Abstract
A 64 year-old male with metastatic prostate adenocarcinoma presented with bilateral hydronephrosis and renal impairment. Bilateral percutaneous nephrostomy drainage followed by ante-grade stenting was done. Shortly afterwards, the patient developed an extensive left-sided pleural effusion. His serum creatinine rose and he became anuric. Emergency pleural aspiration and later, pleural drainage were performed. Pleural aspirate was diagnostic of urinothorax and non contrast CT scan demonstrated a left reno-pleural fistula. The right stent was removed cystoscopically. The left stent could not be removed cystoscopically and was replaced in an ante grade manner through a fresh percutaneous renal approach. This led to cessation of pleural fluid accumulation. The patient was discharged with bilateral ureteric stents and normal renal function. A month later, he had normal renal function, no hydronephrosis and normal chest x-rays.
Keywords: Urinothorax, percutaneous nephrostomy, reno-pleural fistula, complication, prostate cancer, ureteral stent
CASE REPORT
A 64 year-old Caucasian male with previously diagnosed metastatic prostate adenocarcinoma (Gleason’s Grade 5+4, stage T 4 N1 M1- bone ), was re-referred with renal impairment of recent onset (serum creatinine 251 μmol/l) (normal range 49–92 μmol/l). This was investigated and found to be due to bilateral hydronephrosis (Fig 1), secondary to bilateral ureteric involvement by the prostate cancer at the trigone. Bilateral percutaneous nephrostomy drainage was carried out under ultrasound guidance, with the left kidney being accessed through a subcostal upper pole puncture (Fig. 2). Four days later, he underwent bilateral ante- grade stenting when 24 cm, 6 Fr stents were sited under x-ray control. The nephrostomy on the left side was removed, however the right nephrostomy was retained due to bleeding and intraluminal clots (Fig. 3). Shortly after this procedure, the patient experienced increasing dyspnoea and chest pain. His serum creatinine rose rapidly over the next 48 hrs to 445 μmol/l. Examination and imaging revealed an extensive left sided pleural effusion (Fig. 4). Initially he had a urine output of 835 ml from his right-sided nephrostomy and no urine output urethrally. However, the output from the right nephrostomy gradually tapered off such that finally there was no urine output at all. The high serum creatinine level observed was due to blockage of the right nephrostomy tube and both ureteric stents.
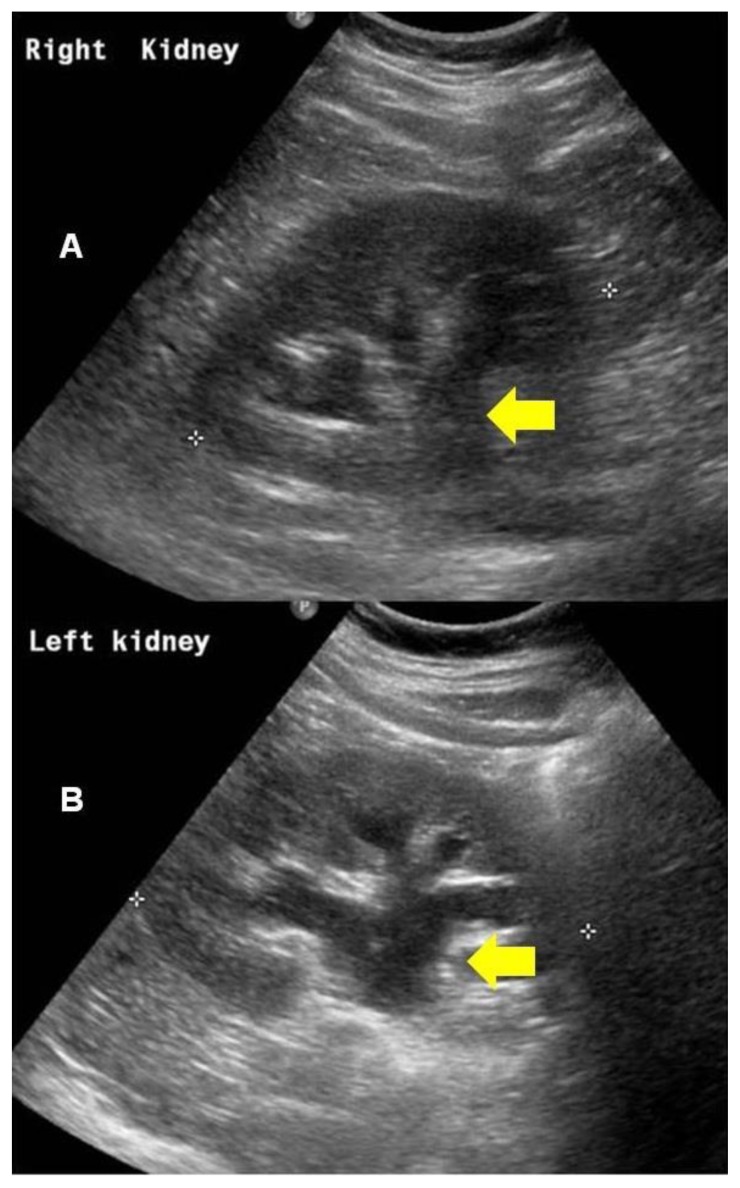
Figure 1.
Right kidney (A) and left kidney (B). 64 year old male with an iatrogenic left urinothorax. Renal ultrasound scans showing bilateral moderate hydronephrosis secondary to bilateral ureteric obstruction by prostate cancer at bladder trigone. Arrows show dialted renal collecting systems. (Transabdominal scan using a 2–5MHz curvilinear probe).
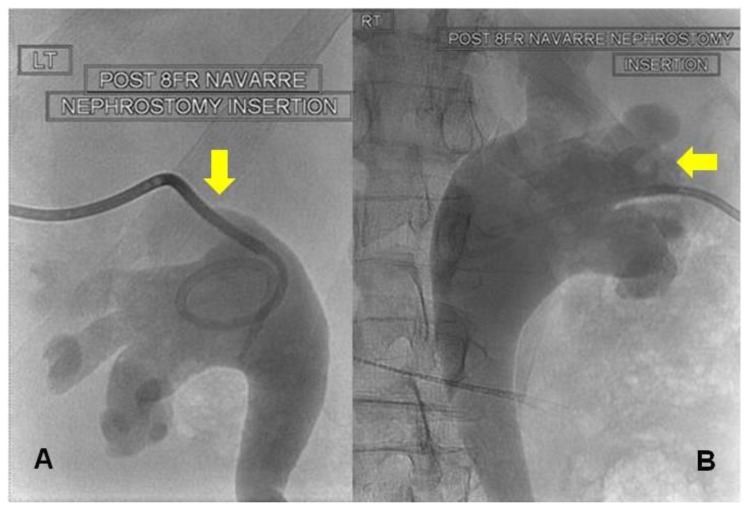
Figure 2.
64 year old male with an iatrogenic left urinothorax. Bilateral nephrostograms at initial nephrostomy placement with 8Fr Navarre nephrostomy tubes (patient prone). Note bilateral moderate hydroureteronephrosis and superior calyceal puncture on left (A). The puncture on the right is lower (B). Fluoroscopic images were obtained after the percutaneous injection of non-ionic water-soluble contrast material through the nephrostomy catheters.
Figure 3.
64 year old male with an iatrogenic left urinothorax. Nephrostogram after initial antegrade stenting (patient prone). No covering nephrostomy has been placed after stenting on the left. Contrast can be seen extravasating from the left superior calyx (arrows) and a large clot lies within the lumen of the dilated right ureter (arrowheads). Spot fluoroscopic image was obtained after the anterograde injection of non-ionic water-soluble contrast material.
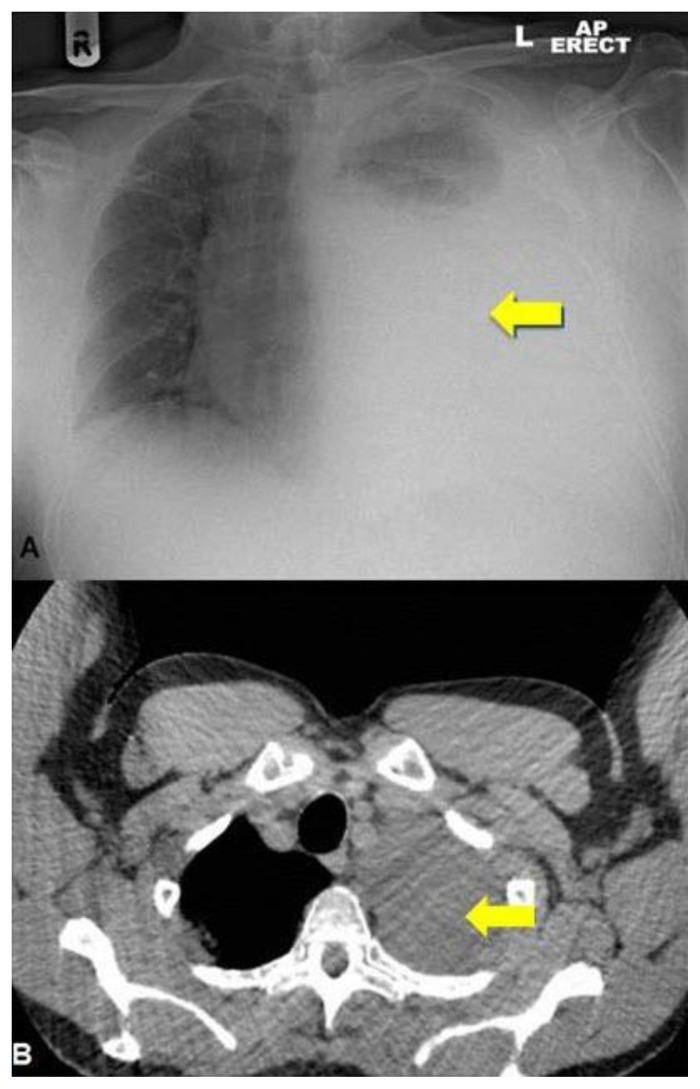
Figure 4.
Chest X-ray (A) and thoracic CT (B). 64 year old male with an iatrogenic left urinothorax. Composite thoracic x-ray and CT images showing the large urinothorax (arrows) which has increased in the lower image (4B). Erect AP Chest x-ray. CT protocol: unenhanced volumetric CT scan of the chest in the axial plane using a Phillips Brilliance 64 slice CT scanner.
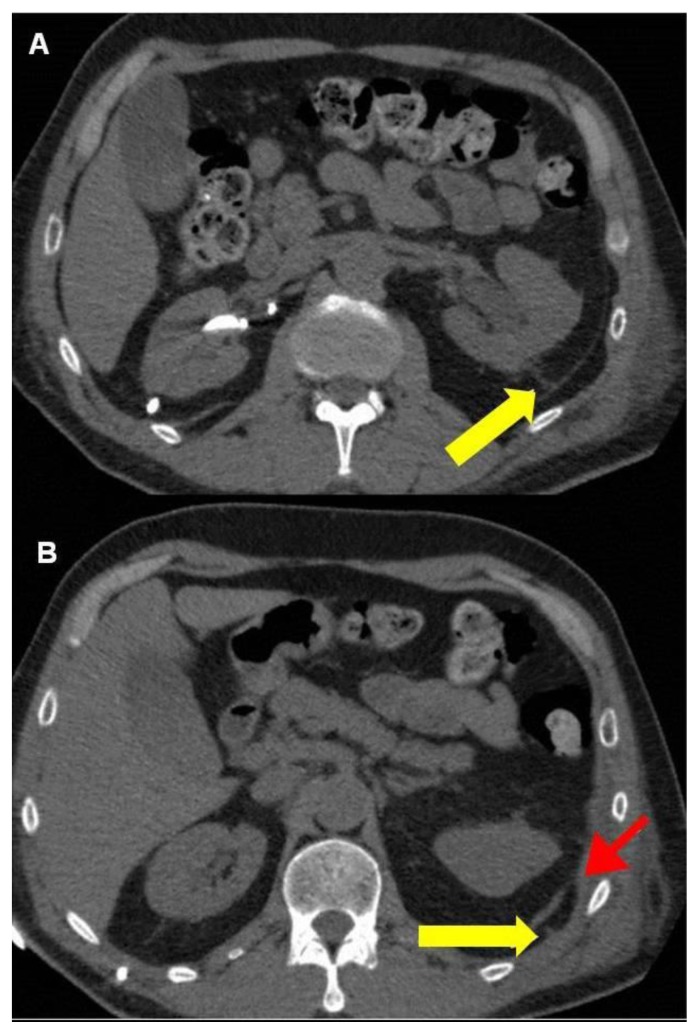
Pleural aspiration was performed and 3500ml of straw coloured fluid removed. As the effusion rapidly recurred, a wide bore chest drain was inserted but removed within a few minutes due to pain. Instead an 8 Fr pigtail catheter was inserted into the pleural cavity under ultrasound guidance. Pleural aspirate had a protein content of <10 g/l (normally < 25 g/l if transudative and >29 g/l if exudative) and a creatinine level of 1337 μmol/l, (serum creatinine 409 μmol/l) (normal pleural/ serum creatinine ratio is <1 ). This was diagnostic of urinothorax. Furthermore, non contrast CT scan demonstrated a fistula between the left kidney and the pleural cavity (Fig. 5).
Figure 5.
64 year old male with an iatrogenic left urinothorax. Non-contrast CT images showing the reno-pleural fistula. The track can be seen both in the retoperitoneum (arrow, A) in the thoracic cavity (yellow arrow, B). The red arrow in the lower image (B) points to the diaphragm where it attaches to the chest wall. (Protocol: Phillips Brilliance 64 slice CT scanner, 2 mm slice thickness, no intravenous, or oral contrast).
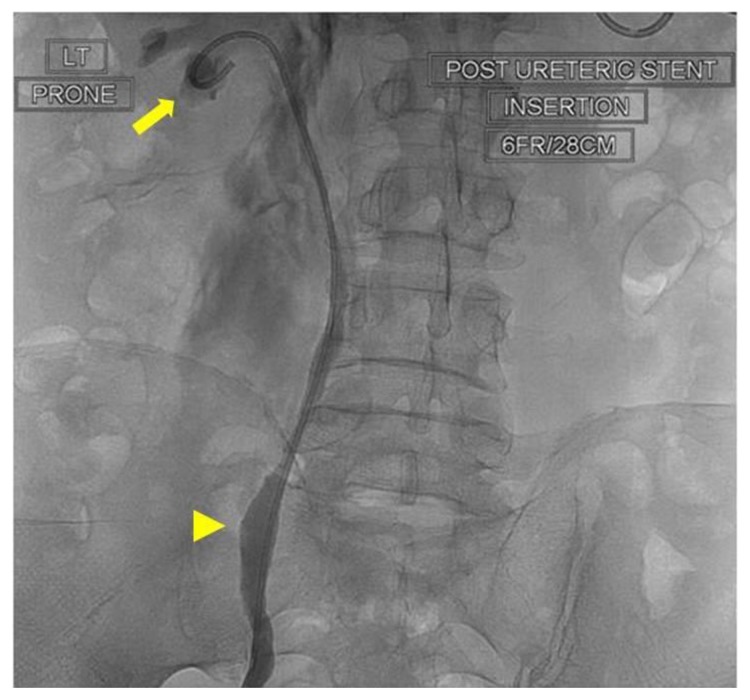
In view of the blockage, the right stent was removed at flexible cystoscopy. The left stent could not be removed at cystoscopy as the distal end was positioned in the ureter and not visible in the bladder. Therefore this stent was replaced in an ante grade manner through a percutaneous renal approach. The left kidney was accessed percutaneously through a lower pole calyx under ultrasound control. Under X-ray guidance, the previous stent was manoeuvred out through this track and replaced with a fresh 28 cm 6Fr stent (Fig 6). The blocked right nephrostomy tube was replaced simultaneously. This led to cessation of fluid accumulation in the pleura. Three days later, his right stent was replaced antegradely and the right nephrostomy tube removed. The patient was discharged from hospital with bilateral ureteric stents when his renal function returned to normal. A month later, he had normal renal function and no hydronephrosis. His symptoms resolved and chest x-rays did not show presence of pleural effusion or any other sequel.
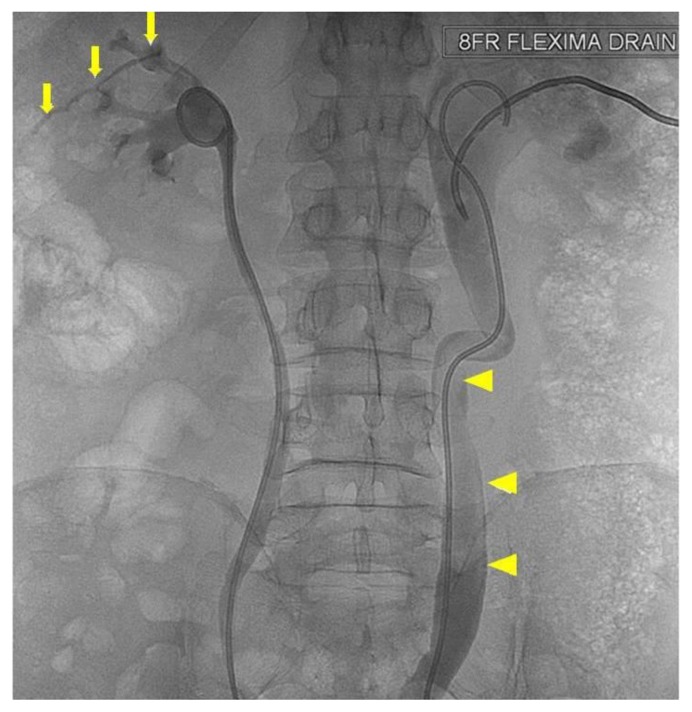
Figure 6.
64 year old male with an iatrogenic left urinothorax. Nephrostogram showing the left stent (6Fr, 28cm) (arrowhead) replaced through a lower calyx (arrow). Spot fluoroscopic image was obtained after the percutaneous injection of non-ionic water-soluble contrast material through the fresh (lower pole calyceal) puncture.
DISCUSSION
Urinothorax is the presence of urine in the pleural cavity. It is a rare and frequently unrecognised cause of transudative pleural effusion and may be classified as obstructive or traumatic [1]. It has been reported in the context of accidental [2] or iatrogenic [3] urinary system trauma, stone or other obstruction [4] and in extra-urinary malignancies like Hodgkin’s disease [5]. It may also occur after extracorporal shock wave lithotripsy [6] or following percutaneous nephrolithtomy[7]. Its occurrence has been documented in congenital urinary obstructive conditions like posterior urethral valves [8] and the VURD syndrome, comprising of congenital anomalies combining posterior urethral valves, unilateral vesicoureteral reflux and renal dysplasia [9].
Our patient developed urinothorax following a percutaneous nephrostomy in the presence of non draining stents. To our knowledge, only one case has been previously reported in a patient who developed urinothorax secondary to ureteric obstruction and a previous percutaneous nephrostomy [10].
Differential diagnosis includes pleural effusion, haemothorax, chylothorax or pulmonary consolidation. Chest X-ray by itself is unable to diagnose urinothorax as it only informs about pleural fluid accumulation but not the cause. Thus it is unable to differentiate between pleural effusions, haemothorax, chylothorax, urinothorax, and sometimes between pulmonary consolidations. Broken ribs, a widened mediastinum and pneumothorax may point to a haemothorax however.
Chest ultrasonography may demonstrate anechoic or hypoechoic area which moves on thoracic cycling and bounded by thoracic structures in patients with pleural effusion. Echoes may be present if chronic. Similarly, echoes or loculations may be present in haemothorax. Echoes may also be present in chylothorax due to lymphatic fluid. In pulmonary consolidation, a hypoechoic area with internal hyperechoic structures and an indistinct margin may occur.
While CT may diagnose pulmonary consolidation, it is likely to show a crescent-shaped attenuation in the dependent portion of the hemithorax in pleural effusion, chylothorax and haemothorax. In haemothorax, the presence of associated injuries or pneumothorax may be helpful. Although intravenous contrast enhanced CT may demonstrate a fistula in the case of urinothorax, in our patient we were able to demonstrate the reno-pleural fistula with non-contrast CT, a fact not mentioned in earlier reports.
Radionuclide renal scintigraphy using Tc 99m is also useful in demonstrating reno-pleural fistula[13]. Radionuclide scanning in pleural effusion is not specific. Increased activity in the hemithorax on Tc-99m methylene-diphosphonate (MDP) bone scans may be present in some malignant effusions, however. Similarly, a Tc-99m RBC scan would be diagnostic in haemothorax, but this modality is rarely ever used in practice for haemothorax. In chylothorax, Tc-99m lymphoscintigraphy can show abnormal lymphatic drainage and in pulmonary consolidation, hypermetabolic PET scans may be present.
In practice, diagnosis of urinothorax depends upon a high index of suspicion in the clinical context and on analysis of pleural fluid. The pleural fluid has the character of a transudate, low glucose and a creatinine concentration that is higher than creatinine levels in serum[11]. The average pleural fluid to serum creatinine ratio reported in 12 cases was 9.15 (range 1.09–19.8)[12]. In this patient this ratio was 3.27.
Prompt recognition of urinothorax and early re establishment of appropriate diversion or distal drainage are important principles of management.
The best approach for needle entry into the renal collecting system is via a subcostal oblique posterolateral approach into the end of a posterior lower-pole or middle-pole calyx under ultrasound or fluoroscopic control. However, in our patient, the initial percutaneous access to place a nephrostomy on the left was sub costal but through a superior calyx (fig 3). As the pleural reflection is lower on the left, in all likelihood the track transgressed the pleural cavity, in spite of using ultrasound guidance. Moreover, because the stent was not properly positioned in the bladder, distal urine drainage could not occur. It is conceivable that as distal drainage could not occur, the trans-pleural track persisted and a reno-pleural fistula formed. Other mechanisms by which retroperitoneal or intra-peritoneal fluid collections may spread to the thorax across an intact diaphragm are excess pressure leading to a direct transdiaphragmatic spread [14] or through lymphatics[2]. However, in this case the mechanism was probably secondary to the initial nephrostomy puncture as we were able to demonstrate the fistulous communication on CT.
The rapid reaccumulation of pleural fluid and worsening renal parameters led to a clinical suspicion of a urinothorax. This was confirmed by pleural fluid biochemical analysis which demonstrated protein content consistent with a transudate. Pleural fluid creatinine was markedly raised as compared to the serum creatinine and this was diagnostic.
Stent change was complicated by the fact that the left stent was not retrievable through cystoscopy. This led to the left kidney having to be percutaneously re-accessed. Furthermore, the old stent had to be manipulated proximally through the percutaneous route before it could be replaced. This was a technically challenging manouver and placed additional demands on the management of the case. Correct replacement of the stent re-established distal drainage and led to cure.
TEACHING POINT
Urinothorax may occur as a rare but important treatable complication of percutaneous nephrostomy despite image guidance. Hence patients need to be appraised of this complication when obtaining informed consent. Early diagnosis is critical as appropriate urological diversion and pleural drainage result in cure. Non contrast CT may be valuable in the diagnosis especially when the clinical situation does not permit the use of contrast.
Table 1.
Summary table for urinothorax
| Etiology | A rare cause of transudative pleural effusion occurring as an aftermath of ureteric obstruction or reno-ureteric trauma. |
| Incidence | Rare. Mostly described in literature as isolated case reports. |
| Gender ratio | No gender predilection described. |
| Age predilection | Varies with the underlying cause. Can occur in childhood when posterior urethral valves or the VURD syndrome are present. Most others are linked to the age of presentation of the primary condition |
| Risk Factors | Renal trauma, ureteric obstruction, stone disease or as a complication of procedures on the urinary tract. |
| Treatment |
|
| Prognosis |
|
| Findings on imaging |
|
Table 2.
Differential diagnosis table for urinothorax
| Differential Diagnosis | X-Ray | Ultrasound | CT | Radionuclide Study |
|---|---|---|---|---|
| Pleural effusion |
|
|
|
|
| Urinothorax |
|
|
|
|
| Haemothorax |
|
|
|
|
| Chylothorax |
|
|
|
|
| Pulmonary consolidation |
|
|
|
|
ABBREVIATIONS
- CT
Computed Tomography
- Fr
French
- MDP
Methylene diphosphonate
- PET
Positron Emission Tomography
- RBC
Red blood cell
- Tc
Technetium
- TNM
Tumour, Node, Metastasis
- VURD
posterior urethral Valves, Unilateral vesicoureteral Reflux and renal Dysplasia
REFERENCES
- 1.Garcia-Pachon E, Romero S. Urinothorax: a new approach. Curr Opin Pulm Med. 2006;12(4):259–63. doi: 10.1097/01.mcp.0000230628.65515.86. [DOI] [PubMed] [Google Scholar]
- 2.Lahiry SK, Alkhafaji AH, Brown AL. Urinothorax following blunt trauma to the kidney. J Trauma. 1978;18(8):608–10. doi: 10.1097/00005373-197808000-00010. [DOI] [PubMed] [Google Scholar]
- 3.Amro O, Webb-Smith F, Sunderji S. Urinothorax: a rare complication of total abdominal hysterectomy. Obstet Gynecol. 2009;114(2 Pt 2):482–4. doi: 10.1097/AOG.0b013e31819bda90. [DOI] [PubMed] [Google Scholar]
- 4.Izzo L, Caputo M, De TG, Izzo P, Bolognese A, Basso L. Urinoma and urinothorax: report of a case. Am Surg. 2008;74(1):62–3. [PubMed] [Google Scholar]
- 5.Karkoulias K, Sampsonas F, Kaparianos A, Tsiamita M, Tsoukalas G, Spiropoulos K. Urinothorax: an unexpected cause of pleural effusion in a patient with non-Hodgkin lymphoma. Eur Rev Med Pharmacol Sci. 2007;11(6):373–4. [PubMed] [Google Scholar]
- 6.Oguzulgen IK, Oguzulgen AI, Sinik Z, Kokturk O, Ekim N, Karaoglan U. An unusual cause of urinothorax. Respiration. 2002;69(3):273–4. doi: 10.1159/000063633. [DOI] [PubMed] [Google Scholar]
- 7.Handa A, Agarwal R, Aggarwal AN. Urinothorax: an unusual cause of pleural effusion. Singapore Med J. 2007;48(11):e289–e292. [PubMed] [Google Scholar]
- 8.Friedland GW, Axman MM, Love T. Neonatal “urinothorax” associated with posterior urethral valves. Br J Radiol. 1971;44(522):471–4. doi: 10.1259/0007-1285-44-522-471. [DOI] [PubMed] [Google Scholar]
- 9.Lee CC, Fang CC, Chou HC, Tsau YK. Urinothorax associated with VURD syndrome. Pediatr Nephrol. 2005;20(4):543–6. doi: 10.1007/s00467-004-1755-y. [DOI] [PubMed] [Google Scholar]
- 10.Deel S, Robinette E., Jr Urinothorax: a rapidly accumulating transudative pleural effusion in a 64-year-old man. South Med J. 2007;100(5):519–21. doi: 10.1097/01.smj.0000242789.87832.24. [DOI] [PubMed] [Google Scholar]
- 11.Stark DD, Shanes JG, Baron RL, Koch DD. Biochemical features of urinothorax. Arch Intern Med. 1982;142(8):1509–11. [PubMed] [Google Scholar]
- 12.Garcia-Pachon E, Padilla-Navas I. Urinothorax: case report and review of the literature with emphasis on biochemical diagnosis. Respiration. 2004;71(5):533–6. doi: 10.1159/000080642. [DOI] [PubMed] [Google Scholar]
- 13.Agranovich S, Cherniavsky E, Tiktinsky E, Horne T, Lantsberg S. Unilateral urinothorax due to nephropleural fistula detected on Tc-99m diethylenetriamine pentaacetic acid renal scintigraphy. Clin Nucl Med. 2008;33(12):889–91. doi: 10.1097/RLU.0b013e31818bf181. [DOI] [PubMed] [Google Scholar]
- 14.Gurtner B. [Urine in the wrong place: urothorax] Schweiz Rundsch Med Prax. 1994;83(2):30–5. [PubMed] [Google Scholar]