [DOI] [PubMed] [Google Scholar]
- 16.Pope CA, III, Thun MJ, Namboodiri MM, et al. Particulate air pollution as a predictor of mortality in a prospective study of US adults. Am J Respir Crit Care Med. 1995;151:669–674. doi: 10.1164/ajrccm/151.3_Pt_1.669. [DOI] [PubMed] [Google Scholar]
- 17.Krewski D, Burnett RT, Goldberg MS, et al. Re-analysis of the Harvard Six Cities Study and the American Cancer Society Study of Particulate Air Pollution and Mortality: Special Report. Health Effects Institute; Cambridge, Mass: 2000. [Google Scholar]
- 18.Chao A, Thun MJ, Jacobs E, Henley SJ, Rodriguez C, Calle EE. Cigarette smoking and colorectal cancer mortality in the Cancer Prevention Study II. J Natl Cancer Inst. 2000;92:1888–1896. doi: 10.1093/jnci/92.23.1888. [DOI] [PubMed] [Google Scholar]
- 19.Calle EE, Terrell DD. Utility of the National Death Index for ascertainment of mortality among Cancer Prevention Study II participants. Am J Epidemiol. 1993;137:235–241. doi: 10.1093/oxfordjournals.aje.a116664. [DOI] [PubMed] [Google Scholar]
- 20.US Postal Service . 1989 National Five Digit Zip Code and Post Office Directory. National Information Data Center; Washington, DC: 1989. [Google Scholar]
- 21.Fleming TR, Harrington DP. Counting Processes and Survival Analysis. John Wiley & Sons; New York, NY: 1991. [Google Scholar]
- 22.Burnett R, Ma R, Jerrett M, et al. The spatial association between community air pollution and mortality: a new method of analyzing correlated geographic cohort data. Environ Health Perspect. 2001;109(suppl 3):375–380. doi: 10.1289/ehp.01109s3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Easton DF, Peto J, Babiker GAG. Floating absolute risk: an alternative to relative risk in survival and case-control analysis avoiding an arbitrary reference group. Stat Med. 1991;10:1025–1035. doi: 10.1002/sim.4780100703. [DOI] [PubMed] [Google Scholar]
- 24.Burnett RT, Ross WH, Krewski D. Non-linear mixed regression models. Environmetrics. 1995;6:85–99. [Google Scholar]
- 25.Siemiatycki J, Nadon L, Lakhani R, Beegin D, Geerin M. Exposure assessment. In: Siemiatycki J, editor. Risk Factors for Cancer in the Workplace. CRC Press; Baton Rouge, La: 1991. pp. 45–114. [Google Scholar]
- 26.Cleveland WS, Devlin SJ. Robust locally weighted regression and smoothing scatterplots. J Am Stat Assoc. 1988;74:829–836. [Google Scholar]
- 27.Hastie T, Tibshirani R. Generalized Additive Models. Chapman & Hall; London, England: 1990. [Google Scholar]
- 28.S-Plus 2000 Programmer's Guide. Math Soft; Seattle, Wash: 2000. [Google Scholar]
- 29.Priestly MB. Spectral Analysis and Time Series. Academic Press; London, England: 1981. [Google Scholar]
- 30.Calle EE, Thun MJ, Petrelli JM, Rodriguez C, Heath CW., Jr Body-mass index and mortality in a prospective cohortofUSadults. NEnglJMed. 1999;341:1097–1105. doi: 10.1056/NEJM199910073411501. [DOI] [PubMed] [Google Scholar]
- 31.Physical status: the use and interpretation of anthropometry: report of a WHO expert committee. WHO Tech Rep Ser. 1995;854:1–452. [PubMed] [Google Scholar]