Abstract
Myelolipoma is an uncommon benign tumour composed of mature fat tissue and haematopoietic elements and is most commonly found in the adrenal gland. We report a case, which was discovered incidentally on chest X-ray, of a rare occurrence of multifocal extra-adrenal myelolipoma in the thoracic paravertebral region. This was further investigated with multi-detector computed tomography and magnetic resonance imaging. The presumed diagnosis, of extra-adrenal myelolipoma, was histologically confirmed via tissue sample obtained by computed tomography guided biopsy. We compare the adrenal and extra-adrenal entities from the perspective of published literature and also review the cases, published in Pubmed, of extra-adrenal myelolipomas in order to summarize the different locations of this lesion.
Keywords: Extra-adrenal, adrenal, paravertebral, myelolipoma, incidental, CT, MRI, fat
CASE REPORT
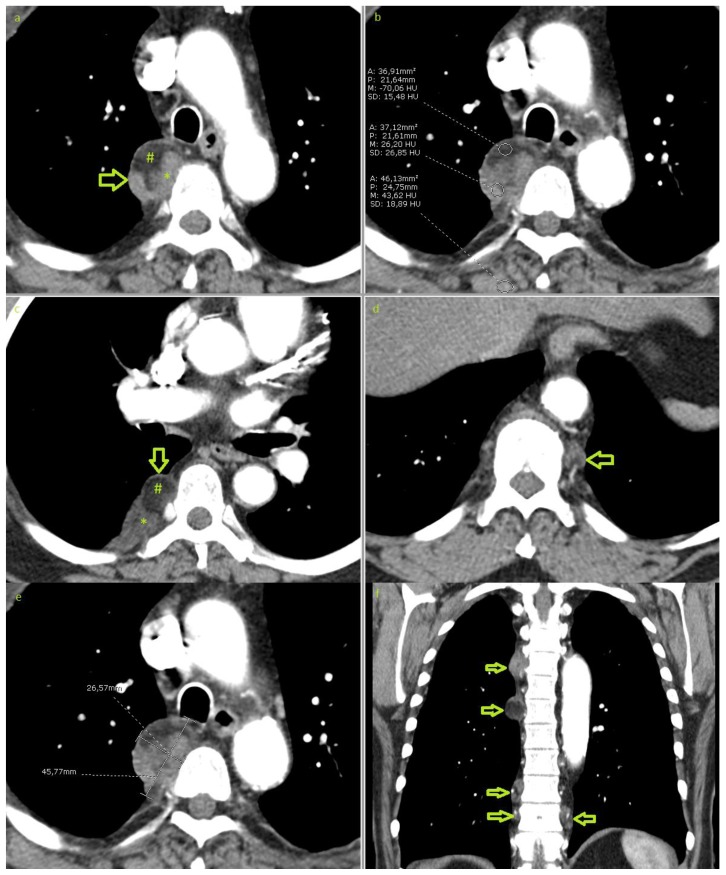
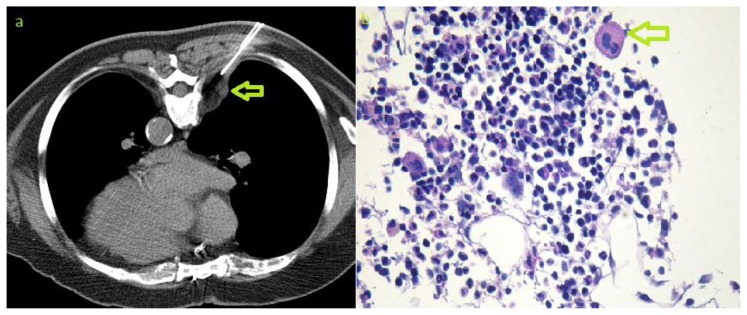
An asymptomatic 70-year-old European male presented with a right paravertebral mass, that had been detected incidentally on routine chest x-ray (fig 1). The patient underwent a computed tomography of the chest with intravenous contrast media, revealing 7 paravertebral lesions: 5 lesions on the right side and 2 lesions on the left (fig 2). The largest lesion, visible on the chest x-ray, was located at the level of T 5/6 with 45×26×56 mm in diameter (fig 2e), and the smallest lesion was located on the right side at the level of T11, with a diameter of 10×7×11mm. Some of these lesions were slightly lobulated and all lesions were encapsulated with well-defined borders and no infiltration of the neighbouring tissue. Lesions showed predominantly mixed densities, ranging from soft tissue, isodense to muscle; to fat-equivalent densities of about −70 Hounsfield units (fig 2b). Neither calcification nor active bleeding was found and there was no splenomegaly or evidence of bone marrow expansion. The patient then underwent a magnetic resonance imaging of the thoracic spine (fig 3). Lesions were partially hyperintense on T1 and T2 weighted images, with signal drop-out on fat suppression technique; and partially hypointense on T1 WI and isointense on T2 WI, with moderate enhancement after intravenous injection of gadolinium. No invasion of the neighbouring structures, no extension to the spinal canal or the neuroforamina was observed. CT-guided biopsy was performed under CT-fluoroscopy guidance, in prone position (fig 4). In the sample tube, the cylinders of tissue taken had a fat like appearance; being pale yellowish in colour and were floating over the formalin surface. Pathological examination revealed bone marrow elements and megakaryocytes (fig 4b). The final diagnosis was bilateral myelolipoma with location in the paravertebral region. Due to the benign nature and the asymptomatic presentation of these multiple tumours no further treatment was required.
Figure 1.
70-year-old male with bilateral multifocal paravertebral extra-adrenal myelolipoma. Chest X-ray showing incidentally detected right paravertebral mass (arrows). Plain chest radiography, posteranterior (a) and lateral (b), erect posture.
Figure 2.
70-year-old male with bilateral multifocal extra-adrenal myelolipoma. Contrast enhanced CT of the chest in an arterial phase demonstrating a well-circumscribed right paravertebral mass at the level of the aortic arch measuring 45×26×56mm(a & e). The mass shows mixed density, partially hypodense with −70 hounsfield units (b) due to fat content (#) and partially hyperdense due to myeloid tissue (*). Multiple smaller lesions are shown bilaterally (arrows in c, d & f). No infiltration of the neighbouring tissues. (Technique: 16-MDCT scanner, Automatic tube current modulation ranging between 131 and 250 mA, 120KVp. 5mm slice thickness. Soft tissue window, level/width: 15/350. i.v. injection of 60ml of 300mg/ml iodine concentration non-ionic contrast. a)–e) axial plane, (f) coronal reformation).
Figure 3.
70-year-old male with bilateral multifocal paravertebral extra-adrenal myelolipoma. MRI of the thoracic spine showing a right paravertebral mass with mixed signal intensity, partially hyperintense on T2 (a) and T1WI (b) due to the fat content of the mass (#) which shows signal drop-out on the fat suppression images (c & e) and on STIR sequence (f), and partially isointense on T2 (a) and hypointense on T1WI (b) due to the myeloid component (*) which shows hyperintensity on STIR (f), and moderate enhancement (c & e). Other similar lesions (arrows) are shown on the coronal plane (d). (Technique: 1.0 T scanner. a) axial T2WI: TR/TE: 3990/105, slice thickness 8, spacing 11.20, acquisition matrix: 256×205. b) axial T1WI: TR/TE: 509/11, slice thickness: 8, spacing 11.20, acquisition matric: 256×205. c) axial contrast enhanced T1WI with fat suppression: TR/TE: 742/12, slice thickness: 8, spacing: 11.20, acquisition matrix: 256×205. Administration of 15ml gadolinium based contrast agent. d) coronal non-enhanced T1WI: TR/TE: 863/13, slice thickness: 6, spacing 7.20, acquisition matrix: 187×512. e) coronal contrast enhanced T1WI with fat suppression: TR/TE: 1020/11, slice thickness: 6, spacing 7.20, acquisition matrix: 205×256. f) Sagittal Short tau inversion recovery (STIR): TR/TE: 3330/63, slice thickness: 3, spacing 3.60, acquisition matrix: 256×205)
Figure 4.
70-year-old male with bilateral multifocal paravertebral extra-adrenal myelolipoma. CT-guided biopsy. a) axial non-enhanced CT obtained during biopsy procedure showing the needle that was advanced in a dorsal approach for biopsy of the paravertebral lesion (arrow). b) Photomicrography (Periodic acid Schiff stain, magnification 200x) showing bone marrow cells and megakaryocyte (arrow). Photomicrograph image courtesy of Prof. Alexander Tzankov, pathology department, University Hospital Basel, Switzerland)
A comparative case with adrenal myelolipoma is provided in the figures section (fig 5).
Figure 5.
(comparative case) 68-year-old female with left adrenal myelolipoma. Contrast enhanced CT of the abdomen in a venous phase showing a well-circumscribed mass in the region of the left adrenal gland measuring 86×99×95mm. The mass is composed of fat cells (#) with density of -109 HU(b) and myeloid tissue with relatively higher attenuation. The mass shows a pseudocapsule (arrows in a, c &d). No infiltration of the neighboring structures. (Technique: 16-MDCT scanner. Automatic tube current modulation ranging between 300 and 440 mA, 120 KVp, 5mm slice thickness. Soft tissue window, level/width: 40/400. i.v. injection of 100 ml Imeron 300. Coronal and sagittal reformatted CT with 5mm slice thickness. a) &b) axial planes, c) coronal reformation and d) sagittal reformation).
DISCUSSION
Myelolipoma is an uncommon benign tumour, composed of mature fat tissue and haematopoietic elements (myeloid and erythroid cells) [1]. The German pathologist Edgar von Gierke first described it in 1905 when he noticed such a lesion in the adrenal gland, but it was not until 1929, that the lesion was given its name, “myelolipoma”, by the French pathologist Charles Oberling [2,3]. Generally, myelolipomas are adrenal lesions and extra-adrenal myelolipomas (EAMLs) are a very rare entity.
Epidemiology
The incidence of myelolipoma is less than 1% on post-mortem and accounts for 7–15% of adrenal masses [1,4]. Nowadays it is incidentally discovered at ultrasound or computed tomography [4]. In the future, the incidence of these lesions may increase due to the expanding use of radiological investigation. The incidence of EAMLs is not exactly known but in an autopsy series, of 67 cases with myelolipomas, submitted to the archives of the Armed Forces Institute of Pathology (AFIP) between 1981 and 1997, only 10 cases (about 14% of the cases in this study) were observed with extra-adrenal location [4]. In our search in Pubmed, over 100 cases of EAMLs were identified (table 1). Myelolipomas are usually found between the sixth and eighth decade, although, EAMLs have also been described in younger patients [1,5,6].
Table 1.
Summary of different locations and number of corresponding cases of Extra-adrenal myelolipomas (EAMLs) found on PubMed.
| Single EAML | No. of cases | Multiple EAML | No. of cases |
|---|---|---|---|
| Head and Neck | |||
| -Mandible | 1 | ||
| -Nasal Cavity | 1 | ||
| Chest | |||
| -Chest wall | 1 | -Thoracic | 2 |
| -Endobronchial | 1 | -Posterior mediastinum | 2 |
| -Mediastinum | 8 | ||
| -Pleural | 2 | ||
| -Pulmonary | 2 | ||
| -Thoracic | 1 | ||
| Abdomen and Pelvis | |||
| -Hepatic | 13 | -Bilateral renal | 2 |
| -Presplenic | 1 | -Retroperitoneal | 1 |
| -Stomach | 1 | -Greater omentum | 1 |
| -Mesentery | 2 | ||
| -Perirenal | 8 | ||
| -Renal hilum | 2 | ||
| -Renal sinus | 3 | ||
| -Retroperitoneal | 10 | ||
| -Presacral | 33 | ||
| -Lower pelvis | 1 | ||
| -Ovarian | 1 | ||
| -Prevesical | 2 | ||
| Intralymphonodular | 1 | ||
| Intraspinal | 1 | ||
| EAML with simultaneous involvement of multiple organs | |||
| -Retroperitoneal and intrathoracic | 2 | ||
| -Renal and posterior mediastinum | 1 | ||
| -Generalized (retroorbital, chest, abdomen and pelvis) | 1 | ||
| -Adrenal and EAML (retroperitoneal) | 1 | ||
Etiology
Although several theories have been discussed, the origin of myelolipoma remains unclear. Theories include remnants of fetal bone marrow, embolism of bone marrow cells, and hyperplasia of heterotopic reticulum cells [7,8]. Chang et al. described a case of adrenal myelolipoma with a translocation t(3;21)(q25;p11). A similar change, t(3;21)(q26;p11), is found in haematopoietic neoplasms, such as myelodysplastic syndromes and chronic myeloid leukaemia. This finding suggests that myelolipoma is a derivative from misplaced haematopoietic cells [9]. A study performed by Bishop et al. showed myelolipomas which display X-chromosome inactivation in both fat and haematopoietic elements, which suggests clonal origin of myelolipoma [10].
Pathology
Myelolipomas are usually unilateral, and very rarely bilateral [1]. EAMLs are very rare, about half of which occur at the presacral region and retroperitoneal space. Other, uncommon, locations have been described and Table 1 summarizes all locations of EAMLs that have been found to date in our search in Pubmed. According to this summary, based on Pubmed cases, ours is the fourth published case, representing multifocal EAML in the posterior mediastinum [11,12,13].
Adrenal myelolipomas vary in size, from several millimetres to more than 30 cm, and usually in the range of 2–10 cm in diameter [14,1]. The mean diameter of the EAML, in the series of the AFIP, was 8.2 cm; ranging from 4–15 cm. The largest documented EAML, recorded in the studied literature, was 26×15×11cm in diameter [15]. Most myelolipomas are well circumscribed; a true capsule is not seen, rather, compressed connective tissue surrounding the lesions represents a pseudocapsule [16]. The cut sections show fat and soft tissue components. Fat amount is variable, ranging from almost complete fat to a few tiny foci in the soft tissue mass [17]. Haemorrhage is more common in larger lesions of more than 10 cm in diameter, with male predominance of 89% [16]. Calcification may also be found. Microscopically, myelolipomas contain mature fat cells and megakaryocytes (fig 4b) [1,14]. They are different from true bone marrow in that they contain no reticular sinusoids or bone spicules [16]. Adrenal myelolipomas may be found coincidentally with other lesions in the adrenal glands, such as adenomas and less commonly with pheochromocytoma or metastases. These cases are described as “collision tumours” [18]. Pathological features of EAMLs are very similar to adrenal myelolipomas, except that haemorrhage and calcifications are very rare.
Clinical issue
Myelolipomas are usually asymptomatic and discovered incidentally on routine radiological examination. About 10% become large and can cause vague symptoms, such as pain [1,17]. EAMLs are usually asymptomatic, although symptoms may occur due to mass effect according to localization. For example; renal failure in the case of compression from perirenal lesions, sciatic pain or urinary retention in presacral lesions or numbness and gait disturbance in case of intraspinal lesions [19,20,21,22]. The most significant complication that could occur with large myelolipomas is acute haemorrhage, which can be present with pain and that localizes in the back or flanks, with nausea, vomiting, hypotension and anaemia [16]. Haemorrhage in EAMLs is a very rare complication; no reported case in the studied literature records a clinical picture of acute haemorrhage. Myelolipomas are hormonally inactive. About 10% only are associated with endocrine disorders, such as Cushing’s syndrome, congenital adrenal hyperplasia, Conn’s syndrome, pheochromocytoma, hyperparathyroidism or adrenogenital syndrome [17,23,24,25,26]. No case of a hormonally active EAML was found.
Imaging
General features
Due to the identical tissue composition of adrenal and extra-adrenal myelolipomas pathologically, imaging features are the same.
Conventional radiology
Adrenal myelolipomas are difficult or even impossible to detect on plain radiography, except in cases of very large lesions with fat predominance where a lucent mass may be seen [4]. They can also be seen if calcified [14]. EAMLs may be seen depending on their location, as in our case of a paravertebral thoracic lesion (fig 1).
Ultrasound
The well-defined fatty masses in myelolipomas appear echogenic in ultrasound [27]. Myeloid tissue is heterogeneous in appearance [1]. Haemorrhage and calcification change the sonographic pattern. Haemorrhage appears usually as low-echo areas compared to fat, and calcification appears as hyperechoic areas with acoustic shadowing [4]. According to location endosonography can be applied in the work up [28].
Cross sectional imaging
Cross sectional imaging is necessary to confirm the origin of myelolipoma and crucial to differentiate these rare lesions from other, more common, tumours. Multiplanar reconstruction (MPR) may be helpful in determining the exact location of the lesion.
Computed Tomography
Understanding the histological tissue composition helps in understanding the CT appearance. The fatty part of the tumour has a negative attenuation value (between −30 and −90 Hounsfield units) (fig 2b & 5b) [1]. Myeloid component of the tumour appear higher in attenuation than the fatty part or other surrounding fat, for example retroperitoneal fat (fig 5). Enhancement is seen in the myeloid tissue after injection of contrast media [14]. When fat and myeloid tissue is diffusely mixed, the attenuation value will be between fat and water [17]. Myelolipomas usually show a recognizable pseudocapsule (fig 5a, c & d) [1,16,14]. CT is superior in detecting haemorrhage, which appears as areas of high densities. It is useful in detecting the size of haemorrhage and also the causative mass in cases of acute abdomen [4]. Calcification is seen in about 20% of adrenal myelolipomas, and only in very few cases of EAMLs [1,29,30]. Calcification is hyperdense and often punctate [17]. It may be related to previous haemorrhage.
Magnetic Resonance Imaging
The key feature on MRI is the hyperintense signal of fat on both T1WI and T2 WI (fig 3a&b). The myeloid elements have low signal intensity on T1WI and moderate signal on T2 WI (fig 3a&b). According to the admixture of fat and myeloid tissue, MRI imaging features can be categorized in 3 groups: Group 1, where fat predominates, so lesions will be homogenous hyperintense on T1WI. Group 2, where there is mixed fatty and myeloid components, so lesions will be heterogeneous with contrast enhancement. Group 3, where myeloid cells predominate, so lesions will show nodules with hypointense T1 signal and moderate T2 signal with enhancement after contrast injection [31]. Macroscopic fat is easily detected on MRI using fat suppression techniques (fig 3c&e) or on Short-Tau Inversion Recovery (STIR) (fig 3f), by loss of signal in the fatty parts of the mass [1,14]. Fat will also show chemical shift artefacts, or loss of signal in the out-of-phase sequence in voxels containing both fat and water, resulting in an appearance called “Indian ink” or “etching artefacts” [32]. In case of haemorrhage, MRI features vary widely, according to the age and size of haemorrhage [4].
Nuclear medicine
Nuclear medicine examinations can play a role in the diagnosis of myelolipoma, such as bone marrow scintigraphy using Technetium-labelled monoclonal antibodies directed against myeloid elements [33].
Angiography
In cases of unclear origin of the tumour, angiography can help in defining the blood supply [1]. It may also help in delineating the vasculature of the mass prior to surgery. The morphology of adrenal myelolipoma in angiography is predominantly avascular with a peripheral rim of vascularity from branching vessels [14].
Differential diagnosis
Adrenal myelolipoma
Adrenal myelolipomas must be differentiated from other fat containing lesions of the adrenal glands, such as adenomas, which lack myeloid components and also lack true fat density even in the presence of intracytoplasmic lipid, which can be detected by chemical shift imaging. Adrenal adenomas classically show a washout of contrast on the delayed imaging phase.
Adrenal myelolipomas with low fat content need to be differentiated from other adrenal lesions, such as: Pheochromocytomas, which are usually highly vascular; adrenal carcinoma and metastases, which usually have a higher intensity than myelolipomas; adrenal lymphomas, which are usually of a homogenous soft tissue-density, and lastly, from ganglioneuroma and neuroblastoma which are seen in children and young adults and are commonly associated with syndromes like neurofibromatosis, multiple endocrine neoplasia, von Hippel-Lindau disease or paraganglioma-syndromes [1].
Extra-adrenal lesions from neighbouring structures can be confused with adrenal myelolipomas, such as retroperitoneal lipomas, liposarcomas and renal angiomyolipomas [34].
Extra-adrenal myelolipoma
Generally EAMLs should be differentiated from extra-medullary haematopoiesis (EMH), as there are similar morphological aspects between these two entities, the differences will be discussed later. Otherwise the differential diagnosis of EAMLs depends on the anatomic localization of the lesions. Many other lesions in the retroperitoneal space, being the most common location of EAMLs, should be easily to differentiate: Lipomas lack the myeloid tissue; well-differentiated liposarcoma are poorly marginated with infiltrative growth pattern and teratomas are usually detected in a younger age group and may be associated with vertebral anomalies.
In our case the two main differential diagnoses are EMH and neurogenic tumours; both of which are typically located in the paraspinal region and can also show multiplicity. Therefore a comprehensive discussion of this differential diagnosis is warranted (table 3).
Table 3.
Comparison between extra-adrenal myelolipoma (EAML), extra-medullary haematopoiesis (EMH) and Neurofibroma (as an example of neurogenic tumour)
| Extra-adrenal myelolipoma | Extra-medullary haematopoiesis | Neurofibroma (Example of neurogenic tumors) | |
|---|---|---|---|
| Definition | Benign tumour composed of fat and myeloid tissues | Formation of blood elements outside the bone marrow cavity | Benign nerve sheath tumour |
| Etiology | Still unclear |
|
Unknown or syndromic |
| Incidence | Extremely rare | Rare | Uncommon |
| Pathological features | |||
| Site |
|
|
|
| Multiplicity | Usually single | Multiple | 10% multiple |
| Capsule | Pseudocapsule | Non capsulated | Non capsulated |
| Macroscopic | Yellow fat areas on cut section | Discrete flesh coloured mass |
|
| Microscopic | Megakaryocytes | Marrow elements outside the bone marrow |
|
| Associated (Related) conditions | Usually non |
|
|
| Clinical features | |||
| Age peak | 6th–8th decade | 3rd–4th decade | 3rd–4th decade |
| Demographics | -- |
|
-- |
| Symptoms |
|
|
|
| Symptoms of associated conditions | -- |
|
|
| Treatment | Asymptomatic lesions (confidently diagnosed by imaging) should be left alone |
|
Surgical excision in localized lesions. |
| Prognosis | Very good | Excision is associated with high recurrence rate and may cause anaemic crisis | Recurrence in case of multiple or plexiform lesions |
| Imaging features | |||
| General features | Well marginated Fat & myeloid tissues |
Well marginated (Fatty deposition may occur in long standing lesions) (Usually >5cm) |
|
| X-ray (depending on site & size) | Well demarcated mass | Well demarcated mass | Well demarcated mass |
| CT |
|
|
Homogeneous hypodense |
| T1 WI |
|
Iso to mildly hyperintense to muscles | Centre of the lesion have higher signal than periphery |
| T2 WI |
|
Iso-to mildly hyperintense to muscle | Periphery has higher signal than the centre |
| Contrast | Enhancement of myeloid components |
|
|
| Scintigraphy | Monoclonal antibodies directed against myeloid elements | Technetium 99m sulfur colloid marrow scan | FDG-PET Higher SUV in malignant transformation |
| Other findings | --- |
|
Neurofibromatosis:
|
EMH is a compensatory mechanism in chronic anaemia by formation of blood elements outside the bone marrow cavity, due to marrow failure or ineffective circulating mature blood cells. This is encountered in the liver and spleen, occasionally, forming paravertebral masses [35]. Any other mesenchymal tissue can be involved. In the pathological specimen, it is a non-capsulated mass with discrete flesh colour. Histologically, it is best described as marrow elements obtained from outside the bone marrow [36]. It is seen between the third and fourth decades in patients with chronic anaemia such as thalassaemia (common in the Mediterranean region), or sickle cell anaemia (common in Africa) [37]. Patients have usually received repeated blood transfusions. The lesion itself is asymptomatic, and it rarely compresses the spinal cord. Patients suffer from signs and symptoms of chronic anaemia. Various lines of treatment are described, for example: Blood transfusion, hydroxyurea which increases the production of haemoglobin F, Irradiation in cases of cord compression, and excision, which, has a high risk of recurrence and may cause anaemic crisis [36]. On imaging, EMH-lesions appear well circumscribed, usually more than 5 cm in diameter. Fatty deposition occurs only in long standing lesions so they are usually homogenous and iso-to mildly hyperintense to muscles on both T1 and T2 WI. They show homogenous enhancement, only in cases of fatty deposition they show heterogeneity. Technetium 99m sulfur colloid can detect EMH-lesions. Other helpful findings on imaging include hepatosplenomegaly and signs of intramedullary hyperplasia, like thickened trabeculae and widened ribs.
The second differential diagnosis of our case is the neurogenic tumours. These include nerve sheath tumours (schwannomas, neurofibromas, and their malignant counterparts) and ganglion cell tumours (neuroblastoma, ganglioneuromas and ganglioneuroblastomas).
For simplicity we will discuss only the neurofibroma (NF) as an example. NF is a benign nerve sheath tumour, and is the most common cause of posterior mediastinal tumours [38]. It can also be found along any peripheral nerve. 10% of these tumours are multiple [39]. Macroscopically it is a non-encapsulated, solid tumour of variable appearance and may show cystic degeneration [39,40]. Microscopically it is formed of nerve sheath cells and collagen bundles [41]. NFs may be associated with von Recklinghausen’s disease (neurofibromatosis I) with increased incidence of other neurogenic tumours such as optic glioma [42]. Malignant transformation may occur especially in cases of neurofibromatosis. Peak manifestation age is 20–30 years [39]. The lesion may be asymptomatic or may cause pain due to nerve entrapment or malignant transformation [40]. Patients may show other signs of von Recklinghausen’s disease such as café au lait spots and neurofibromatous nodules. Surgical excision of the localized lesion may be advised. Recurrence rate is higher in cases of multiple or plexiform lesions [39,40]. On imaging, it may be localized, diffuse or plexiform [39]. Neurofibromas are typically smooth round tumours, which may show cystic degeneration or calcification. Extension into neuroforamina may give the dumbbell shape appearance [40]. On CT they are homogenously hypodense. On T1 WI the centre of the lesion may have higher signal than the periphery in contrary to T2 WI where the periphery has a higher signal [40]. After contrast injection, they show mild to moderate homogenous enhancement or a target appearance [40]. FDG-PET can differentiate malignant transformation by the higher standardized uptake value (SUV) in malignant lesions [39]. Other manifestations of neurofibromatosis may be visible in imaging such as cutaneous nodules, bone deformities, remodelling, erosions and scalloping as well as widened neuroforamina [40].
Other entities, with paravertebral location, should be easy to differentiate. Malignant pleural lesions, such as pleural mesothelioma, have an infiltrative nature and volume reducing effect on the lung. Secondary lymphoma is more common than primary lymphoma of the chest wall, usually accompanied by mediastinal lymph node enlargement and sclerosis of the adjacent ribs. Subpleural lipomas, as well as fibrin bodies lack myeloid elements. Intrathoracic splenic tissue occurs only on the left side with other signs of previous trauma, such as rib fractures or diaphragmatic rupture.
Diagnosis, prognosis & treatment
The key to the diagnosis of adrenal and EAMLs is the presence of fat intermingled with myeloid elements and also the benign nature of the tumour. In most cases, diagnosis of adrenal myelolipomas can be confidently based on CT or MRI features alone [17]. Due to the rarity of EAMLs, it may occasionally be difficult to establish the diagnosis from imaging features only. In cases where diagnosis is uncertain and in those with low fat content, a histological sample will be helpful. Percutaneous fine needle biopsy (FNA) is simple, safe and effective. It will demonstrate numerous trilineage haematopoietic cells and a variable proportion of mature adipose cells. The presence of megakaryocytes is also an important feature [17,43,44]. Intraoperative cytodiagnosis, on touch preparation, can also be used in diagnosis. This is an important adjunct to frozen sections of fresh tissues, especially when tissue is technically unsuitable for cryostat section [45].
The prognosis is excellent, and therefore when diagnosis is certain, surgery is not necessary for small non-haemorrhagic myelolipomas [1,23]. In some cases a follow up, with CT or MRI, is appropriate [6]. Surgery is recommended in symptomatic patients, or when there is progressive growth with mass effect [6]. Some surgeons recommend surgery for large tumours (over 9 cm in diameter) due to the increased risk of haemorrhage with consecutive acute abdomen [27]. Other surgeons recommend laparoscopic adrenalectomy for tumours larger than 4 cm [46]. In fact, less than 40 published cases of myelolipomas have presented with signs and symptoms that required surgical resection [6].
Due to the rare incidence of EAMLs, no general surgical guidelines are mentioned in the studied literature and most surgical excisions were performed because of the uncertainty regarding the nature of the tumour or because of mass effect, for example in the case of intraspinal myelolipoma [21]. Risk of recurrence after excision is minimal due to the benign nature of these tumours, so there is usually no need for postoperative follow up [47]. Interventional radiology and embolization can play a role in the treatment of haemorrhage [47,48].
TEACHING POINT
Extra-adrenal myelolipomas are rare lesions, but the likelihood of detecting such lesions may increase with the increasing use of radiological investigations, therefore awareness of the typical location and appearance of these benign tumours is important in the differential diagnosis of fat-containing lesions. Fine needle biopsy is helpful in confirming the diagnosis in uncertain cases so that an asymptomatic patient will avoid unnecessary surgery.
Table 2.
Facts and figures about myelolipoma
| Facts and figures about myelolipomas: |
|---|
|
Table 4.
Comparison between adrenal and extra-adrenal myelolipomas
| Adrenal | Extra-adrenal | |
|---|---|---|
| Incidence |
|
|
| Age predilection | 6th–8th decades | Younger patients may be also involved |
| Etiology | Not quite understood | |
| Multiplicity |
|
Multiple lesions are extremely rare |
| Site | Adrenal glands |
|
| Size | Usually 2–10cm | Usually 4–15cm |
| Gross pathology |
|
|
| Hemorrhage | In larger lesions (>10cm) | Extremely rare |
| Calcification | 20 % usually due to hemorrhage |
Extremely rare |
| Microscopic picture | Mature fat cells & megakaryocytes | |
| Clinical Picture |
|
|
| Imaging key findings |
|
|
| Differential diagnosis | Adenoma |
|
| Prognosis | Excellent | Excellent |
| Indication for surgery |
|
|
ACKNOWLEDGEMENTS
The authors would like to thank Prof. Dr. Alexander Tzankov, Pathology Department, University Hospital Basel, Switzerland, for his kind permission to use the histopathology image.
ABBREVIATIONS
- AFIP
Armed Forces Institute of Pathology
- CECT
Contrast material Enhanced Computed Tomography
- CT
Computed tomography
- EAML
Extra-adrenal myelolipoma
- EMH
Extra-medullary Hematopoiesis
- FDG-PET
Fluordeoxyglucose Positron Emission Tomography
- FNA
Fine Needle Biopsy
- HU
Hounsfield Units
- MPR
Multiplanar reconstruction
- MRI
Magnetic resonance imaging
- NF
Neurofibroma
- STIR
Short-Tau Inversion Recovery
- SUV
Standardized Uptake Value
- WI
Weighted Images
REFERENCES
- 1.Federle M, Anne V. Adrenal Myelolipoma. In: Federle M, editor. Diagnostic Imaging: Abdomen. 1st ed. Salt lake: Amirsys; 2004. pp. III 2 24–25. [Google Scholar]
- 2.Gierke E. Uber Knochenmarksgewebe in der Nebenniere. Beitr Pathol Anat. 1905;7:311–25. [Google Scholar]
- 3.Oberling C. Les formation myelo-lipomateuses. Bull Assoc Fr Etud Cancer. 1929;18:234–246. [Google Scholar]
- 4.Rao P, Kenney PJ, Wagner BJ, Davidson AJ. Imaging and pathologic features of myelolipoma. Radiographics. 1997 Nov-Dec;17(6):1373–85. doi: 10.1148/radiographics.17.6.9397452. [DOI] [PubMed] [Google Scholar]
- 5.Arzanian MT, Khaleghnejad-Tabari A, Shamsian BS, Jadali F, Gharib A, Esfahani H. Generalized myelolipoma. Arch Iran Med. 2006 Jul;9(3):274–6. [PubMed] [Google Scholar]
- 6.Sawhney R, McRae B, Lazarchick J. A rare case of multifocal extra-adrenal myelolipoma with markedly hypocellular bone marrow. Ann Clin Lab Sci. 2006 Spring;36(2):208–11. [PubMed] [Google Scholar]
- 7.Gao B, Sugimura H, Sugimura S, Hattori Y, Iriyama T, Kano H. Mediastinal myelolipoma. Asian Cardiovasc Thorac Ann. 2002 Jun;10(2):189–90. doi: 10.1177/021849230201000227. [DOI] [PubMed] [Google Scholar]
- 8.Karam AR, Nugent W, Falardeau J, Desai D, Khan A, Shankar S. Multifocal extra-adrenal myelolipoma arising in the greater omentum. Radiol Case Rep. 2009 Oct;3(11):20–23. doi: 10.3941/jrcr.v3i11.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang KC, Chen PI, Huang ZH, Lin YM, Kuo PL. Adrenal myelolipoma with translocation (3;21)(q25;p11) Cancer Genet Cytogenet. 2002 Apr 1;134(1):77–80. doi: 10.1016/s0165-4608(01)00592-1. [DOI] [PubMed] [Google Scholar]
- 10.Bischop E, Eble JN, Cheng L, Wang M, Chase DR, Orazi A, O’Malley DP. Adrenal myelolipomas show nonrandom X-chromosome inactivation in hematopoietic elements and fat: support for a clonal origin of myelolipomas. Am J Surg Pathol. 2006 Jul;30(7):838–43. doi: 10.1097/01.pas.0000202044.05333.17. [DOI] [PubMed] [Google Scholar]
- 11.Blake MA, Chaoui AS, Barish MA. Thoracic case of the day. Thoracic myelolipomatosis. Am J Roentgenol. 1999 Sep;173(3):821, 823–4. doi: 10.2214/ajr.173.3.10470940. [DOI] [PubMed] [Google Scholar]
- 12.Krismann M, Reichle G, Müller KM. (Thoracic bilateral myelolipoma) Pneumologie. 1993 Aug;47(8):501–3. [PubMed] [Google Scholar]
- 13.Franiel T, Fleischer B, Raab BW, Füzesi L. Bilateral thoracic extraadrenal myelolipoma. Eur J Cardiothorac Surg. 2004 Dec;26(6):1220–2. doi: 10.1016/j.ejcts.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 14.Ramchandani P. Adrenal Myelolipoma Imaging. Medscape Reference. [Accessed Dec 4, 2012]. Updated: May 25, 2011. Available at: http://emedicine.medscape.com/article/376700-overview.
- 15.Prahlow JA, Loggie BW, Cappellari Jo, Scharling ES, Teot LA, Iskandar SS. Extra-adrenal myelolipoma: report of two cases. South Med J. 1995 Jun;88(6):639–43. doi: 10.1097/00007611-199506000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Kenny PJ, Wagner BJ, Rao O, Heffess CS. Myelolipoma: CT and pathologic features. Radiology. 1998 Jul;208(1):87–95. doi: 10.1148/radiology.208.1.9646797. [DOI] [PubMed] [Google Scholar]
- 17.Goldman S, Kenney P. The adrenal gland. In: LEE J, editor. Computed body tomography with MRI correlation. 4th ed. Philadelphia [Sl]: Lippincott Williams & Wilkins; 2006. pp. 1311–1373. [Google Scholar]
- 18.Otal P, Escourrou G, Mazerolles C, et al. Imaging features of uncommon adrenal masses with histopathologic correlation. Radiographics. 1999 May-Jun;19(3):569–81. doi: 10.1148/radiographics.19.3.g99ma07569. [DOI] [PubMed] [Google Scholar]
- 19.Kumar M, Duerinckx AJ. Bilateral extraadrenal perirenal myelolipomas: an imaging challenge. Am J Roentgenol. 2004 Sep;183(3):833–6. doi: 10.2214/ajr.183.3.1830833. [DOI] [PubMed] [Google Scholar]
- 20.Spizzirri A, Napolitano V, La Mura F, et al. (Presacral myelolipoma: a case report) G Chir. 2010 Oct;31(10):451–5. [PubMed] [Google Scholar]
- 21.Omdal DG, Baird DE, Burton BS, Goodhue WW, Jr, Giddens EM. Myelolipoma of the thoracic spine. Am J Neuroradiol. 1997 May;18(5):977–9. [PMC free article] [PubMed] [Google Scholar]
- 22.Orsola A, Raventos C, Trias I, Espanol I, Orsola I. Urinary retention secondary to presacral myelolipoma; first reported case diagnosed by prostate TUR and requiring a cystectomy. Int Urol Nephrol. 2005;37(4):717–9. doi: 10.1007/s11255-005-0928-3. [DOI] [PubMed] [Google Scholar]
- 23.Skucas J. Advanced Imaging of the Abdomen. 1st ed. London: Springer; 2006. Adrenals; pp. 953–974. [Google Scholar]
- 24.Oliva A, Duarte B, Hammadeh R, Ghosh L, Baker RJ. Myelolipoma and endocrine dysfunction. Surgery. 1988 Jun;103(6):711–5. [PubMed] [Google Scholar]
- 25.Miyazaki Y, Yoshida M, Doi J. (A case of adrenal myelolipoma associated with adrenogenital syndrome) Hinyokika Kiyo. 1990 Jan;36(1):35–9. [PubMed] [Google Scholar]
- 26.Ukimura O, Inui E, Ochiai A, Koljima M, Watanabe H. Combined adrenal myelolipoma and pheochromocytoma. J Urol. 1995 Oct;154(4):1470. [PubMed] [Google Scholar]
- 27.Sutton D, Robinson P. Textbook of radiology and Imaging. 7th ed. London: Churchill Livingstone; 2002. Adrenal glands; pp. 836–838. [Google Scholar]
- 28.Rossi M, Ravizza D, Fiori G, et al. Thoracic myelolipoma diagnosed by endoscopic ultrasonography and fine needle aspiration cytology. Endoscopy. 2007 Feb;39( Suppl 1):E114–5. doi: 10.1055/s-2007-966147. Epub 2007 Apr. 18. [DOI] [PubMed] [Google Scholar]
- 29.Vaziri M, Sadeghipour A, Pazooki A, Shoolami LZ. Primary mediastinal myelolipoma. Ann Thorac Surg. 2008 May;85(5):1805–6. doi: 10.1016/j.athoracsur.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 30.Savoye-Collet C, Goria O, Scotte M, Hemet J. MR imaging of hepatic myelolipoma. Am J Roentgenol. 2000 Feb;174(2):574–5. doi: 10.2214/ajr.174.2.1740574. [DOI] [PubMed] [Google Scholar]
- 31.Elsayes KM, Mukundan G, Narra VR, et al. Adrenal masses: MR imaging features with pathologic correlation. Radiographics. 2004 Oct;24( Suppl 1):S73–86. doi: 10.1148/rg.24si045514. [DOI] [PubMed] [Google Scholar]
- 32.Pereira JM, Sirlin CB, Pinto PS, Casola G. CT and MR imaging of extrahepatic fatty masses of the abdomen and pelvis: techniques, diagnosis, differential diagnosis, and pitfalls. Radiographics. 2005 Jan-Feb;25(1):69–85. doi: 10.1148/rg.251045074. [DOI] [PubMed] [Google Scholar]
- 33.Adam S, Liermann D, Mruck S. Functional and morphological imaging of extraadrenal myelolipoma. Clin Nucl Med. 2009 Apr;34(4):226–9. doi: 10.1097/RLU.0b013e31819a1f55. [DOI] [PubMed] [Google Scholar]
- 34.Alcala A, Potolicchio M, Mara L. Geniturinary, gynecology and obstetrical imaging. In: Ribes R, Luna A, Ros P, editors. Learning diagnostic imaging 100 essential cases. 1st ed. Berlin Heidelberg: Springer; 2008. pp. 104–105. [Google Scholar]
- 35.Armstrong P. Imaging of the diseases of the Chest. 3rd ed. London: Mosby; 2000. Mediastinal and hilar disorders; pp. 849–853. [Google Scholar]
- 36.Chen M. Extramedullary hematopoiesis. In: Ross J, editor. Diagnostic Imaging: Spine. Vol. 2. Salt lake: Amirsys; 2004. pp. 16–18. [Google Scholar]
- 37.Gurney J. Diagnostic Imaging: Chest. Salt lake: Amirsys; 2006. Extramedullary hematopoiesis, Mediastinum; pp. II 1 66–69. [Google Scholar]
- 38.Müller N, Rosado de Christenson M, Reed J, Section F. Chest. In: Reeder M, editor. Reeder and Felson’s Gamuts in Radiology comprehensive lists of roentgen differential diagnosis. 4th ed. New york: Springer; 2003. p. 565. [Google Scholar]
- 39.Chen M. Neurofibroma. In: Ross J, editor. Diagnostic Imaging: Spine. Salt lake: Amirsys; 2004. pp. IV 1 90–93. [Google Scholar]
- 40.Ravenel J. Nerve sheath tumors, Mediastinum. In: Gurney J, editor. Diagnostic Imaging: Chest. Salt Lake: Amirsys; 2006. pp. II 1 58–61. [Google Scholar]
- 41.Rha SE, Byun JY, Jung SE, Chun HJ, Lee HG, Lee JM. Neurogenic tumors in the abdomen: tumor types and imaging characteristics. Radiographics. 2003 Jan-Feb;23(1):29–43. doi: 10.1148/rg.231025050. [DOI] [PubMed] [Google Scholar]
- 42.Fortman BJ, Kuszyk BS, Urban BA, Fishman EK. Neurofibromatosis type 1: a diagnostic mimicker at CT. Radiographics. 2001 May-Jun;21(3):601–12. doi: 10.1148/radiographics.21.3.g01ma05601. [DOI] [PubMed] [Google Scholar]
- 43.Spanta R, Saleh HA, Khatib G. Fine needle aspiration diagnosis of extraadrenal myelolipoma presenting as a pleural mass. A case report. Acta Cytol. 1999 Mar-Apr;43(2):295–8. doi: 10.1159/000330997. [DOI] [PubMed] [Google Scholar]
- 44.Evans GW, Olinde HD, Kozdereli E. Extraadrenal myelolipoma. A lesion that can be diagnosed by fine needle aspiration biopsy. Acta Cytol. 1990 Jul-Aug;34(4):536–8. [PubMed] [Google Scholar]
- 45.Dusenbery D. Extraadrenal myelolipoma. Intraoperative cytodiagnosis on touch preparations. Acta Cytol. 1990 Jan-Feb;34(1):89–91. [PubMed] [Google Scholar]
- 46.Porcaro AB, Novella G, Ficarra V, Cavalleri S, Antoniolli SZ, Curti P. Incidentally discovered adrenal myelolipoma. Report on 3 operated patients and update of the literature. Arch Ital urol Androl. 2002 Sep;74(3):146–51. [PubMed] [Google Scholar]
- 47.Asuquo SE, Nguyen SQ, Scordi-Bello I, Divino CM. Laparoscopic management of presacral myelolipoma. JSLS. 2011 Jul-Sep;15(3):406–8. doi: 10.4293/108680811X13125733356792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chng SM, Lin MB, Ng FC, Chng HC, Khoo TK. Adrenal myelolipoma presenting with spontaneous retroperitoneal haemorrhage demonstrated on computed tomography and angiogram--a case report. Ann Acad Med Singapore. 2002 Mar;31(2):228–30. [PubMed] [Google Scholar]
- 49.Nakajo M, Onohara S, Shinmura K, Fujiyoshi F, Nakajo M. Embolization for spontaneous retroperitoneal hemorrhage from adrenal myelolipoma. Radiat Med. 2003 Sep-Oct;21(5):214–9. [PubMed] [Google Scholar]