Abstract
Heterotopic ossification is a benign process of mature laminar bone formation in the soft tissues. A synonymous term used to describe this pathology in muscle is myositis ossificans. The pathogenesis is unclear, but is likely multifactorial. The basic pathology is thought to be ectopic production of osseous tissue as part of a repair process in response to tissue injury. This report describes a case of heterotopic ossification of the quadratus lumborum muscle as an incidental finding. This case highlights that treatment is based on symptoms and conservative management might be appropriate for the asymptomatic patient.
Keywords: Quadratus lumborum muscle, idiopathic ossification, imaging, computed tomography
CASE REPORT
This 29-year-old male presented to the emergency department complaining of left flank and groin pain. He described the pain as being of sudden onset and rated it 7/10 in severity. There was no recollection of a traumatic event to this area. He did not have any significant past medical or surgical history.
Physical exam revealed moderate tenderness of the left flank area but was otherwise unremarkable. Laboratory values showed a slight WBC elevation of 13.5 ×109/L (normal range: 4.0 – 11.0 ×109/L) and a mild increase in creatinine at 152 umol/L (normal range: 60 – 104 umol/L). Urinalysis revealed a trace of hemoglobin, with no evidence of bacturia. Serum calcium and phosphate levels were not ordered in the emergency setting.
Computed Tomography (CT) renal calculus study demonstrated a dense 5 mm diameter calcification in the cranial end of the left kidney at the corticomedullary junction. There was no evidence of hydronephrosis, perinephric stranding or perinephric edema and the left kidney was not enlarged. The upper left ureter was mildly dilated with a dense 3 mm diameter calcification in the distal left ureter, consistent with mild hydroureter. This calcification was located approximately 8 cm cranial to the ureterovesicular junction. The calcifications were consistent with collecting system calculi.
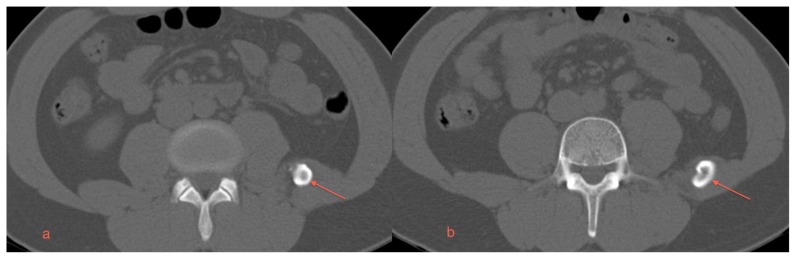
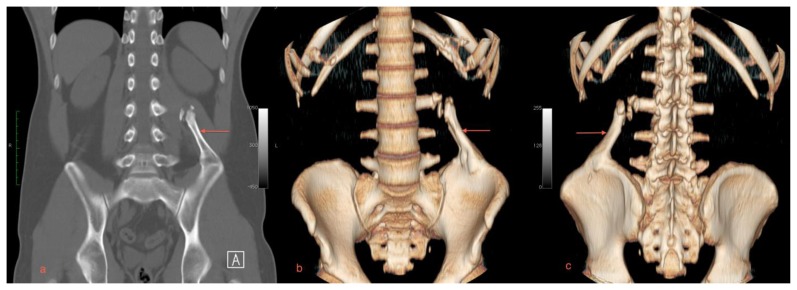
Axial CT images also demonstrated a focus of mature bone in the quadratus lumborum muscle (Figures 1a, 1b). Reformatted CT images revealed a mass measuring 10 cm × 3.2 cm (craniocaudal by transverse) arising from the left iliac crest, extending obliquely superiormedially towards the left L3 transverse process (Figures 2a, 2b, 2c). There were small corticated calcifications seen between the mass and the L3 transverse process. The patient was treated for renal colic with IV hydration and analgesics and discharged following relief of his pain.
Figure 1.
A 29 year old male with myositis ossificans of the quadratus lumborum muscle. Protocol: Non-contrast enhanced CT, Siemens Somatom. Images were acquired kVp 120, mAs 51, helical acquisition, pitch 0.8, the original image collimation was 1.2 mm, the final images were reformatted to 5 mm thickness.
a) An axial CT scan in the mid-portion of the abnormality reveals a thick cortical type of bone and a central lucency within the region of ossification (arrow).
b) An axial CT scan in the caudal aspect of the ossification near the junction with the left iliac bone again reveals dense cortical type bone with more lucent bone centrally (arrow).
Figure 2.
A 29 year old male with myositis ossificans of the quadratus lumborum muscle. Protocol: Non-contrast enhanced CT, Siemens Somatom. Images were acquired kVp 120, mAs 51, helical acquisition, pitch 0.8, the original image collimation was 1.2 mm, the final images were reformatted to 5 mm thickness.
a) A coronal plane MIP reformat demonstrates the mature bone arising from the left iliac crest projecting toward the upper lumbar spine (arrow).
b) An anteriorly oriented 3D volume rendered CT image shows the relationship of the quadratus lumborum lesion with the L3 transverse process (arrow).
c) A posteriorly oriented 3D volume rendered CT image shows the relationship of the quadratus lumborum lesion with the L3 transverse process (arrow).
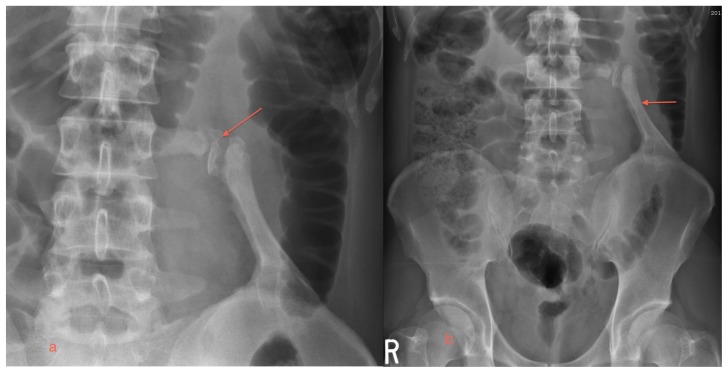
The patient returned to the emergency department two days later with a similar episode. A plain x-ray of the abdomen did not reveal any calculi and note was made of the mature bone mass arising from the left iliac crest with the corticated fragments between the mass and the transverse process of L3 (Figures 3a, 3b). The patient was treated conservatively and discharged pain free.
Figure 3.
A 29 year old male with myositis ossificans of the quadratus lumborum muscle.
a) A magnified view of the L3 pseudoarthrosis is provided (arrow).
b) The plain x-ray of the abdomen reveals abnormal ossified mass arising from the left iliac crest forming a pseudoarthrosis with the L3 transverse process (arrow).
At one year follow up the patient remains asymptomatic with no pain, restriction of motion or impaired function.
DISCUSSION
This case report demonstrates a rare location of presumed Heterotopic Ossification (HO). The patient had no recollection of previous trauma or any other health issues. The ossification arises from the left iliac crest and involved the majority of the left Quadratus Lumborum (QL) muscle.
Review of the literature revealed only one other reported case of HO in the same anatomical location of the QL - this was associated with a history of local trauma in the region of ossification [1].
HO is the term used to describe the process of mature laminar bone formation in soft tissues. The synonymous term used to describe the same pathology specifically located in muscle is Myositis Ossificans (MO). Both HO and MO are used interchangeably in the literature. MO is a self limiting, benign condition that usually affects young adults [2]. Men are known to develop ossifications more frequently than women [3,4]. Studies have found an increased incidence of two to three times greater in men compared to women [4]. The incidence is dependent upon classification of the ossification (posttraumatic, nontraumatic and myositis ossificans progressiva). The incidence of posttraumatic ossification has been cited by location and type of injury ranging from 0.6 to 100%. The incidence of nontraumatic ossification depends on the type of pathology and ranges from 3.4 to 89%. Myositis ossificans progressiva is a rare hereditary metabolic bone disease and occurs 1 in 2 million live births [5].
The pathogenesis of MO is unclear, but is likely multifactorial. The basic pathology is thought to be ectopic production of osseous tissue as part of a repair process in response to tissue injury [6]. Posttraumatic ossification most commonly affects young athletes [5]. Current understanding is that tissue injury initiates transformation of pluripotent mesenchymal cells into osteoblasts resulting in ectopic bone formation [7]. The four factors necessary for this process to occur are an inciting event, a protein signal from the site of injury, adequate supply of mesenchymal cells, and an appropriate environment for growth [8]. Factors shown to be important in this process are hypercalcemia, prolonged immobilization, parathyroid hormone and calcitonin imbalance, tissue hypoxia, and sympathetic nervous system dysfunction [9].
MO is associated with a number of conditions, but most commonly follows traumatic injuries such as spinal cord injury, hip surgery, traumatic brain injury, and burns [4]. The most common locations include the arm, shoulder, thigh and hand. MO typically involves a single muscle or muscle group [10]. Although the patient in this case did not recall any traumatic event to this area, injury of the QL muscle is often implicated as the cause of mechanical lower back pain. With MO the traumatic event can be minimal and may consist of only a small number of torn muscle or collagen fibers [8].
Management of MO is dependent upon the patient’s symptoms. If asymptomatic, MO can be treated conservatively. Once significant impairment of functional capacity is reached, surgical excision becomes the lone treatment option [11].
The differential diagnostic considerations for this pattern of calcification includes pelvic digits, and less likely, but more worrisome, extraskeletal osteosarcoma and chondrosarcoma.
The pelvic digit is a rare, benign congenital anomaly in which bone forms in the soft tissues near the pelvis. The bone closely resembles a digit and often will include one or more segments and pseudoarticulations. It is thought that the pelvic digit originates from the mesenchymal stage of bone growth during the first six weeks of embryogenesis [12]. As the name implies the most common location is within the pelvis, but similar lesions have been reported in the lower ribs and abdominal wall [13]. Most pelvic digits are found incidentally and do not require treatment.
Extraskeletal osteosarcoma and chondrosarcoma must be ruled out as the prognosis and treatment are radically different -- depending on the stage and subtype of the cancer treatment may require surgery, chemotherapy, radiation or a combination of these modalities.
Imaging features can help distinguish MO from other pathologies. The most important diagnostic feature of mature MO by CT scan is mature peripheral mineralization with central lucency.
The radiologic appearance of a pelvic digit varies, usually resembling a digit with multiple segments and pseudoarticulations [13].
The peripheral mineralization associated with central lucency distinguish MO from osteosarcoma [14] which is the most frequent misdiagnosis [15]. Further testing by biopsy can be avoided if this pattern of ossification is demonstrated by CT [16]. Careful interpretation of the imaging appearance alone may help avoid a false positive pathological diagnosis of osteosarcoma as a biopsy from the central portion of the lesion may be very cellular, show abundant osteoid and mitotic activity resembling osteosarcoma [17,18]. Osteosarcomas vary in appearance, commonly having lytic and sclerotic areas also known as a ‘moth eaten’ pattern. Lesions are ill defined and can involve the periosteum and adjacent soft tissues [19]. CT is useful for further delineating the intra and extra-osseous components of the tumor and characterizing the mineralization of the osseous matrix. CT is used for staging the tumor [19].
Chondrosarcomas are commonly lytic lesions with destructive cortical thickening or thinning. Endosteal scalloping may be present [20]. CT is useful for further delineating intraosseous chondroid matrix mineralization, extraosseous tumor extension and associated soft tissue mass [21].
In this case, the ossification was an incidental finding and a diagnosis of MO is favored. The patient was asymptomatic and did not warrant an invasive biopsy for definite diagnosis. The ossification was specifically confined to the anatomic location of the QL muscle - this strongly supports the primary pathology originating in muscle. By contrast, pelvic digits do not correspond to the anatomy of individual muscles. The center of the lesion is radiolucent and has a well-defined cortex, supporting the diagnosis of MO. Pelvic digits vary in location, shape, number of bony structures and pseudoarticulations. This lesion is larger in size and does not resemble a phalanx as most cases described in the literature. There are small bony fragments proximally, which could be interpreted as pseudoarticulations, avulsion of bony fragments from the primary lesion, or as possible ossifications within the QL tendon. Although a pelvic digit is a possibility, the diagnosis of MO was favored. The benign circumscription, extra osseous location and clinical course effectively exclude a diagnosis of osteosarcoma and chondrosarcoma.
This case demonstrates that MO of the QL muscle can be managed conservatively in an asymptomatic patient. The use of further invasive diagnostic studies and treatment is unnecessary for such patients. This is in sharp contrast with the only other reported case of MO of the QL muscle in the literature, where surgical excision and radiation therapy were employed for a symptomatic patient [1]. Although spontaneous regression and resolution of these lesions has been reported to occur, the actual occurrence of this may be only 30% [22].
TEACHING POINT
Myositis ossificans can occur in the distribution of the quadratus lumborum muscle and can be diagnosed with imaging alone. Treatment is based on symptoms and conservative management might be appropriate for the asymptomatic patient.
Table 1.
Summary table for Heterotopic Ossification
| Etiology |
|
|
| Gender Ratio |
|
|
| Age Predilection |
|
|
| Incidence |
|
|
| Risk factors |
|
|
| Treatment |
|
|
| Prognosis |
|
|
| Findings of Imaging | X-ray | An area of mature peripheral ossification and central lucency. Lesion is only visualized on plain films when mature. |
| CT | Mature peripheral mineralization with central lucency. | |
Table 2.
Differential diagnosis table for Heterotopic Ossification
| X-ray | CT | |
|---|---|---|
| Myositis Ossificans |
|
|
| Pelvic Digit |
|
|
| Osteosarcoma |
|
|
| Chondrosarcoma |
|
|
ABBREVIATIONS
- CT
Computed Tomography
- HO
Heterotopic Ossification
- QL
Quadratus Lumborum
- MO
Myositis Ossificans
REFERENCES
- 1.Karam M, McKinley T. Traumatic heterotopic ossification of the quadratus lumborum: a case report. Iowa Orthopaedic Journal. 2008;28:88–90. [PMC free article] [PubMed] [Google Scholar]
- 2.Nishio J, Nabeshima K, Iwasaki H, Naito M. Non-traumatic myositis ossificans mimicking a malignant neoplasm in an 83-year-old woman: a case report. J Med Case Rep. 2010;4:270. doi: 10.1186/1752-1947-4-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vanden Bossche L, Vanderstraeten G. Heterotopic ossification: a review. J Rehabil Med. 2005 May;37(3):129–36. doi: 10.1080/16501970510027628. [DOI] [PubMed] [Google Scholar]
- 4.Sawyer J, Myers M, Rosier R, Puzas JE. Heterotopic ossification: clinical and cellular aspects. Calcified Tissue International. 1991 Sep;49(3):208–15. doi: 10.1007/BF02556120. [DOI] [PubMed] [Google Scholar]
- 5.Mavrogenis AF, Soucacos PN, Papagelopoulos PJ. Heterotopic ossification revisited. Orthopedics. 2011;34(3):177. doi: 10.3928/01477447-20110124-08. [DOI] [PubMed] [Google Scholar]
- 6.Isaacson B, Stinstra J, MacLeod R, Pasquina P, Bloebaum R. Developing a quantitative measurement system for assessing heterotopic ossification and monitoring the bioelectric metrics from electrically induced osseointegration in the residual limb of service members. Annals of Biomedical Engineering. 2010 Sep;38(9):2968–78. doi: 10.1007/s10439-010-0050-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hastings H, Graham T. The classification and treatment of heterotopic ossification about the elbow and forearm. Hand Clinics. 1994 Aug;10(3):417–37. [PubMed] [Google Scholar]
- 8.McCarthy E, Sundaram M. Heterotopic ossification: a review. Skeletal Radiology. 2005 Oct;34(10):609–19. doi: 10.1007/s00256-005-0958-z. [DOI] [PubMed] [Google Scholar]
- 9.Balboni T, Gobezie R, Mamon H. Heterotopic ossification: Pathophysiology, clinical features, and the role of radiotherapy for prophylaxis. International Journal of Radiation Oncology, Biology, Physics. 2006 Aug;65(5):1289–99. doi: 10.1016/j.ijrobp.2006.03.053. [DOI] [PubMed] [Google Scholar]
- 10.Aneiros-Fernandez J, Caba-Molina M, Arias-Santiago S, Ovalle F, Hernandez-Cortes P, Aneiros-Cachaza J. Myositis ossificans circumscripta without history of trauma. J Clin Med Res. 2010;2:142–144. doi: 10.4021/jocmr2010.05.364w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Subbarao J, Garrison S. Heterotopic ossification: diagnosis and management, current concepts and controversies. Journal of Spinal Cord Medicine. 1999;22(4):273–83. doi: 10.1080/10790268.1999.11719580. [DOI] [PubMed] [Google Scholar]
- 12.Goyen M, Barkhausen J, Markschies NA, Debatin JF. The pelvic digit--a rare developmental anomaly. A case report with CT correlation and review of the literature. Acta Radiol. 2000;41:317–319. doi: 10.1080/028418500127345569. [DOI] [PubMed] [Google Scholar]
- 13.Nguyen VD, Matthes JD, Wunderlich CC. The pelvic digit: CT correlation and review of the literature. Comput Med Imaging Graph. 1990;14:127–131. doi: 10.1016/s0895-6111(05)80047-3. [DOI] [PubMed] [Google Scholar]
- 14.Ginedele A, Schwamborn D, Tsironis K, Bentz-Bohm G. Myositis ossificans traumatica in young children: report of three cases and review of the literature. Pediatr Radiol. 2000;30:451–9. doi: 10.1007/s002479900168. [DOI] [PubMed] [Google Scholar]
- 15.Micheli A, Trapani S, Brizzi I, Campanacci D, Resti M, de Martino M. Myositis ossificans circumscripta: a pediatric case and review of the literature. Eur J Pediatr. 2009 May;168(5):523–529. doi: 10.1007/s00431-008-0906-8. [DOI] [PubMed] [Google Scholar]
- 16.Parikh J, Hyare H, Saifuddin A. The imaging features of post-traumatic myositis ossificans, with emphasis on MRI. Clin Radiol. 2002 Dec;57:1058–1066. doi: 10.1053/crad.2002.1120. [DOI] [PubMed] [Google Scholar]
- 17.Kransdorf MJ, Meis JM, Jelinek JS. Myositis ossificans: MR appearance with radiologic-pathologic correlation. AJR Am J Roentgenol. 1990;157:243–248. doi: 10.2214/ajr.157.6.1950874. [DOI] [PubMed] [Google Scholar]
- 18.Resnick D, Niwayama G. Soft tissues. In: Resnick D, editor. Diagnosis of bone and joint disorders. 3rd ed. Philadelphia, Pa: Saunders; 1995. pp. 4491–4622. [Google Scholar]
- 19.White LM, Kandel R. Osteoid-producing tumors of bone. Semin Musculoskelet Radiol. 2000;4(1):25–43. doi: 10.1055/s-2000-6853. [DOI] [PubMed] [Google Scholar]
- 20.Ollivier L, Vanel D, Leciere J. Imaging of chondrosarcomas. Cancer Imaging. 2003 Oct 22;4(1):36–38. doi: 10.1102/1470-7330.2003.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Littrell LA, Wenger DE, Wold LE, et al. Radiographic CT, and MR imaging features of dedifferentiated chondrosarcomas: a retrospective review of 174 de novo cases. Radiographics. 2004 Sep-Oct;24(5):1397–409. doi: 10.1148/rg.245045009. [DOI] [PubMed] [Google Scholar]
- 22.Wang XL, Malghem J, Parizel PM, Gielen JL, Vanhoenacker F, De Schepper AM. Myositis ossificans circumscripta. JBR - BTR. 2003 Sep-Oct;86(5):278–285. [PubMed] [Google Scholar]