Abstract
Dengue is the most prevalent arthropod-borne viral illness in humans. A MHC class I polypeptide-related sequence B (MICB) single nucleotide polymorphism (SNP) was previously associated with symptomatic dengue compared to non-dengue causes of acute febrile illnesses in infants. We measured circulating levels of soluble (s)MICB in the sera of infants with symptomatic primary dengue virus infections. We found that serum levels of sMICB increased between pre-infection and acute illness among infants with symptomatic primary dengue virus infections. The likelihood of being hospitalized with an acute primary DENV infection during infancy also tended to be higher with increasing acute illness sMICB levels. The elevation of sMICB during acute primary DENV infections in infants likely represents an immune evasion strategy and contributes to the severity of the acute illness.
Introduction
Dengue is the most prevalent arthropod-borne viral illness in humans with half of the world's population at risk. The global burden of symptomatic dengue is on the order of 100 million cases/year [1]. The dengue viruses (DENVs) are single-stranded, positive-sense, RNA-containing enveloped viruses belonging to the Flavivirus genus within the Flaviviridae family [2]. There are four serotypes of DENVs (DENV1-4). DENV infections produce a wide spectrum of clinical illness. It ranges from asymptomatic or mild illness to a severe and potentially life threatening disease, dengue hemorrhagic fever (DHF)/dengue shock syndrome (DSS).
A genome-wide association study (GWAS) found that polymorphisms in only two genes were associated with the development of DSS compared to milder forms of symptomatic dengue in children and adults [3]. One of those genes was MHC class I polypeptide-related sequence B (MICB). The MICB single nucleotide polymorphism (SNP) rs3132468 was also associated with symptomatic dengue compared to non-dengue causes of acute febrile illnesses in children and adults, and in infants [4]. Proteolytic cleavage of MICB on cell surface membranes produces soluble (s)MICB that can be measured in sera [5]. We have been conducting a prospective clinical study of DENV infections during infancy in the Philippines [6]. We therefore examined circulating levels of sMICB in the sera of infants with symptomatic primary dengue virus infections. We found that serum levels of sMICB increased between pre-infection and acute illness among infants with symptomatic primary dengue virus infections. The likelihood of being hospitalized with an acute primary DENV infection during infancy also tended to be higher with increasing acute illness sMICB levels.
Methods
Ethics Statement
The study protocol was approved by the institutional review boards of the Research Institute for Tropical Medicine, Philippines, and the University of Massachusetts Medical School. Mothers and their healthy infants were recruited and enrolled after providing written informed consent.
Clinical Study
The study began in January 2007 in San Pablo, Laguna, Philippines, and has been previously described [6]. Blood samples were collected from the infant and mother at the first study visit when the infant was between approximately 6-18 weeks old. Clinical and epidemiological information were collected at the study visit. We conducted surveillance year-round for hospitalized acute febrile illnesses in study infants across the seven hospitals serving San Pablo, Philippines. During the rainy season (June-November), mothers were encouraged to bring their infants to the San Pablo City Health Office for evaluation of outpatient febrile illnesses. Acute- and convalescent-phase (day 14) blood samples were obtained from study infants with febrile illnesses that did not have an obvious source at time of presentation (e.g. lobar pneumonia, bacterial meningitis, pyelonephritis).
A DENV infection was identified in febrile infants by serotype-specific RT-PCR in acute-phase sera and DENV IgM/IgG ELISA in paired acute and convalescent phase sera. Primary or secondary DENV infections were identified by previously established serologic criteria for the paired IgM/IgG ELISA results [7]. The infecting DENV serotype was identified by RT-PCR for all the symptomatic infants.
sMICB ELISA
Briefly, capture Ab to human MICB (R&D Systems, 20 µg/ml) was coated on 96-well flat-bottomed microtiter plates (Thermo Scientific). Plates were blocked with bovine serum albumin (BSA). Undiluted sera (50 µl/well) were added to the plates and incubated for 2 h at room temperature. Then, biotin-conjugated detecting Ab to human MICB (R&D Systems, 2 µg/ml) was added followed by streptavidin-horseradish peroxidase (HRP). The ELISA plates were developed using SuperSignal ELISA Femto Substrate (Pierce Protein Biology). sMICB levels were determined using a luminometer (Envision 2012 plate reader, Perkin Elmer).
Statistical Analysis
The SPSS software package (version 21.0) was used for statistical analyses. Comparisons between pre-infection and acute illness paired sMICB levels were made using the Wilcoxon signed rank non-parametric statistical test. P<0.05 was considered significant; 0.05≤p<0.10 was considered a significant trend.
Results
Symptomatic primary DENV infections in infants
The characteristics of the infants with symptomatic primary DENV infections are shown in Table 1.
Table 1. Characteristics of infants with symptomatic primary dengue virus (DENV) infections.
| Number of infants | n = 46 |
| Age at time of DENV infection (median [95% confidence interval]) | 7.4 [5.8–8.6] months |
| Gender (male:female) | 23∶23 |
| Serotype of dengue virus infection | DENV1: n = 5 DENV2: n = 6 DENV3: n = 33 DENV4: n = 2 |
| Disease severity | Hospitalized: n = 24 Outpatient: n = 22 |
Circulating levels of sMICB in infants with symptomatic primary DENV infections
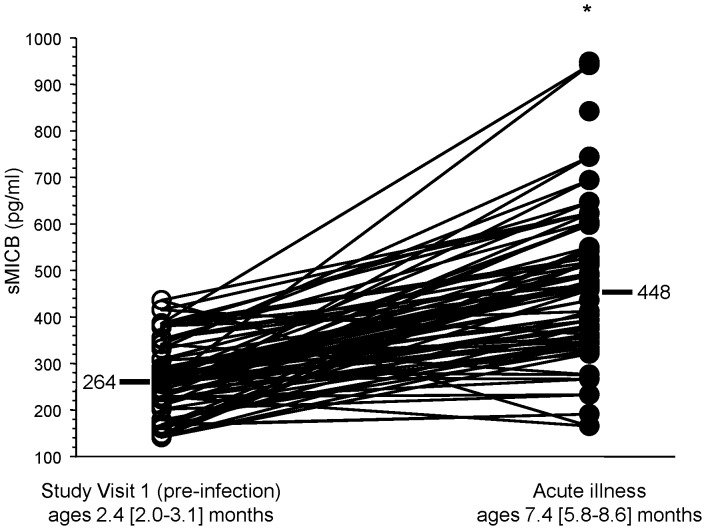
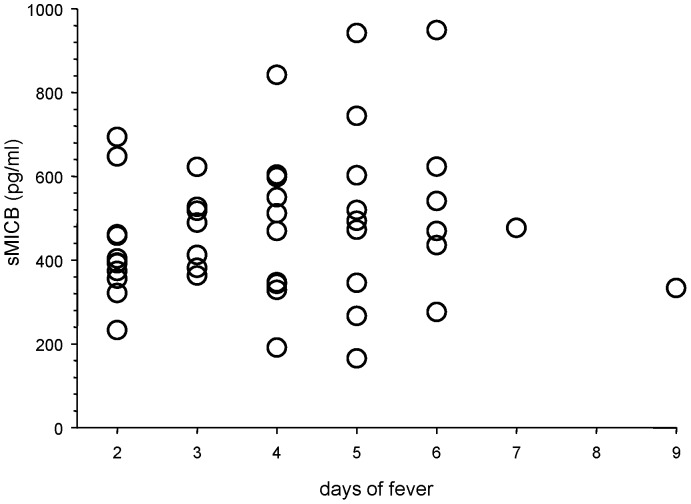
Circulating levels of sMICB increased between the pre-infection levels at study visit 1 and acute illness levels among infants with symptomatic primary DENV infections (Figure 1). sMICB levels were not different over the first week of illness among infants with symptomatic primary DENV infections (Figure 2).
Figure 1. Serum levels of soluble (s)MICB pre-infection and during acute illness among infants with symptomatic primary dengue virus infections (n = 46).
Ages are shown as median [95% confidence interval]. Bars are median values. * p<0.001 for comparison between the two groups.
Figure 2. Serum levels of soluble (s)MICB during acute illness by day of fever among infants with symptomatic primary dengue virus infections (n = 46).
sMICB levels trend higher in infants hospitalized with primary DENV infections
In a binary logistic regression model, the odds of a symptomatic primary DENV infection during infancy that led to hospitalization trended higher for every 100 pg/ml increase in acute illness serum sMICB (odds ratio (OR) [95% confidence interval] for hospitalization compared to outpatient illness: 1.4 [0.97–2.1], p = 0.075, n = 46).
Discussion
MICB is a cell surface protein and a ligand for the natural killer group 2 member D (NKG2D) receptor, a stimulatory receptor on NK cells and a co-stimulatory receptor on CD8+ T-cells [8]. sMICB blocks the activating signal produced by MICB-NKG2D, and is felt to be an immune evasion strategy [5]. We found that circulating levels of sMICB increased during acute primary symptomatic DENV infections in infants compared to pre-infection levels. The elevation of sMICB during acute DENV infections is likely an immune evasion strategy in a systemic viral infection. It could inhibit activation of the anti-viral effects of NK cells, CD8+ T-cells, or both, in infants with primary DENV infections and thereby contribute to the development of an acute febrile illness. We also noted that the circulating levels of sMICB in healthy 2 month old infants were higher than what has been reported in healthy adults [9], [10]. This may contribute to the susceptibility of infants to severe dengue with primary infections.
The likelihood of being hospitalized with an acute primary DENV infection during infancy tended to be higher with increasing acute illness sMICB levels. The MICB polymorphism (MICB SNP rs3132468) associated with symptomatic dengue in infants is an A/G change in an intron region of the human MICB gene (dbSNP database, NCBI). The effect of this SNP is unknown, but we postulate it could promote the proteolytic cleavage of cell surface MICB leading to increased sMICB levels. Further experiments to examine the effect of this SNP are planned.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All data are included within the manuscript.
Funding Statement
This work was supported by a grant from National Institutes of Health/National Institute of Allergy and Infectious Diseases R01 AI091820. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, et al. (2013) The global distribution and burden of dengue. Nature 496: 504–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Henchal EA, Putnak JR (1990) The dengue viruses. Clin Microbiol Rev 3: 376–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Khor CC, Chau TN, Pang J, Davila S, Long HT, et al. (2011) Genome-wide association study identifies susceptibility loci for dengue shock syndrome at MICB and PLCE1. Nat Genet 43: 1139–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Whitehorn J, Chau TN, Nguyet NM, Kien DT, Quyen NT, et al. (2013) Genetic variants of MICB and PLCE1 and associations with non-severe dengue. PLoS One 8: e59067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chitadze G, Bhat J, Lettau M, Janssen O, Kabelitz D (2013) Generation of soluble NKG2D ligands: proteolytic cleavage, exosome secretion and functional implications. Scand J Immunol 78: 120–129. [DOI] [PubMed] [Google Scholar]
- 6. Libraty DH, Acosta LP, Tallo V, Segubre-Mercado E, Bautista A, et al. (2009) A prospective nested case-control study of Dengue in infants: rethinking and refining the antibody-dependent enhancement dengue hemorrhagic fever model. PLoS Med 6: e1000171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Innis BL, Nisalak A, Nimmannitya S, Kusalerdchariya S, Chongswasdi V, et al. (1989) An enzyme-linked immunosorbent assay to characterize dengue infections where dengue and Japanese encephalitis co-circulate. Am J Trop Med Hyg 40: 418–427. [DOI] [PubMed] [Google Scholar]
- 8. Gonzalez S, Groh V, Spies T (2006) Immunobiology of human NKG2D and its ligands. Curr Top Microbiol Immunol 298: 121–138. [DOI] [PubMed] [Google Scholar]
- 9. Chung HW, Lim JB (2011) Clinical significance of serum levels of immune-associated molecules, uric acid and soluble MHC class I chain-related molecules A and B, as diagnostic tumor markers for pancreatic ductal adenocarcinoma. Cancer Sci 102: 1673–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tamaki S, Kawakami M, Ishitani A, Kawashima W, Kasuda S, et al. (2010) Soluble MICB serum levels correlate with disease stage and survival rate in patients with oral squamous cell carcinoma. Anticancer Res 30: 4097–4101. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All data are included within the manuscript.