Abstract
Immunization against self-tumor antigens can induce T-regulatory cells which inhibit proliferation of Type I CD4+ T-helper (Th1) and CD8+ cytotoxic T-cells. Type I T-cells are required for potent anti-tumor immunity. We questioned whether immunosuppressive epitopes could be identified and deleted from a cancer vaccine targeting IGFBP-2 and enhance vaccine efficacy. Screening breast cancer patient lymphocytes with IFN-γ and IL-10 ELISPOT, we found epitopes in the N-terminus of IGFBP-2 that elicited predominantly Th1 while the C-terminus stimulated Th2 and mixed Th1/Th2 responses. Epitope-specific Th2 demonstrated a higher functional avidity for antigen than epitopes which induced IFN-γ (p=0.014). We immunized TgMMTV-neu mice with DNA constructs encoding IGFBP-2 N-and C-termini. T-cell lines expanded from the C-terminus vaccinated animals secreted significantly more Type II cytokines than those vaccinated with the N-terminus and could not control tumor growth when infused into tumor-bearing animals. In contrast, N-terminus epitope-specific T-cells secreted Th1 cytokines and significantly inhibited tumor growth, as compared with naïve T-cells, when adoptively transferred (p=0.005). To determine whether removal of Th2 inducing epitopes had any effect on the vaccinated anti-tumor response, we immunized mice with the N-terminus, C-terminus and a mix of equivalent concentrations of both vaccines. The N-terminus vaccine significantly inhibited tumor growth (p<0.001) as compared to the C-terminus vaccine which had no anti-tumor effect. Mixing the C-terminus with the N-terminus vaccine abrogated the anti-tumor response of the N-terminus vaccine alone. The clinical efficacy of cancer vaccines targeting self-tumor antigens may be greatly improved by identification and removal of immunosuppressive epitopes.
Keywords: cancer vaccine, IGFBP-2, Th1, Th2, self-protein
Introduction
Cancer vaccines have progressed slowly from clinical testing into standard of care use despite decades of development and evaluation. The majority of vaccines that have advanced to later stage clinical trials have been protein- or tumor cell-based. Both vaccine platforms supply intact antigen for presentation to the immune system. In general, Phase III studies reported with these types of vaccines have shown little or modest clinical efficacy in definitive randomized trials (1-3). Obstacles to improved efficacy have included the poor immunogenicity of self-proteins which are cancer associated antigens, low to moderate immune responses elicited by vaccination, and the observation that active immunization against self-antigens can induce immune suppressive cells, such as T-regulatory cells (Treg), to proliferate (4).
In the last several years there has been an increased understanding of the type of immune response that is needed to elicit tumor destructive immunity. Cancer patients who endogenously develop Type I T-cells (both IFN-γ-secreting CD4+ and activated cytotoxic T-cells) capable of infiltrating their tumors have a significantly improved disease outcome as compared to individuals who do not mount such an immune response (5, 6). Recent studies of a variety of cancer vaccines have reported a correlation of vaccine-induced Type I T-cell levels, specifically T-helper (Th) 1 cells, with disease resolution or overall survival benefit after immunization (7-9).
For cancer vaccines, the main mode of stimulating the development and activation of Th1-cells has been vaccine adjuvants to induce the appropriate co-stimulatory signals or alter the cytokine milieu of the vaccine environment to elicit Th1 (10, 11). There has been little exploration of the modulation of the antigenic epitope within the T-cell receptor (TCR) (12). Investigations have well documented that the nature of the Th epitope presented to the TCR is the first signal in the differentiation of a naïve T helper-cell into a specific Th phenotype (13). As Th1 cytokines have been shown to be much more effective than Th2 cytokines in activating antigen presenting cells (APC) to stimulate tumor specific adaptive immunity and Th2 have been shown to suppress Th1 cells, it would be a benefit to develop a vaccine that included only antigenic amino acid sequences that elicited primarily or exclusively Th1 responses (14, 15).
We questioned whether screening epitopes derived from a self-tumor antigen for sequences that may induce antigen-specific Treg or Th2, and then eliminating those epitopes from a sub-unit vaccine, would enhance vaccine efficacy. Studies described below demonstrate that specific peptides of self-proteins can preferentially elicit IFN-γ or IL-10 secretion by T-cells. Elimination of epitopes that elicit IL-10 secretion assures the anti-tumor potency of an IGFBP-2 directed vaccine.
Materials and Methods
Evaluation of antigen-specific T-cell phenotype and functional avidity
IGFBP-2 peptides, predicted to bind promiscuously to human MHCII, were selected using web-based algorithms as previously described (16). Peripheral blood mononuclear cells (PBMC) from 20 female breast cancer patients, obtained by a University of Washington institutional review board approved informed consent, were cryopreserved and evaluated by ELISPOT for antigen specific IFN-γ or IL-10 secretion, according to our published methods (17, 18). Data are reported as the mean number of spots for each experimental antigen minus the mean number of spots detected in no antigen control wells (corrected spots per well: CSPW). Positive responses were defined by a statistically significant difference (p<0.05) between the mean number of spots from five replicates in the experimental wells and the mean number from no antigen control wells for an individual.
Some donors, who demonstrated either an IFN-γ restricted (n=5) or IL-10 restricted epitope-specific response (n=4), had their PBMCs assessed for the appropriate cytokine secretion induced by a single epitope at varying concentrations (10 μg/ml, 1 μg/ml, 0.1 μg/ml and 0.01 μg/ml) (18). For each donor, data are graphed as best-fit non-linear regression curves for the mean CSPW generated at all peptide concentrations. The mean half-maximal effective peptide concentration (EC50) is calculated for each curve using the “log(agonist) vs. response -Find ECanything” analysis in GraphPad Prism.
Antigen-specific IFN-γ production by mouse spleen cells was quantitated by ELISPOT as we have reported (18), with the modification that PVDF plates (Millipore) were coated with 10 μg/ml anti-mouse IFN-γ (clone AN-18; Mabtech) and 5 μg/ml biotinylated anti-mouse IFN-γ (clone R4-6A2; Mabtech). Data are reported as CSPW as defined above.
Generation of human and murine IGFBP-2-specific Th1 and Th2 cell lines
Human antigen-specific T-cell lines were generated for phenotyping using our published methods (18). Spleen cells from IGFBP-2 (1-163) (N-terminus)-vaccinated mice were stimulated with a pool of peptides; p8-22, p17-31, p67-81, p99-113, p109-123 and p121-135 (10 μg/ml each) and IGFBP-2 (164-328) (C-terminus)-vaccinated mice were stimulated with p164-178, p190-204, p213-227, p235-249, p251-265, p266-280, p291-305, p307-321 (10 μg/ml each) peptides. The T-cells were subjected to a second in vitro stimulation on day 8 by adding equivalent numbers of peptide-loaded (10 μg/ml) autologous irradiated (3000 rads) splenic cells to the original culture. 10 ng/ml recombinant mouse IL-7 (R & D Systems), 5 ng/ml recombinant human IL-15 (PreproTech, Inc.) and 10 U/ml recombinant human IL-2 (Hoffman-LaRoche) were added on days 5 and 12, with additional IL-2 on days 15 and 18 for T-cell expansion (16).
Assessment of T-cell phenotype
Antibodies were obtained from eBioscience (murine) or Biolegend (human). Receptor expression was documented in the expanded T-cells by adding PE-Cy7-conjugated anti-mouse CD49b (clone: DX5; 0.5 μg), APC-conjugated anti-mouse CD4 (clone: GK1.5; 0.2 μg) or APC-conjugated anti-human CD4 (clone: OKT4; 20 μl), PerCP-conjugated anti-mouse CD3 (clone: 145-2C11; 0.2 μg) or PE-Cy7-conjugated anti-human CD3 (clone: UCHT1; 20 μl) and FITC-conjugated anti-mouse CD19 (clone: 1D3; 0.5 μg). For extracellular staining, cells were incubated 30 minutes with the receptor antibodies. Intracellular expression of FOXP3 was documented after permeablization and fixation with the FOXP3 Buffer Set (Biolegend) according to the manufacturer's instructions and staining with PE-conjugated anti-mouse FOXP3 (clone: 150D/E4; 0.5 μg) and anti-mouse CD4 or PE-conjugated anti-human FOXP3 (clone: 206D; 20 μl) and anti-human CD4. Flow cytometry was performed on the FACSCanto (BD Biosciences) and data analyzed using FlowJo software (BD Biosciences). Typically, 100,000 cells were collected per sample. Results are reported as a percentage of total cell number or a percentage of a specific cell population.
Cytokine levels in the murine T-cell cultures were assessed according to manufacturer's instructions using the appropriate ELISA (eBioscience) on medium collected from the splenic T-cell lines on day 10 of culture. Data are expressed as mean ng/ml ± SD of 3 separate expansions.
Vaccine construction
IGFBP-2 (1-163) (N-terminus) and IGFBP-2 (164-328) (C-terminus) were amplified using the primers and conditions listed in Supplementary Table S1 with the Herculase II polymerase (Stratagene). For the IGFBP-2 (1-328) (full length) construct, cDNA was made from RNA extracted from the human breast cancer cell line, MCF-7 (ATCC). The cDNA was amplified using primers and conditions listed in Supplementary Table S1. The insert and eukaryotic expression vector, pUMVC3 (National Gene Vector Biorepository), were cut with EcoRI and BamHI restriction enzymes and ligated using E. coli ligase (New England Biolabs). Transformation of XL1 Blue competent bacteria (Stratagene) allowed kanamycin resistant clone selection. Sequencing (www.agencourt.com) was performed on each clone and each large scale DNA prep (Qiagen) to confirm identity. All DNA plasmids were determined to express the correct sized protein in vitro by transfecting HEK-293 (ATCC) cells using Polyfect reagent (Qiagen) and Western blot probing with anti-IGFBP-2 polyclonal antibodies (Santa Cruz Biotechnology, Inc) (data not shown).
Sequence Alignment
N-terminal (amino acids 1-163) or C-terminal IGFBP-2 (amino acids 164-328) sequences were aligned with human, viral, bacterial or fungal proteins searching the ref seq_protein database in NCBI's DELTA-BLAST algorithm using the default parameters. Alignments with less than 35% positivity (identical amino acids or conservative amino acid substitutions) over 80 amino acids were excluded as insignificant homology (19). Thirty-five percent represents a conservative assessment for identification of potential cross-reactive sequences for allergens (19). Data are reported as the % positivity (number of positive amino acids/total amino acids examined x100).
Vaccination, adoptive transfer, and assessment of tumor growth
Animal care and use were in accordance with institutional guidelines. Female FVB/N-TgN (MMTVneu)-202Mul mice (Tg-MMTVneu) (6-8 weeks old; mean weight: 18.5 g, range: 15.4-23.1 g) (Jackson Laboratory) were immunized with IGFBP-2 DNA constructs or pUMVC3 vector alone (50 μg plasmid) as a mixture in complete Freund's adjuvant/incomplete Freund's adjuvant (Sigma). Three immunizations were given two weeks apart. For tumor challenge, a syngeneic mouse mammary tumor cell line, MMC, (0.5 x 106 cells) was implanted into the mammary fat pad two weeks after the last vaccine or seven days before T-cell adoptive transfer (n=10/group) (20). Tumors were measured as previously described (16). Briefly, measurements on length, width, and depth were assessed by Vernier caliper 2-3x per week by the same technician at each time point. The measurements were multiplied by the volume constant of an ellipsoid shape, or π/6, in order to calculate tumor volumes. All tumor growth is presented as mean tumor volume (mm3 ± SEM). Data are representative of two independent experiments.
For adoptive transfer, 5x106 IGFBP-2 N-terminal- or C-terminal-specific T-cells were transferred into tumor-bearing mice by i.v. tail vein injection. The same number of splenocytes derived from naïve mice were used as a control infusion. Data are representative of two independent experiments.
in vivo T-cell depletion
Depletions were performed as previously described (21). Briefly, mice were vaccinated with the N-terminus vaccine three times, 14 days apart as described above. MMC cells were implanted two weeks from the last vaccine. Monoclonal antibodies were used for in vivo depletion (250 μg of anti-CD4; clone GK1.5 and 100 μg of anti-CD8; clone 2.43, UCSF Monoclonal Antibody Core) via intraperitoneal injection of the specific antibody three consecutive days before MMC implant and twice per week until the experiment was terminated. Rat IgG2b was used as a control. Data are shown as the mean ± SEM of 5 mice/group.
Statistical analysis
The unpaired, two-tailed Student's t-test, Fischer's exact test or χ2 test was used to evaluate differences between groups. p<0.05 was considered significant. in vivo studies were designed based on a priori power calculations generated by in vivo modeling of previous experiments with the same dose of MMC (Sample Size Predictor, Stata I2.0 IC). When control tumors reached a size that achieved 80% power with a significance level of 0.05 using a two sided, two sample test to reject the null hypothesis (Stata IC), the experiments were terminated. All statistical analyses were performed using GraphPad Prism 5.04 (GraphPad Software).
Results
The IGFBP-2 C-terminus is enriched for epitopes that induce IL-10-secreting T-cells as compared to the N-terminus
Investigations indicate the predominant cellular immune response in most patients with breast cancer is of a Th2 phenotype (22, 23). As epitope motifs have been shown to influence Th phenotype, we questioned whether we could identify sequences within a self-antigen that were specific for eliciting Th1 vs. Th2 or Treg for the purpose of excluding immune suppressive sequences from an epitope-based vaccine construct (13). We chose to analyze Th2/Treg by examining IL-10 secretion, as IL-10 has been shown to negatively regulate Th1 activity and enhance the expression of TGF-β, mediating the conversion of Th1 to Th2 (24, 25). Further, IL-10 is also secreted by Treg, which can proliferate via self-peptide stimulation (26). IGFBP-2 epitope-induced IL-10 and IFN-γ secretion was variable in breast cancer PBMC (Fig. 1A). We noted that epitopes within the C-terminus (p190-p307) of the protein were more immunogenic, stimulating a greater magnitude IL-10 and IFN-γ response than epitopes in the N-terminus. The mean IL-10 epitope-specific response (18 CSPW; range, 0-129 CSPW) in the C-terminus was 6-fold greater than the mean IL-10 epitope-specific response in the N-terminus (3 CSPW; range, 0-44 CSPW; p<0.001). The mean IFN-γ epitope-specific response (12 CSPW; range, 0-82 CSPW) in the C-terminus was 2-fold greater than the mean IFN-γ epitope-specific response in the N-terminus (6 CSPW; range, 0-70 CSPW; p=0.022). Epitopes in the C-terminus equally elicited the same magnitude of IFN-γ and IL-10 secreting cells by ELISPOT (p=0.132). In contrast, epitopes derived from the N-terminus of IGFBP-2 induced 3-fold more IFN-γ secreting cells than IL-10 secreting cells by ELISPOT (p=0.012).
Figure 1.
The IGFBP-2 C-terminus is enriched for epitopes that induce IL-10-secreting T-cells as compared to the N-terminus. (A) ELISPOT for IFN-γ (white) and IL-10 (black) in breast cancer patient PBMC for IGFBP-2 peptides presented as interquartile box plots with Tukey whiskers (n=20). Median corrected spots per well (CSPW) are indicated by the horizontal bar. (B) Percent of PBMC stimulated with IGFBP-2 peptides that induced only an IFN-γ (white bars) response, only an IL-10 (black bars) response, or both (gray bars) in ELISPOT.
Figure 1.B. shows the % of patients with a specific response to individual epitopes. In some patients, individual epitopes were shown to induce exclusively IFN-γ or IL-10 secreting cells by ELISPOT. In some patients, individual epitopes induced a mixed response with evidence of both IFN-γ and IL-10 secreting cells. When responses were pooled and grouped by N-and C-terminus rather than by individual epitopes, a significantly greater number of patients responded to the C-terminus of the protein (mean responder, 42%), compared to the N-terminus (mean responder, 31%; p=0.007). The C-terminal epitopes induced a mix of both IL-10 and IFN-γ secretion in response to antigen in a higher percentage of patients (mean responder, 20%) than that induced by the N-terminal epitopes (mean responder, 7%; p=0.003) where responses appeared to be more restricted to either Th1 or Th2.
To assess whether the T-cells elicited were Th2 or FOXP3+ Treg, we evaluated the phenotype of cultured T-cell lines. T-cells generated were CD3+ (mean: 90%, range 86-94%) composed primarily of CD4+ (mean: 73%, range: 70-77%) with fewer CD8+ (mean: 27%, range: 24-30%). No culture demonstrated an outgrowth of Treg (mean CD4+ FOXP3+: 1.7%, range: 0.9-2.5%) as compared to baseline.
IGFBP-2 epitope-specific Th2 demonstrate a higher functional avidity and homology to a greater number of bacterial and self-proteins than IGFBP-2
epitope-specific Th1
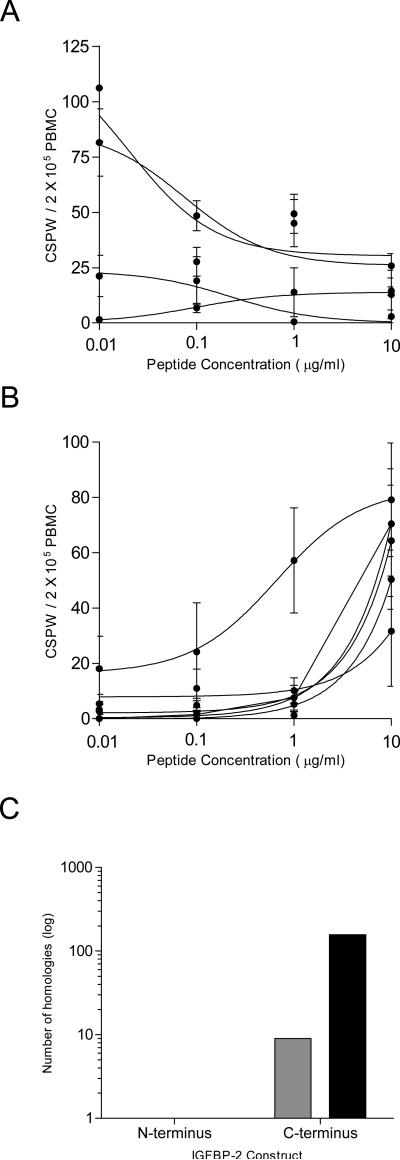
Titration studies documented that the peptides that induced IL-10 secretion were recognized by T-cells with a higher functional avidity (mean EC50 concentration: 0.11 ± 0.04 μg/ml) (Fig. 2A) than those peptides that induced an IFN-γ response (mean EC50 concentration 2.4 ± 0.56 μg/ml; p=0.014) (Fig. 2B).
Figure 2.
IGFBP-2 epitope-specific Th2 demonstrate a higher functional avidity and homology to a greater number of bacterial and self-proteins than IGFBP-2 epitope-specific Th1. Best-fit non-linear regression curves for the mean CSPW (•) generated at all peptide concentrations for each donor for (A) IL-10 (n=4) and (B) IFN-γ (n=5). Error bars: ± SEM of 4 replicates. (C) Number of homologous proteins for human (non-IGFBP family members) (gray bars) and bacteria (black bars) for the IGFBP-2 domains.
Our previous studies, which identified IGFBP-2 as a human antigen, had demonstrated that some epitopes derived from murine IGFBP-2 shared a high degree of homology with bacterial proteins (16). Indeed, T-cells specific for IGFBP-2 sequences were demonstrated as cross reactive with highly homologous sequences from antigens such as C. albicans and P. aeruginosa (16). We questioned whether this homology was associated with the Th2 induction observed with the use of the C-terminus vaccine. The N- and C-terminus differed in the amount of sequence homologies shared with foreign antigens. We identified 157 bacterial species that demonstrated 35% shared amino acid positivity over 80 or more amino acids (range 35-43%) for the human IGFBP-2 C-terminus (Fig. 2C; Supplementary Table S2). In contrast, the N-terminus demonstrated no sequence homology with bacterial proteins, a difference of over 100-fold. There was no difference in the number of viral homologies between the two termini (N-term, 0 and C-term, 0).
The IGFBP-2 N-terminus shared significant homology with other IGFBP proteins, and only one additional self-protein, CYR61 (Fig. 2C; Supplementary Table S3). The C-terminus also demonstrated significant homology with other IGFBP proteins but also to nine additional self-proteins including thyroglobulin, nidogens, and testicans (Fig. 2C; Supplementary Table S4). Only 16% of all homologous sequences for the N-terminus were non-IGFBP related while 64% of homologous sequences for the C-terminus were self-proteins other than IGFBP family members.
An N-terminus, but not IGFBP-2 C-terminus, vaccine both stimulates Type I immunity and inhibits tumor growth
Human and murine IGFBP-2 are highly homologous (82%) and tumors that arise in the TgMMTV-neu overexpress IGFBP-2 (21). We immunized mice with DNA constructs encoding the N-terminus (1-163), the C-terminus (164-328) and the full length (1-328) of IGFBP-2. The N-terminus vaccine could elicit peptide-specific Th1 (mean, 73 CSPW; range, 0-190 CSPW) compared to the C-terminus vaccine (mean, 10 CSPW; range, 0-89 CSPW; p=0.023) or the IGFBP-2 full length sequence (mean, 0 CSPW; p=0.007) (Fig. 3A). The mean tumor volume of N-terminus vaccinated mice (104.2 ± 8.4 mm3) was significantly less than that observed in the empty vector control (319.1 ± 33.2 mm3), C-terminus immunized (295.8 ± 15.5 mm3) and IGFBP-2 full length (278.3 ± 33 mm3) vaccinated mice, (p<0.001 for all) (Fig. 3B). Indeed, tumor growth after vaccination with the C-terminus and full length constructs was no different than control (p>0.15 for all). We also found that hIGFBP-2 (1-163) tumor volumes were significantly smaller than the vector control group, as determined by a two-way ANOVA with repeated measures with a Bonferroni's post-test, at multiple time points. Significance was observed as early as 22 days after tumor implant (p<0.001), and additionally at 26 and 28 days after tumor implant (p<0.0001).
Figure 3.
An N-terminus, but not C-terminus, IGFBP-2 vaccine both stimulates Type I immunity and inhibits tumor growth. (A) IFN-γ ELISPOT in splenocytes from mice immunized with the indicated vaccine. The data are presented as corrected spots per well (CSPW). The horizontal bar indicates the mean CSPW ± SEM. n=10 mice/group; *p<0.01. (B) Mean tumor volume (mm3 ± SEM) from mice injected with pUMVC3 alone (•), pUMVC3-hIGFBP2 (1-328) (■), pUMVC3-hIGFBP2 (164-328) (▲) or pUMVC3- hIGFBP2 (1-163) (○). n=5 mice/group; **p<0.001. (C) Mean tumor volume (mm3 ± SEM) from mice injected with pUMVC3 (•) or pUMVC3-hIGFBP2 (1-163) with isotype control IgG (○), CD8+ depletion (■) or CD4+ depletion (▲). #p<0.05 compared to pUMVC3-hIGFBP2 (1-163) vaccine with isotype control IgG.
Tumor inhibition was mediated by CD4+ T-cells (Fig. 3C). Elimination of CD8+ T-cells had no impact on the anti-tumor response elicited by vaccination with isotype control (p=0.291). Depletion of CD4+ T-cells within 2 weeks of completing immunizations, however, resulted in a significant loss of the tumor inhibitory effect of the N-terminus vaccine (p=0.027) compared to vaccine without depletion and was no different than the administration of empty vector alone (p=0.291).
IGFBP-2 vaccine-induced Th2 can abrogate the anti-tumor effect of IGFBP-2-specific Th1
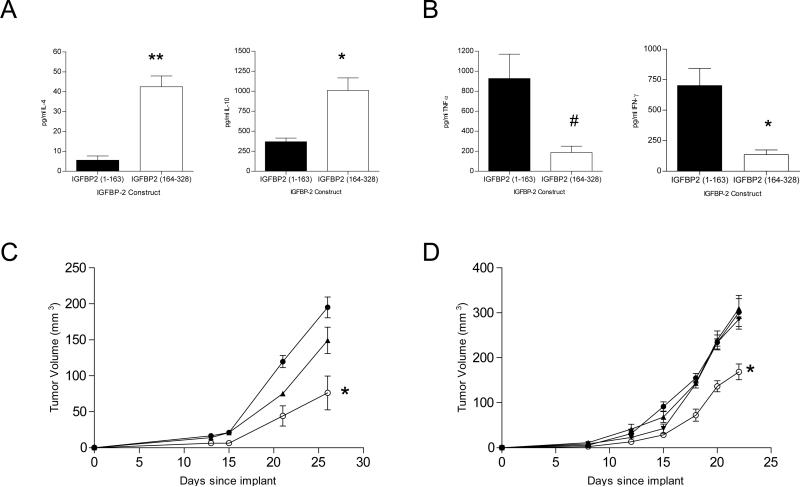
To confirm that the inability of the C-terminus vaccine to inhibit tumor growth was not due to the lack of an immune response, we evaluated cytokine secretion from T-cell lines generated after vaccination. T-cell lines derived from mice vaccinated with the N-terminus (mean, 77% CD3+ cells) were divided equally between CD4+ (mean, 50%) and CD8+ (mean, 50%) cells. T-cell lines generated from mice vaccinated with the C-terminus (mean, 65% CD3+ cells) were predominantly CD4+ (mean, 64%) with fewer CD8+ (mean, 36%) cells. Less than 0.5% of B cells, NK cells or FOXP3+CD4+ T-cells were detected in any culture. Expanded T-cell lines from the C-terminus secreted significantly more of the Type II cytokines IL-4 (mean, 42.4 ± 5.4 ng/ml; p<0.001) and IL-10 (mean, 1011 ± 154 ng/ml; p=0.002) than those from the N-terminus (mean IL-4, 5.5 ± 1.9 ng/ml; mean IL-10, 368.8 ± 45.5 ng/ml) (Fig. 4A). T-cell lines from mice vaccinated with the N-terminus construct secreted significantly more Th1 cytokines, IFN-γ (mean, 702.5 ± 125.7 ng/ml; p=0.008) and TNFα (mean, 926 ± 244 ng/ml; p=0.015) than T-cells from mice vaccinated with the C-terminus construct (mean IFN-γ, 135.8 ± 33.4 ng/ml; mean TNFα, 186.5 ± 64.4 ng/ml) (Fig. 4B). T-cells from mice vaccinated with the N-terminus adoptively transferred into tumor-bearing mice inhibited tumor growth (mean, 76.1 ± 23.6 mm3) compared to naïve T-cells (mean, 195 ± 14.4 mm3; p=0.005) (Fig. 4C). Conversely, tumor growth in mice treated with T-cells derived from animals vaccinated with the C-terminus construct (mean, 149.2 ± 18.3 mm3) was not statistically different than the naïve T-cell treated mice (p=0.09). Immunization with a vaccine which mixed both N- and C- terminus constructs in equivalent amounts abrogated the anti-tumor effect (mean, 292.3±16.7 mm3) of the N-terminus construct when used alone (mean, 178.7±16.6 mm3; p=0.001). Mean tumor growth after immunization with the combination vaccine was not significantly different than the empty vector control (313±41.3 mm3; p=0.712) or the C-terminus vaccine alone (300.4±23.4 mm3; p=0.409) (Fig. 4D).
Figure 4.
IGFBP-2 vaccine-induced Th2 abrogates the anti-tumor effect of IGFBP-2-specific Th1. Type II cytokines IL-4 and IL-10 (A) and Type I cytokines TNFα and IFN-γ (B) secretion from T-cell lines expanded with peptides in IGFBP2 (1-163) or IGFBP2 (164-328) (mean ng/ml ± SD); **p<0.001, *p<0.01 and #p<0.05. (C) Mean tumor volume (mm3 ± SEM) from mice infused with CD3+ T-cells expanded from mice vaccinated with pUMVC3-hIGFBP2 (1-163) (○), pUMVC3-hIGFBP2 (164-328) (▲) or naïve T-cells (•). n=4 mice/group; *p<0.01. (D) Mean tumor volume (mm3 ± SEM) from mice injected with pUMVC3 alone (•), pUMVC3-hIGFBP2 (164-328) (▲), pUMVC3-hIGFBP2 (1-163) (○) or pUMVC3-hIGFBP2 (1-163) + pUMVC3-hIGFBP2 (164-328) (▼). n=5 mice/group; *p<0.01.
Discussion
The generation of tumor-specific Th1, via vaccination, can result in the activation of both innate immune cells and CD8+ cytotoxic T-cells (CTL). Vaccine-stimulated antigen-specific Th1 secrete Type I cytokines, such as IFN-γ, which enhance the function of local APC and augment endogenous antigen presentation (27). An increased processing of tumor cells by the APC results in epitope spreading, which is associated with tissue destruction. Many current cancer vaccine approaches, especially those which employ the use of whole intact antigen, elicit Th2 or mixed Th1/Th2 immunity (28). Subunit or epitope-based vaccines may be much more effective for preferentially inducing Th1 than whole antigen approaches. Data presented here demonstrate that a self-tumor antigen contains sequences that are capable of specifically stimulating either a Th1 or Th2 response. Moreover, the Th2 generated by such epitopes are of a higher functional avidity than the Th1 cells elicited, thus may compete more effectively for antigen/MHC complexes at the site of the tumor. Removal of Th2 inducing sequences from a vaccine construct, however, will allow Th1 dominance and an effective anti-tumor response.
The differentiation of a naïve Th-cell into one with a mature phenotype is influenced by the binding of a particular peptide to the MHC (signal 1), the co-stimulation provided at the time of antigen recognition (signal 2), and the cytokine environment in which the immune response is generated (signal 3) (29). Signals 2 and 3 can be influenced by the adjuvants provided with vaccination. We chose CFA/IFA as a vaccine adjuvant as this agent has been shown to stimulate both Th1 and Th2 responses, thus, would be less likely to bias to a specific Th phenotype (30). We sought to influence signal 1 by determining whether immunosuppressive epitopes could be identified within a tumor antigen protein sequence and then removed. There have been several reports of tumor antigen-specific Treg present in the peripheral blood of cancer patients and volunteer donors. Two recent studies identified both mammoglobin-specific (31) and p53-specific-Treg (32) in the blood of breast cancer patients and volunteers respectively. The Treg in both these models had common characteristics; they recognized specific class II binding peptide sequences and suppressed the response of conventional T-cells and CTL. Presumably, p53 Treg in volunteer donor blood represents one mechanism of peripheral tolerance. The mammoglobin-specific Treg were enriched in the blood of breast cancer patients as compared to controls indicating those cells were educated and stimulated to proliferate by endogenously expressed antigen. Similarly, antigen-specific Treg can be induced by active immunization against a cancer antigen. Investigators, immunizing patients with melanoma, demonstrated Treg specific for a 17-amino acid peptide derived from NY-ESO expanded in vivo with administration of an NY-ESO targeting vaccine (4). In some cases, both effector T-cells as well as Treg could be stimulated by the same NY-ESO peptide sequence. We did not identify IGFBP-2 specific Treg in our T-cell expansions, rather conventional Th2. Vaccine-induced Th2 can have the same immunosuppressive effects as Treg. Th2 cytokines have been shown to markedly reduce the secretion of IFN-γ by leukemic-specific Th1 when present in the same co-culture (15). Similar to NY-ESO Treg, we also found IGFBP-2 sequences that stimulated both IFN-γ as well as IL-10. However, it was possible to identify sequences that elicited predominantly IFN-γ secretion in response to antigen, allowing epitopes that generated mixed responses to be removed from the vaccine construct.
The IGFBP-2 N- and C-termini differed significantly in the prevalence of Th1- vs. Th2-inducing epitopes. We had previously reported that individual epitopes of IGFBP-2 shared significant sequence homology with foreign proteins, in particular bacterial antigens (16). As the primary Th response to bacteria is Th2, with Type II cytokine secretion driving the development of antibodies needed to bind to and clear bacteria, we questioned whether the C-terminus may contain greater areas of homology with bacteria than the N-terminus. We used a standard methodology for predicting the potential for cross-reactivity to allergens which requires a minimum of 35% identity over 80 amino acid sequences to define risk for cross-interaction (19, 33). The IGFBP-2 C-terminus harbored over 100-fold greater sequences with potential cross-reactivity to bacterial antigens than the N-terminus. Potentially, the sequence similarity of the C-terminus to bacteria could be steering Th differentiation to Type II. We also identified that the C-terminus had a greater sequence homology with numerous self-proteins outside of the insulin like growth factor receptor family, in contrast to the N-terminus whose homology was restricted. It has recently been reported that memory Treg differentiate into Th2 after down regulation of FOXP3 following in vitro expansion (34). The C-terminus Th2 could represent a pool of higher avidity T-cells which have been stimulated by numerous self-antigens presented endogenously in the periphery and may play a role in maintaining self-tolerance.
IGFBP-2 Th2 demonstrated a higher functional avidity than Th1 responding to antigen at lower concentrations. In all likelihood, tumor antigen is presented in the context of MHC in low concentrations in the tumor bed. Subsequently, vaccine-induced Th2 will more effectively compete for those peptide-MHC complexes than lower avidity Th1. Type II cytokines secreted in response to antigen stimulation will prevent APC activation and the development of CTL. Removing the Th2 dominant sequences and allowing a Th1 dominant response resulted in inhibition of tumor growth after administration of an IGFBP-2 vaccine. In humans, although of a lower avidity, the Th1 population boosted with active immunization may still be quite functional. Recent evidence suggests that CD4+ T-cells with lower functional avidity produce cytokines for longer periods of time and are less prone to experiencing exhaustion in the face of chronic antigen exposure (35). In contrast, higher avidity T-cells have been shown to be more likely to experience antigen induced cell death which could be a particular problem in situations of chronic antigen stimulation such as with cancer (36). Further, higher avidity T-cells can become tolerized in the tumor microenvironment (37).
Clinical studies of cancer vaccines often use constructs encoding the full length self-protein or immunize with the complete antigenic self-protein in adjuvant and rarely monitor both the Th1 and Th2 immune response generated with active immunization. Studies described here raise the question as to whether the lack of efficacy seen with many cancer vaccines might lie in the augmentation of Th2 concurrent with Th1. Selection of particular portions of a tumor antigen which specifically stimulate Type I, but not Type II, T-cells provides the capability of developing vaccines that preferentially elicit Th1 and CTL. Combination of a Th1-generating cancer vaccine with potent adjuvants and/or checkpoint blocking agents can have the potential to drive the Type I immune response to therapeutic levels in vivo.
Supplementary Material
Acknowledgments
Financial Support: This work was supported by a grant from the NCI P50 CA083636, Ovarian Cancer Research Fund 17624550-36370-A and a DOD Postdoctoral Fellowship Award W81XWH-10-1-0700. MLD is supported by the Athena Distinguished Professorship for Breast Cancer Research.
Footnotes
COI: MLD is an inventor on patents held by University of Washington that pertain to data presented in this manuscript.
References
- 1.Amato RJ, Hawkins RE, Kaufman HL, Thompson JA, Tomczak P, Szczylik C, et al. Vaccination of metastatic renal cancer patients with MVA-5T4: a randomized, double-blind, placebo-controlled phase III study. Clin Cancer Res. 2010;16:5539–47. doi: 10.1158/1078-0432.CCR-10-2082. [DOI] [PubMed] [Google Scholar]
- 2.Wood C, Srivastava P, Bukowski R, Lacombe L, Gorelov AI, Gorelov S, et al. An adjuvant autologous therapeutic vaccine (HSPPC-96; vitespen) versus observation alone for patients at high risk of recurrence after nephrectomy for renal cell carcinoma: a multicentre, open-label, randomised phase III trial. Lancet. 2008;372:145–54. doi: 10.1016/S0140-6736(08)60697-2. [DOI] [PubMed] [Google Scholar]
- 3.Sondak VK, Liu PY, Tuthill RJ, Kempf RA, Unger JM, Sosman JA, et al. Adjuvant immunotherapy of resected, intermediate-thickness, node-negative melanoma with an allogeneic tumor vaccine: overall results of a randomized trial of the Southwest Oncology Group. J Clin Oncol. 2002;20:2058–66. doi: 10.1200/JCO.2002.08.071. [DOI] [PubMed] [Google Scholar]
- 4.Ebert LM, Macraild SE, Zanker D, Davis ID, Cebon J, Chen W. A Cancer Vaccine induces expansion of NY-ESO-1-specific regulatory T cells in patients with advanced melanoma. PloS One. 2012;7:e48424. doi: 10.1371/journal.pone.0048424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–64. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 6.Fridman WH, Pages F, Sautes-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12:298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 7.Kenter GG, Welters MJ, Valentijn AR, Lowik MJ, Berends-van der Meer DM, Vloon AP, et al. Vaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplasia. New Engl J Med. 2009;361:1838–47. doi: 10.1056/NEJMoa0810097. [DOI] [PubMed] [Google Scholar]
- 8.Madan RA, Bilusic M, Heery C, Schlom J, Gulley JL. Clinical evaluation of TRICOM vector therapeutic cancer vaccines. Semin Oncol. 2012;39:296–304. doi: 10.1053/j.seminoncol.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Disis ML, Wallace DR, Gooley TA, Dang Y, Slota M, Lu H, et al. Concurrent trastuzumab and HER2/neu-specific vaccination in patients with metastatic breast cancer. J Clin Oncol. 2009;27:4685–92. doi: 10.1200/JCO.2008.20.6789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Curtsinger JM, Mescher MF. Inflammatory cytokines as a third signal for T cell activation. Curr Opin Immunol. 2010;22:333–40. doi: 10.1016/j.coi.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perret R, Sierro S, Botelho NK, Corgnac S, Donda A, Romero P. Adjuvants that improve the ratio of antigen-specific effector to regulatory T cells enhance tumor immunity. Cancer Res. 2013;73:6597–608. doi: 10.1158/0008-5472.CAN-13-0875. [DOI] [PubMed] [Google Scholar]
- 12.Linterman MA, Vinuesa CG. Signals that influence T follicular helper cell differentiation and function. Semin Immunopathol. 2010;32:183–96. doi: 10.1007/s00281-009-0194-z. [DOI] [PubMed] [Google Scholar]
- 13.Pfeiffer C, Stein J, Southwood S, Ketelaar H, Sette A, Bottomly K. Altered peptide ligands can control CD4 T lymphocyte differentiation in vivo. J Expl Med. 1995;181:1569–74. doi: 10.1084/jem.181.4.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luheshi N, Davies G, Poon E, Wiggins K, McCourt M, Legg J. Th1 cytokines are more effective than Th2 cytokines at licensing anti-tumour functions in CD40-activated human macrophages in vitro. Eur J Immunol. 2014;44:162–72. doi: 10.1002/eji.201343351. [DOI] [PubMed] [Google Scholar]
- 15.Guenova E, Watanabe R, Teague JE, Desimone JA, Jiang Y, Dowlatshahi M, et al. TH2 cytokines from malignant cells suppress TH1 responses and enforce a global TH2 bias in leukemic cutaneous T-cell lymphoma. Clin Cancer Res. 2013;19:3755–63. doi: 10.1158/1078-0432.CCR-12-3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park KH, Gad E, Goodell V, Dang Y, Wild T, Higgins D, et al. Insulin-like growth factor-binding protein-2 is a target for the immunomodulation of breast cancer. Cancer Res. 2008;68:8400–09. doi: 10.1158/0008-5472.CAN-07-5891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Disis ML, dela Rosa C, Goodell V, Kuan LY, Chang JC, Kuus-Reichel K, et al. Maximizing the retention of antigen specific lymphocyte function after cryopreservation. J Immunol Methods. 2006;308:13–18. doi: 10.1016/j.jim.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 18.Cecil DL, Park KH, Gad E, Childs JS, Higgins DM, Plymate SR, et al. T-helper I immunity, specific for the breast cancer antigen insulin-like growth factor-I receptor (IGF-IR), is associated with increased adiposity. Breast Cancer Res Treat. 2013;139:657–65. doi: 10.1007/s10549-013-2577-z. [DOI] [PubMed] [Google Scholar]
- 19.Harper B, McClain S, Ganko EW. Interpreting the biological relevance of bioinformatic analyses with T-DNA sequence for protein allergenicity. Regul Toxicol Pharmacol. 2012;63:426–32. doi: 10.1016/j.yrtph.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 20.Knutson KL, Almand B, Dang Y, Disis ML. Neu antigen-negative variants can be generated after neu-specific antibody therapy in neu transgenic mice. Cancer Res. 2004;64:1146–51. doi: 10.1158/0008-5472.can-03-0173. [DOI] [PubMed] [Google Scholar]
- 21.Disis ML, Gad E, Herendeen DR, Lai VP, Park KH, Cecil DL, et al. A multi-antigen vaccine targeting neu, IGFBP-2 and IGF-IR prevents tumor progression in mice with pre-invasive breast disease. Cancer Prev Res. 2013;6:1273–82. doi: 10.1158/1940-6207.CAPR-13-0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aspord C, Pedroza-Gonzalez A, Gallegos M, Tindle S, Burton EC, Su D, et al. Breast cancer instructs dendritic cells to prime interleukin 13-secreting CD4+ T cells that facilitate tumor development. J Exp Med. 2007;204:1037–47. doi: 10.1084/jem.20061120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pedroza-Gonzalez A, Xu K, Wu TC, Aspord C, Tindle S, Marches F, et al. Thymic stromal lymphopoietin fosters human breast tumor growth by promoting type 2 inflammation. J Exp Med. 2011;208:479–90. doi: 10.1084/jem.20102131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maeda H, Shiraishi A. TGF-beta contributes to the shift toward Th2-type responses through direct and IL-10-mediated pathways in tumor-bearing mice. J Immunol. 1996;156:73–78. [PubMed] [Google Scholar]
- 25.Salazar-Onfray F, Lopez MN, Mendoza-Naranjo A. Paradoxical effects of cytokines in tumor immune surveillance and tumor immune escape. Cytokine Growth Factor Rev. 2007;18:171–82. doi: 10.1016/j.cytogfr.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 26.Vence L, Palucka AK, Fay JW, Ito T, Liu YJ, Banchereau J, et al. Circulating tumor antigen-specific regulatory T cells in patients with metastatic melanoma. Proc Natl Acad Sci U S A. 2007;104:20884–89. doi: 10.1073/pnas.0710557105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Camacho SA, Carbobe FR, Sarvetnick N, LeBon A, Karlsson L, Peterson PA, Webb SR. A key role for ICAM-1 in generating effector cells mediating inflammatory responses. Nat Immunol. 2001;2:523–29. doi: 10.1038/88720. [DOI] [PubMed] [Google Scholar]
- 28.Li J, Sun Y, Jia T, Zhang R, Zhang K, Wang L. Messenger RNA vaccine based on recombinant MS2 virus-like particles against prostate cancer. Int J Cancer. 2014;134:1683–94. doi: 10.1002/ijc.28482. [DOI] [PubMed] [Google Scholar]
- 29.Kalinski P. Dendritic cells in immunotherapy of established cancer: Roles of signals 1,2,3 and 4. Curr Opin Investig Drugs. 2009;10:526–35. [PMC free article] [PubMed] [Google Scholar]
- 30.Reed SG, Orr MT, Fox CB. Key roles of adjuvants in modern vaccines. Nat Med. 2013;19:1597–608. doi: 10.1038/nm.3409. [DOI] [PubMed] [Google Scholar]
- 31.Schmidt HH, Ge Y, Hartmann FJ, Conrad H, Klug F, Nittel S, et al. HLA Class II tetramers reveal tissue-specific regulatory T cells that suppress T-cell responses in breast carcinoma patients. Oncoimmunology. 2013;2:e24962. doi: 10.4161/onci.24962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mandapathil M, Visus C, Finn OJ, Lang S, Whiteside TL. Generation and immunosuppressive functions of p53-induced human adaptive regulatory T cells. Oncoimmunology. 2013;2:e25514. doi: 10.4161/onci.25514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herman RA, Song P, Thirumalaiswamysekhar A. Value of eight-amino-acid matches in predicting the allergenicity status of proteins: an empirical bioinformatic investigation. Clin Mol Allergy. 2009;7:9. doi: 10.1186/1476-7961-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hansmann L, Schmidl C, Kett J, Steger L, Andreesen R, Hoffmann P, et al. Dominant Th2 differentiation of human regulatory T cells upon loss of FOXP3 expression. J Immunol. 2012;188:1275–82. doi: 10.4049/jimmunol.1102288. [DOI] [PubMed] [Google Scholar]
- 35.Caserta S, Kleczkowska J, Mondino A, Zamoyska R. Reduced functional avidity promotes central and effector memory CD4 T cell responses to tumor-associated antigens. J Immunol. 2010;185:6545–54. doi: 10.4049/jimmunol.1001867. [DOI] [PubMed] [Google Scholar]
- 36.Brentville VA, Metheringham RL, Gunn B, Durrant LG. High avidity cytotoxic T lymphocytes can be selected into the memory pool but they are exquisitely sensitive to functional impairment. PloS One. 2012;7:e41112. doi: 10.1371/journal.pone.0041112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu Z, Singh V, Watkins SK, Bronte V, Shoe JL, Feigenbaum L, et al. High-avidity T cells are preferentially tolerized in the tumor microenvironment. Cancer Res. 2013;73:595–604. doi: 10.1158/0008-5472.CAN-12-1123. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.