Abstract
With the aim of acquiring deeper knowledge about repetitive DNAs chromosomal organization in grasshoppers, we used fluorescent in situ hybridization (FISH) to map the distribution of 16 microsatellite repeats, including mono-, di-, tri- and tetra-nucleotides, in the chromosomes of the species Abracris flavolineata (Acrididae), which harbors B chromosome. FISH revealed two main patterns: (i) exclusively scattered signals, and (ii) scattered and specific signals, forming evident blocks. The enrichment was observed in both euchromatic and heterochromatic areas and only the motif (C)30 was absent in heterochromatin. The A and B chromosomes were enriched with all the elements that were mapped, being observed in the B chromosome more distinctive blocks for (GA)15 and (GAG)10. For A complement distinctive blocks were noticed for (A)30, (CA)15, (CG)15, (GA)15, (CAC)10, (CAA)10, (CGG)10, (GAA)10, (GAC)10 and (GATA)8. These results revealed an intense spreading of microsatellites in the A. flavolineata genome that was independent of the A+T or G+C enrichment in the repeats. The data indicate that the microsatellites compose the B chromosome and could be involved in the evolution of this element in this species, although no specific relationship with any A chromosome was observed to discuss about its origin. The systematic analysis presented here contributes to the knowledge of repetitive DNA chromosomal organization among grasshoppers including the B chromosomes.
Introduction
The accumulation of highly repetitive DNAs that are organized in tandem and dispersed is a common pattern in eukaryotic genomes [1]–[3]. Among tandem repeats, microsatellites or simple sequence repeats (SSRs) are composed of short motifs (∼6 bp) and constitute one of the most dynamic types of sequences; SSRs are abundant and can be located in specific chromosomal areas or widely scattered throughout euchromatic or heterochromatic areas [4]–[7]. In addition to other repetitive DNAs, such as transposable elements (TEs), satellite DNAs and multigene families, these sequences, have had a great impact on the organization and evolution of genomes [3], [5], [8]–[13].
One genome element that is characterized by the accumulation of repetitive DNAs are the B chromosomes (supernumerary or accessory chromosomes), which are dispensable elements that occur as polymorphism in addition to the standard karyotype in more than 2,000 eukaryotic species [14]–[16]. The close relationship between B chromosomes and repetitive DNAs has been demonstrated in some studies, revealing distinct classes of this genomic fraction, including transposons, retrotransposons, satellite DNA and multigene families. The accumulation of these repetitive DNAs may have resulted from a lack of recombination and may have led to B chromosome species-specific evolution [14]–[20].
The occurrence of B chromosomes has been reported in 191 Orthoptera species and in approximately 14.6% of Acridoidea representatives; B chromosomes also prevail in species with acrocentric chromosomes [21]. Although prevalent in grasshoppers, the molecular content of B chromosomes and their relationship with A elements have only been extensively investigated by the chromosomal mapping of repetitive DNAs in a few species, mainly in Eyprepocnemis plorans and to a lesser extent in Locusta migratoria, Abracris flavolineata, Rhammatocerus brasiliensis, Xyleus discoideus angulatus, Dichroplus pratensis, and species of Podisma. In these species, the mapping of repetitive DNAs has revealed variability and the presence of distinct sequences in B chromosomes, such as satellite DNA, rDNAs, histone genes, U2 snDNA and a SCAR marker; these mapping efforts may inform hypotheses about the possible origins and evolution of these elements [22]–[37].
Although a significant fraction of the eukaryotic genome is composed of microsatellites, their chromosomal distribution has been addressed only in specific groups (see for example [6], [7], [38]–[44]). Moreover, the studies that explore the composition of the B chromosome with distinct repetitive sequences do not systematically investigate microsatellite occurrence/accumulation [38], [45].
Grasshoppers possess large genomes and it could be directly related to the proliferation of repetitive DNAs, as recently demonstrated by genome sequencing of Locusta migratoria [46], that revealed ∼60% of repetitive DNAs. On the other hand, the organization of specific repetitive sequences, like microsatellites, are poorly know in this insect group, with chromosomal mapping of (AG)10 and (AC)10 restrict to E. plorans and Chorthippus sp, respectively [38]. Here in order to obtain a deeper knowledge of repetitive DNAs chromosomal organization for standard complement and B chromosome composition/evolution in grasshoppers we mapped 16 distinct microsatellite motifs in the chromosomes of Abracris flavolineata (Acrididae: Ommatolampidinae), a species presenting 2n = 23,X0 (male) and 2n = 24,XX (female) and with occurrence of B chromosomes [37]. Our results revealed intense spreading of distinct motifs in A complement and B chromosome.
Materials and Methods
Cells bearing one B chromosome were obtained from embryos males (2n = 23,X0) or females (2n = 24,XX) following the protocol proposed by Webb et al. [47], with slight modifications. These cells were used in C-banding experiments according to Sumner [48] and in FISH experiments using microsatellites. At least 15 metaphase spreads were analyzed to describe the patterns of microsatellite distribution.
Specific microsatellites were labeled directly with Biotin during synthesis at the 5′ end and were used as probes (Sigma, St. Louis, MO, USA). The microsatellites included mono-, di- tri- and tetra-nucleotides: (C)30, (A)30, (TA)15, (CG)15, (CA)15, (GA)15, (TAA)10, (TAC)10, (GAG)10, (GAA)10, (GAC)10, (CAA)10, (CAC)10, (CGG)10, (GACA)4, and (GATA)8. The FISH experiments were performed with at least 300 ng of DNA according to the protocol proposed by Pinkel et al. [49] and with modifications reported by Cabral-de-Mello et al. [50]. The probes were detected using Streptavidin, Alexa Fluor 488 conjugate (Invitrogen, San Diego, CA, USA), and all preparations were counterstained with DAPI (4′, 6-diamidino-2-phenylindone) and mounted in Vectashield (Vector, Burlingame, CA, USA). The results were observed using an Olympus microscope BX61 that was equipped with a fluorescence lamp and the appropriate filters. Images were photographed using a DP70 cooled digital camera in grayscale, and the images were pseudocolored and posteriorly combined and optimized for brightness and contrast with Adobe Photoshop CS6.
Results
C-positive blocks that correspond to constitutive heterochromatin were observed in pericentromeric regions that extended to the short arms in the A complement. The B chromosome was euchromatic, as previously described by Bueno et al. [37] (Figure 1).
Figure 1. C-banding in embryo female mitotic metaphase of Abracris flavolineata.

The X and B chromosomes are indicated. Note that the C-positive blocks in the centromeric regions extend to the short arms of the A chromosomes and the euchromatic nature of the B element. The inset highlights the euchromatic B chromosome. Bar = 5 µm.
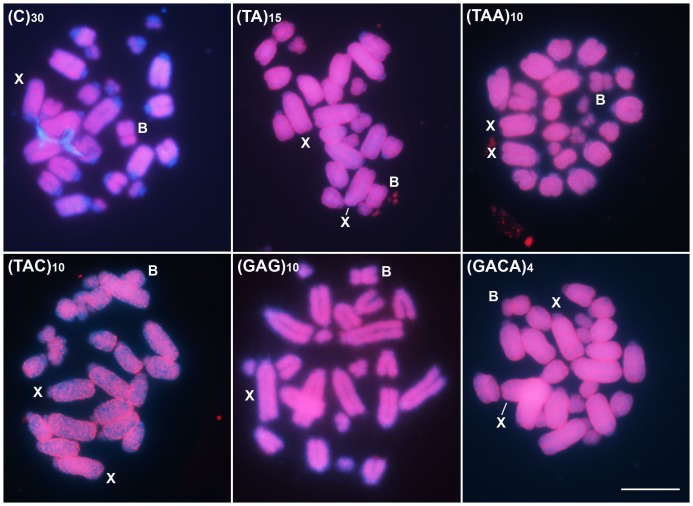
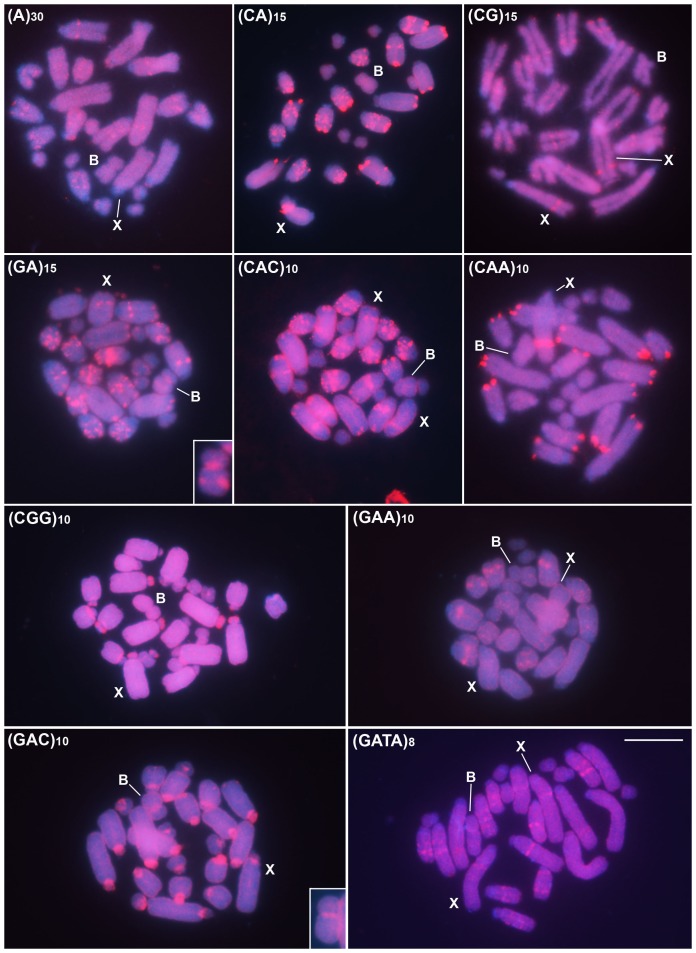
Two distinct general distribution patterns that depended on the microsatellite were observed by FISH: (i) exclusively scattered signals (Figure 2), and (ii) scattered and specific signals, forming evident blocks (Figure 3). Among the microsatellites with scattered distribution slight differences were observed for distribution with signals along the entire chromosomes for (TA)15, (TAA)10, (TAC)10, (GAG)10 and (GACA)4 and restrict to euchromatin for (C)30. Moreover the intensity of signals were distinct, being observed less intensity for (TAC)10 in comparison to the other repeats (Figure 2). Regarding the microsatellites with scattered and specific signals some differences were also remarkable for the evident blocks, as follows: (i) only interstitial signals were observed for (A)30 and (GAA)10; (ii) interstitial and terminal blocks were noticed for (CG)15, (CAA)10 and (CAC)10; (iii) interstitial, terminal and proximal blocks were noticed for (CA)15, (GA)15, (GATA)8; (iv) mainly blocks in the short arms were evidenced for (CGG)10; and finally (v) centromeric blocks, in some chromosomes extending to the short arms were observed for (GAC)10 (Figure 3).
Figure 2. FISH mapping for six microsatellite motifs in embryo mitotic cells of A. flavolineata with scattered distribution.
Each microsatellite is indicated in each image. The B and X chromosomes are pointed in each image and the sex of the embryo can be noticed by the occurrence of one (male X0) or two (female XX) X chromosomes. Note the absence of signals in the heterochromatic areas for (C)30. Bar = 5 µm.
Figure 3. FISH performed using ten microsatellites as probes in embryo mitotic cells of A. flavolineata revealing distribution of repeats in distinct chromosomal regions.
Each microsatellite is indicated in each image. The B and X chromosomes are pointed in each image and the sex of the embryo can be noticed by the occurrence of one (male X0) or two (female XX) X chromosomes. The insets highlight the B chromosome with probes for repeats with distinctive blocks. Bar = 5 µm.
Concerning the B chromosome, in general the microsatellites were scattered along its entire extension. More evident blocks were observed only for (GA)15, located in the centromeric and in the interstitial region of the long arm and for (GAC)10, with centromeric block (Figure 3). The X chromosome showed more intense interstitial hybridization for (CA)15, (CG)15, (GA)15, (CAC)10 and (CAA)10. For (CGG)10 and (GAC)10 more intense signal were noted in the short arm and centromeric positions, respectively (Figure 3).
Discussion
This study provides the first systematic analysis for microsatellites repeats chromosomal mapping in a grasshopper species. On a broad scope, the results indicated that an intense spreading of mono-, di-, tri- and some tetra-nucleotides has occurred in the A. flavolineata genome, including similarly heterochromatic and euchromatic areas of the A complement and the B chromosome. The accumulation of microsatellites in eukaryotic genomes, in general, tends to vary and decrease from vertebrates and plants to fungi and invertebrates. It has also been suggested that the presence of highly repetitive simple sequences can correlate to genome size and in species distantly related the microsatellite quantity and genome size present correlation, as for example human, Drosophila melanogaster and Saccharomyces cerevisae and Arabidospsis thaliana [3], [51]–[54]. For grasshoppers, a survey performed in Chorthippus biguttulus (Acrididae) with expected large genome revealed that the microsatellites are not more frequent, at least the di-nucleotides studied, than in other insect with smaller genomes. On the other hand, the repeat arrays are longer than in other insects and could reflect the genome size increasing [54]. Non-correlation of genome size and abundance of microsatellite was also documented in other insects, as in parasitic wasps that have more microsatellite density than D. melanogaster, although their genomes are comparable in size [55]–[57].
A remarkable distribution/spreading of microsatellites, such as that observed for most of the repeats described herein, has also been reported in other species for distinct motifs, such as in reptiles (Eremias velox), plants (Solanum licopersicum, Silene latifolia and Rumex acetosa) and insects (Drosophila melanogaster), including the grasshopper species Eyprepocnemis plorans [38], [39], [41], [42], [58], [59]. This wide spreading could be attributed to the activity of transposable elements that contain microsatellite sequences and, in some cases, are involved with microsatellites origin during genome integration of TEs [8], [60]–[64]. In the case of A. flavolineata, the occurrence/spreading of some microsatellites in euchromatin resembles the distribution of two isolated Mariner-like transposable elements [65].
In contrast to intense and random distribution patterns, localized intense signals were observed for some microsatellites in A. flavolineata. Specific signals were observed in the chromosomes of the grasshopper Chorthippus sp for AC motif, and cases of non-random distribution for distinct repeats are well documented within and between Drosophila melanogaster chromosomes and in Triticeae plants [7], [38]; these patterns depended on euchromatin, heterochromatin and centromeric areas. Moreover, in some other cases, the density of SSRs varied between chromosomes in the same genome, as observed for A. flavolineata. These results indicate that the organization of SSRs could form a particular pattern for each repeat, which could follow distinct trajectories of expansion, elimination and accumulation at intra- and inter-genomic levels through distinct molecular mechanisms, such as ectopic recombination, slippage replication and transposition [40], [44], [66]–[69].
Concerning the B chromosome, which has been poorly investigated in the context of microsatellite mapping, the (CAA)10 repeat was reported to harbor these elements in rye (Secale cereale) [45], while the B chromosome of E. plorans did not revealed signals for (AG)10, that is abundant in the A chromosomes of the species [38]. As, in general, a non-recombining element with A elements the B chromosomes could be a preferable site for SSR accumulation during its evolution; SSR accumulation may be involved in the differentiation process of these chromosomes, which would favor, for example, the rate of mutability. The mutability rate for microsatellites is very high (10−2 to 10−6 events per locus per generation) in relation to those at coding gene loci [8]; this high mutability could have been occurred in the A complement and B chromosome of A. flavolineata, which exhibit similar enrichment of most microsatellites that have been mapped here. In contrast, there is apparently a paucity of general pool of repetitive DNAs in the B chromosome of A. flavolineata, which was determined by using the C0t-1 DNA fraction (composed of highly and moderately repetitive DNAs) as a probe [37], indicating that not all types of repetitive DNAs have been isolated in this fraction, such as distinct microsatellites. The distinctive block for (GA)15 observed in the long arm of the B chromosome in A. flavolineata indicate that after its origin through isochromosome formation [37] the arms probably have been experienced distinct accumulation of repetitive sequences, as also recently reported for a Mariner-like transposable element (Afmar2) [65].
Although we noted the spreading of SSRs in the non-recombining B chromosome it was, in general, not different from A chromosomes that present distinct degrees of recombination, e.g., recombining (autosomes) and less-recombining (X chromosome that recombine only in females). Furthermore, these differences, in general, were not observed for distinct chromosomal regions with distinct degree of recombination, such euchromatin and heterochromatin. Such differences of microsatellite accumulation between different chromosomes with distinct degrees of recombination have been previously reported. For example, an accumulation of distinct microsatellites was observed in the young Y chromosome of Rumex acetosa [42]. Additionally, Silene latifolia, which has non-recombining genome regions, e.g., the Y chromosome, has also accumulated microsatellites in this chromosome [39]. The accumulation of microsatellites in sex chromosomes has also been reported in animals, such as for example in Eremias velox (lacertid lizard) [41], Aprasia parapulchella (Pygopodid lizard) [43] and Semaprochilodus (Prochilodontid fish) [44], suggesting a role for this type of sequence in sex chromosome differentiation favored by the suppression of recombination among these elements.
Although the results of this study did not provide new insights regarding the specific origin of the B chromosome in A. flavolineata, proposed to be derived from pair one [37], our approach provided novel valuable primary data about the organization of distinct microsatellite sequences in grasshopper genomes, including the A complement and B chromosomes. Moreover, these data increase the knowledge of B chromosome composition in this insect group and in eukaryotes, indicating that microsatellites could play important role in B chromosome evolution. The next step should employ the use of this simple assay, i.e., FISH mapping of microsatellites, in other grasshopper species that bear B chromosomes to determine whether the spreading of these elements commonly occurs in this group and whether there is a correlation with the distribution of euchromatin/heterochromatin.
Acknowledgments
The authors are grateful to the anonymous reviewers for their substantial contributions.
Funding Statement
This study was supported by Fundação de Amparo a Pesquisa do Estado de São Paulo, FAPESP (2011/19481-3), Conselho Nacional de Desenvolvimento Científico e Tecnologico, CNPq (475308/2011-5) and PROPE-UNESP. The scholarships of Milani D were granted by PIBIC/CNPq program (process number 27785). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Charlesworth B, Sniegowski P, Stephan W (1994) The evolutionary dynamics of repetitive DNA in eukaryotes. Nature 371: 215–220. [DOI] [PubMed] [Google Scholar]
- 2. Biémont C, Vieira C (2006) Genetics: Junk DNA as an evolutionary force. Nature 443: 521–524. [DOI] [PubMed] [Google Scholar]
- 3. López-Flores I, Garrido-Ramos MA (2012) The repetitive DNA content of eukaryotic genomes. Genome Dyn 7: 1–28. [DOI] [PubMed] [Google Scholar]
- 4. Tautz D, Renz M (1984) Simple sequences are ubiquitous repetitive components of eukaryotic genomes. Nuc Acids Res 12: 4127–4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schlötterer C (2000) Evolutionary dynamics of microsatellite DNA. Chromosoma 109: 365–371. [DOI] [PubMed] [Google Scholar]
- 6. Cuadrado A, Jouve N (2007) Similarities in the chromosomal distribution of AG and AC repeats within and between Drosophila, human and barley chromosomes. Cytogenet Genome Res 119: 91–99. [DOI] [PubMed] [Google Scholar]
- 7. Cuadrado A, Cardoso M, Jouve N (2008) Physical organization of simple sequence repeats (SSRs) in Triticeae: structural, functional and evolutionary implications. Cytogenet Genome Res 120: 210–219. [DOI] [PubMed] [Google Scholar]
- 8. Li YC, Korol AB, Fahima T, Beiles A, Nevo E (2002) Microsatellites: genomic distribution, putative functions and mutational mechanisms: a review. Mol Ecol 11: 2453–2465. [DOI] [PubMed] [Google Scholar]
- 9. Shapiro JA, Sternberg R (2004) Why repetitive DNA is essential to genome function. Biol Rev 80: 1–24. [DOI] [PubMed] [Google Scholar]
- 10. Nei M, Rooney AP (2005) Concerted and birth-and-death evolution of multigene families. Annu Rev Genet 39: 121–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Feschotte C, Pritham EJ (2007) DNA transposons and the evolution of eukaryotic genomes. Ann Rev Genet 41: 331–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cabrero J, Camacho JP (2008) Location and expression of ribosomal RNA genes in grasshoppers: Abundance of silent and cryptic loci. Chromosome Res 16: 595–607. [DOI] [PubMed] [Google Scholar]
- 13. Montiel EE, Cabrero J, Camacho JP, López-León MD (2012) Gypsy, RTE and Mariner transposable elements populate Eyprepocnemis plorans genome. Genetica 140: 365–74. [DOI] [PubMed] [Google Scholar]
- 14.Camacho JPM (2005) B chromosomes. In: The evolution of the genome (Gregory TR, ed.): 223–286.
- 15. Jones RN, Gonzalez-Sanchez M, Gonzalez-Garcia M, Vega JM, Puertas MJ (2008) Chromosomes with a life of their own. Cytogenet Genome Res 120: 265–280. [DOI] [PubMed] [Google Scholar]
- 16. Houben A, Banaei-Moghaddam AM, Klemme S, Timmis JN (2013) Evolution and biology of supernumerary B chromosomes. Cell Mol Life Sci 71: 467–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Beukeboom LW (1994) Bewildering Bs: An imprecision of the 1st B-chromosome conference. Heredity 73: 928–996. [Google Scholar]
- 18. Jones RN (1995) B chromosomes in plants. New Phytol 131: 411–434. [DOI] [PubMed] [Google Scholar]
- 19. Camacho JPM, Sharbel TF, Beukeboom LW (2000) B chromosome evolution. Phil Trans R Soc Lond B 355: 163–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jones N, Houben A (2003) B chromosomes in plants: escapees from the A chromosome genome? Trends Plant Sci 8: 417–23. [DOI] [PubMed] [Google Scholar]
- 21. Palestis BG, Cabrero J, Trivers R, Camacho JPM (2010) Prevalence of B chromosomes in Orthoptera is associated with shape and number of A chromosomes. Genetica 138: 1181–1189. [DOI] [PubMed] [Google Scholar]
- 22. López-León MD, Neves N, Schwarzacher T, Heslop-Harrison JS, Hewitt GM, et al. (1994) Possible origin of a B chromosome deduced from its DNA composition using double FISH technique. Chromosome Res 2: 87–92. [DOI] [PubMed] [Google Scholar]
- 23. López-León MD, Cabrero J, Dzyubenko VV, Bugrov AG, Karamysheva TV, et al. (2008) Differences in ribosomal DNA distribution on A and B chromosomes between eastern and western populations of the grasshopper Eyprepocnemis plorans . Cytogenet Gen Res 121: 260–265. [DOI] [PubMed] [Google Scholar]
- 24. Cabrero J, Bakkali M, Bugrov A, Warchalowska-Sliwa E, López-León MD, et al. (2003a) Multiregional origin of B chromosomes in the grasshopper Eyprepocnemis plorans . Chromosoma 112: 207–211. [DOI] [PubMed] [Google Scholar]
- 25. Cabrero J, Perfectti F, Gómez R, Camacho JPM, López-León MD (2003b) Population variation in the A chromosome distribution of satellite DNA and ribosomal DNA in the grasshopper Eyprepocnemis plorans . Chromosome Res 11: 375–381. [DOI] [PubMed] [Google Scholar]
- 26. Bidau CJ, Rosato M, Martí DA (2004) FISH detection of ribosomal cistrons and assortment-distortion for X and B chromosomes in Dichroplus pratensis (Acrididae). Cytogenet Genome Res 106: 295–301. [DOI] [PubMed] [Google Scholar]
- 27. Bugrov AG, Karamysheva TV, Rubtsov DN, Andreenkova OV, Rubtsov NB (2004) Comparative FISH analysis of distribution of B chromosome repetitive DNA in A and B chromosomes in two subspecies of Podisma sapporensis (Orthoptera, Acrididae). Cytogenet Genome Res 106: 284–288. [DOI] [PubMed] [Google Scholar]
- 28. Bugrov AG, Karamysheva TV, Perepelov EA, Elisaphenko EA, Rubtsov DN, et al. (2007) DNA content of the B chromosomes in grasshopper Podisma kanoi Storozh. (Orthoptera, Acrididae). Chromosome Res 15: 315–325. [DOI] [PubMed] [Google Scholar]
- 29. Abdelaziz M, Teruel M, Chobanov D, Camacho JPM, Cabrero J (2007) Physical mapping of rDNA and satDNA in A and B chromosomes of the grasshopper Eyprepocnemis plorans from a Greek population. Cytogenet Genome Res 119: 143–146. [DOI] [PubMed] [Google Scholar]
- 30. Cabrero J, Teruel M, Carmona FD, Jiménez R, Camacho JPM (2007) Histone H3 lysine 9 acetylation pattern suggests that X and B chromosomes are silenced during entire male meiosis in a grasshopper. Cytogenet Genome Res 119: 135–142. [DOI] [PubMed] [Google Scholar]
- 31. Loreto V, Cabrero J, López-León MD, Camacho JPM, Souza MJ (2008) Possible autosomal origin of macro B chromosomes in two grasshopper species. Chromosome Res 16: 233–241. [DOI] [PubMed] [Google Scholar]
- 32. Teruel M, Cabrero J, Montiel EE, Acosta MJ, Sánchez A, et al. (2009) Microdissection and chromosome painting of X and B chromosomes in Locusta migratoria . Chromosome Res 17: 11–18. [DOI] [PubMed] [Google Scholar]
- 33. Teruel M, Cabrero J, Perfectti F, Acosta MJ, Sánchez A, et al. (2009) Microdissection and chromosome painting of X and B chromosomes in the grasshopper Eyprepocnemis plorans . Cytogenet Genome Res 125: 286–291. [DOI] [PubMed] [Google Scholar]
- 34. Teruel M, Cabrero J, Perfectti F, Camacho JP (2010) B chromosome ancestry revealed by histone genes in the Locusta migratoria . Chromosoma 119: 217–225. [DOI] [PubMed] [Google Scholar]
- 35. Muñoz-Pajares AJ, Martínez-Rodríguez L, Teruel M, Cabrero J, Camacho JPM, et al. (2011) A Single, Recent Origin of the Accessory B Chromosome of the Grasshopper Eyprepocnemis plorans . Genetics 187: 853–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Oliveira NL, Cabral-de-Mello DC, Rocha MF, Loreto V, Martins C, et al. (2011) Chromosomal mapping of rDNAs and H3 histone sequences in the grasshopper Rhammatocerus brasiliensis (Acrididae, Gomphocerinae): extensive chromosomal dispersion and co-localization of 5S rDNA/H3 histone clusters in the A complement and B chromosome. Mol Cytogenet 4: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bueno D, Palacios-Gimenez OM, Cabral-de-Mello DC (2013) Chromosomal Mapping of Repetitive DNAs in the Grasshopper Abracris flavolineata Reveal Possible Ancestry of the B Chromosome and H3 Histone Spreading. PLoS ONE 8: e66532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cuadrado A, Jouve N (2010) Chromosomal detection of simple sequence repeats (SSRs) using nondenaturing FISH (ND-FISH). Chromosoma 119: 495–503. [DOI] [PubMed] [Google Scholar]
- 39. Kubat Z, Hobza R, Vyskot B, Kejnovsky E (2008) Microsatellite accumulation on the Y chromosome in Silene latifolia . Genome 51: 350–356. [DOI] [PubMed] [Google Scholar]
- 40. Santos J, Lluis Serra L, Solé E, Pascual M (2010) FISH mapping of microsatellite loci from Drosophila subobscura and its comparison to related species. Chromosome Res 18: 213–226. [DOI] [PubMed] [Google Scholar]
- 41. Pokorná M, Kratochvíl L, Kejnovský E (2011) Microsatellite distribution on sex chromosomes at different stages of heteromorphism and heterochromatinization in two lizard species (Squamata: Eublepharidae: Coleonyx elegans and Lacertidae: Eremias velox). BMC Genetics 12: 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kejnovský E, Michalovova M, Steflova P, Kejnovska I, Manzano S, et al. (2013) Expansion of microsatellites on evolutionary young Y chromosome. PLoS ONE 8: e45519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Matsubara K, Knopp T, Sarre SD, Georges A, Ezaz T (2013) Karyotypic analysis and FISH mapping of microsatellite motifs reveal highly differentiated XX/XY sex chromosomes in the pink-tailed worm-lizard (Aprasia parapulchella, Pygopodidae, Squamata). Mol Cytogenet 6: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Terencio ML, Schneider CH, Gross MC, Vicari MR, Farias IP, et al. (2013) Evolutionary dynamics of repetitive DNA in Semaprochilodus (Characiformes, Prochilodontidae): A fish model for sex chromosome differentiation. Sex Dev 7: 325–333. [DOI] [PubMed] [Google Scholar]
- 45. Marques A, Klemme S, Guerra M, Houben A (2012) Cytomolecular characterization of de novo formed rye B chromosome variants. Molecular Cytogenetics 5: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wang, et al. (2014) The locust genome provides insight into swarm formation and long-distance flight. Nature communications 5: 2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Webb GC, White MJD, Contreras N, Cheney J (1978) Cytogenetics of the parthogenetic grasshopper Warramaba (formerly Moraba) virgo and its bisexual relatives. IV. Chromosome banding studies. Chromosoma 67: 309–339. [Google Scholar]
- 48. Sumner AT (1972) A simple technique for demonstrating centromeric heterochromatin. Exp Cell Res 75: 304–306. [DOI] [PubMed] [Google Scholar]
- 49. Pinkel D, Lanlegent J, Collins C, Fuscoe J, Segraves R, et al. (1986) Fluorescence in situ hybridization with human chromosome-specific libraries: detection of trisomy 21 and translocations of chromosome 4. Proc Natl Acad Sci USA 85: 9138–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cabral-de-Mello DC, Moura RC, Martins C (2010) Chromosomal mapping of repetitive DNAs in the beetle Dichotomius geminatus provides the first evidence for an association of 5S rRNA and histone H3 genes in insects, and repetitive DNA similarity between the B chromosome and A complement. Heredity 104: 393–400. [DOI] [PubMed] [Google Scholar]
- 51. Comeron JM (2001) What controls the length of noncoding DNA? Curr Opin Genet Dev 11: 652–659. [DOI] [PubMed] [Google Scholar]
- 52. Tóth G, Gaspari Z, Jurka J (2000) Microsatellites in different Eukaryotic genomes: survey and analysis. Genome Res 10: 967–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Morgante M, Hanafey M, Powell W (2002) Microsatellites are preferentially associated with nonrepetitive DNA in plant genomes. Nat Genet 30: 194–200. [DOI] [PubMed] [Google Scholar]
- 54. Ustinova J, Achmann R, Cremer S, Mayer F (2006) Long Repeats in a Huge Genome: Microsatellite Loci in the Grasshopper Chorthippus biguttulus . J Mol Evol 62: 158–167. [DOI] [PubMed] [Google Scholar]
- 55. Thoren PA, Paxton RJ, Estoup A (1995) Unusually high frequency of (CT)n and (GT)n microsatellite loci in a yellowjacket wasp, Vespula rufa (L) (Hymenoptera:Vespidae). Insect Mol Biol 4: 141–148. [DOI] [PubMed] [Google Scholar]
- 56. Butcher RD, Hubbard SF, Whitfield WG (2000) Microsatellite frequency and size variation in the parthenogenetic parasitic wasp Venturia canescens (Gravenhorst) (Hymenoptera:Ichneumonidae). Insect Mol Biol 9: 375–384. [DOI] [PubMed] [Google Scholar]
- 57.Gregory TR (2001) Animal genome size database; http://www.genomesize.com.
- 58. Chang SB, Yang TJ, Datema E, van Vugt J, Vosman B, et al. (2008) FISH mapping and molecular organization of the major repetitive sequences of tomato. Chromosome Res 16: 919–33. [DOI] [PubMed] [Google Scholar]
- 59. Cuadrado A, Jouve N (2011) Novel simple sequence repeats (SSRs) detected by ND-FISH in heterochromatin of Drosophila melanogaster . BMC Genomics 12: 205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Messier W, Li S-H, Stewart C-B (1996) The birth of microsatellites. Nature 381: 483. [DOI] [PubMed] [Google Scholar]
- 61. Wilder J, Hollocher H (2001) Mobile elements and the genesis of microsatellites in dipterans. Molec Biol Evol 18: 384–392. [DOI] [PubMed] [Google Scholar]
- 62. Nadir E, Margalith H, Gallily T, Ben-Sasson SA (1996) Microsatellite spreading in the human genome: evolutionary mechanisms and structural implications. Proc Natl Acad Sci USA 93: 6470–6475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Johnson PCD, Webster LMI, Adam A, Buckland R, Dawson DA, et al. (2006) Abundant variation in microsatellites of the parasitic nematode Trichostrongylus tenuis and linkage to a tandem repeat. Mol Biochem Parasitol 148: 210–218. [DOI] [PubMed] [Google Scholar]
- 64. Grandi FC, An W (2013) Non-LTR retrotransposons and microsatellites: Partners in genomic variation. Mob Genet Elements 3: e25674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Palacios-Gimenez O, Bueno D, Cabral-de-Mello DC (2014) Chromosomal mapping of two Mariner-like elements in the grasshopper Abracris flavolineata reveals enrichment in euchromatin. Eur J Entomol. doi: 10.14411/eje.2014.000 [Google Scholar]
- 66. Subramanian S, Mishra RK, Singh L (2003) Genome-wide analysis of microsatellite repeats in humans: their abundance and density in specific genomic regions. Genome Biol 4: R13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Carmona A, Friero E, de Bustos A, Jouve N, Cuadrado A (2013) The evolutionary history of sea barley (Hordeum marinum) revealed by comparative physical mapping of repetitive DNA. Ann Bot 112: 1845–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Nanda I, Feichtinger W, Schmid M, Schroder JH, Zischler H, et al. (1990) Simple repetitive sequences are associated with the differentiation of the sex chromosomes in the guppy fish. J Mol Evol 30: 456–462. [Google Scholar]
- 69. Vanzela ALL, Swarça AC, Dias AL, Stolf R, Ruas PM, et al. (2002) Differential distribution of (GA)9+C microsatellite on chromosomes of some animal and plant species. Cytologia 67: 9–13. [Google Scholar]