Abstract
Congenital adrenal hyperplasia refers to a group of autosomal recessive disorders caused by a deficiency of an enzyme involved in the synthesis of glucocorticoids. The enzyme deficiency generally leads to a deficiency of cortisol and/or aldosterone production within the adrenal cortex. The lack of glucocorticoids generally leads to elevated levels of plasma corticotropin (ACTH), which often results in adrenal hyperplasia. Testicular adrenal rest tumors may develop in males with congenital adrenal hyperplasia due to overstimulation of aberrant adrenal cells within the testes. Recognition of this disease entity is essential when evaluating young males with testicular masses.
Keywords: Congenital adrenal hyperplasia, testicular adrenal rest tumors, testicular ultrasonography
CASE REPORT
A 27 year old male presented to the Urology Department with bilateral testicular fullness and right sided testicular pain that had become progressively severe over the course of several months. The patient was diagnosed with congenital adrenal hyperplasia at age 3 weeks. He had been receiving steroid supplementation over the course of his life, but admitted to being poorly compliant during his teens. He had noticed that his testes felt “lumpy and bumpy” for several years prior to seeking medical care. However, an ultrasound was never performed since the patient had a strong reluctance to seek medical care for fear of receiving an unfavorable prognosis. The patient eventually sought medical care for infertility and testicular pain in his mid twenties at a different facility two years prior to presentation at our facility. He was found to be hypogonadal with azoospermia, and was undergoing B-HCG injections at the time of presentation.
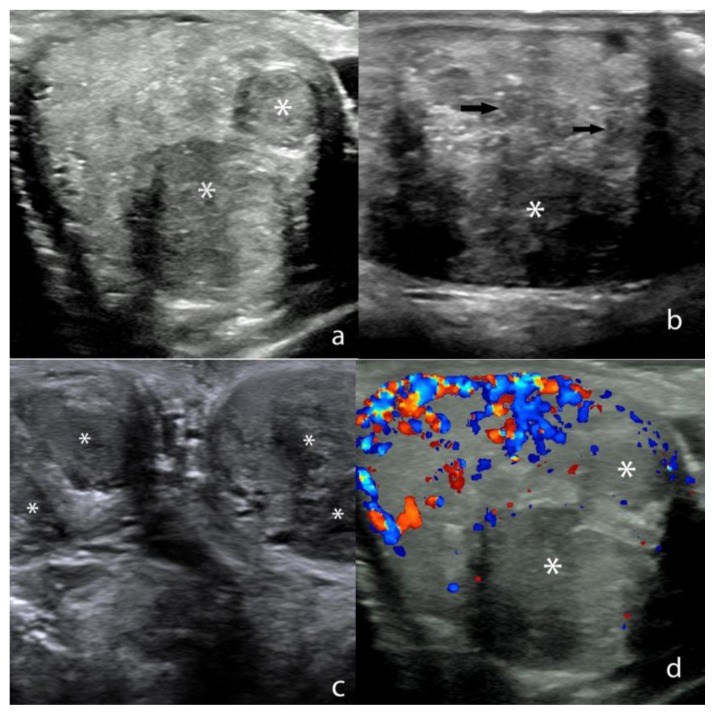
A scrotal ultrasound was subsequently performed for the evaluation of bilateral testicular pain which revealed multiple sharply marginated, hypoechoic masses throughout both testes that was estimated to occupy approximately 70–80% of the testicular parenchyma (Fig. 1). The lesions were mildly heterogeneous and ranged in size from 2 to 18 mm. The lesions were predominantly round and partially coalescent. Doppler interrogation demonstrated no color flow within the majority of the lesions with mild perilesional flow involving a small minority of lesions (Fig. 1). Limited sonographic evaluation of the abdomen was subsequently performed that revealed bilateral adrenal gland enlargement (Fig. 2).
Figure 1.
27 year old male with testicular adrenal rest tumors.
a) Transverse Gray-scale ultrasound image through the right testis shows sharply marginated masses (*) that are hypoechoic relative to the normal background testicular parenchyma. Technique: 18 MHz high frequency linear-array transducer (Acuson).
b) Sagittal Gray-scale ultrasound image through the left testis shows a dominant hypoechoic mass (*) with multiple other smaller, partially coalescent hypoechoic lesions scattered throughout the testis (arrows). Technique: 18 MHz high frequency linear-array transducer (Acuson).
c) Transverse Gray-scale ultrasound image through both testes shows multiple bilateral, partially coalescent hypoechoic masses (*). Technique: 18 MHz high frequency linear-array transducer (Acuson).
d) Transverse ultrasound image through the right testis with color Doppler interrogation shows hypoechoic masses (*) that show little to no vascular flow. Normal color Doppler flow is noted within the uninvolved testicular parenchyma. Technique: 18 MHz high frequency linear-array transducer (Acuson).
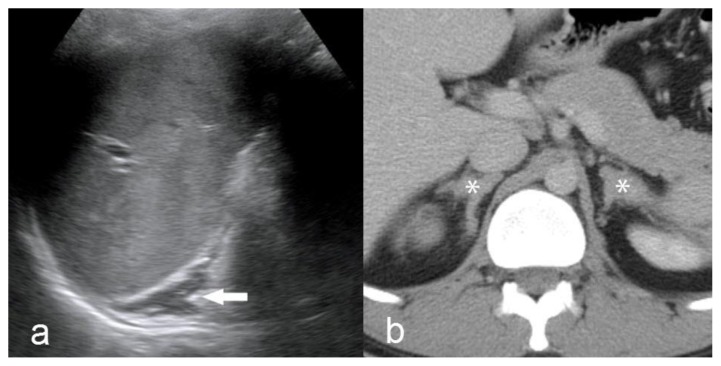
Figure 2.
27 year old male with testicular adrenal rest tumors.
a) Sagittal Gray-scale ultrasound image of the left upper quadrant shows diffuse, uniform enlargement of the left adrenal gland (arrow). Technique: 4.5 MHz vector transducer (Acuson). b) Contrast enhanced axial CT in the portal venous phase shows bilateral, symmetric enlargement of the adrenal glands (*). Technique: CT (Siemens Somatom) mA 380, kV 120, slice thickness 5 mm, 150 ml Isovue-300 IV contrast, portal venous phase.
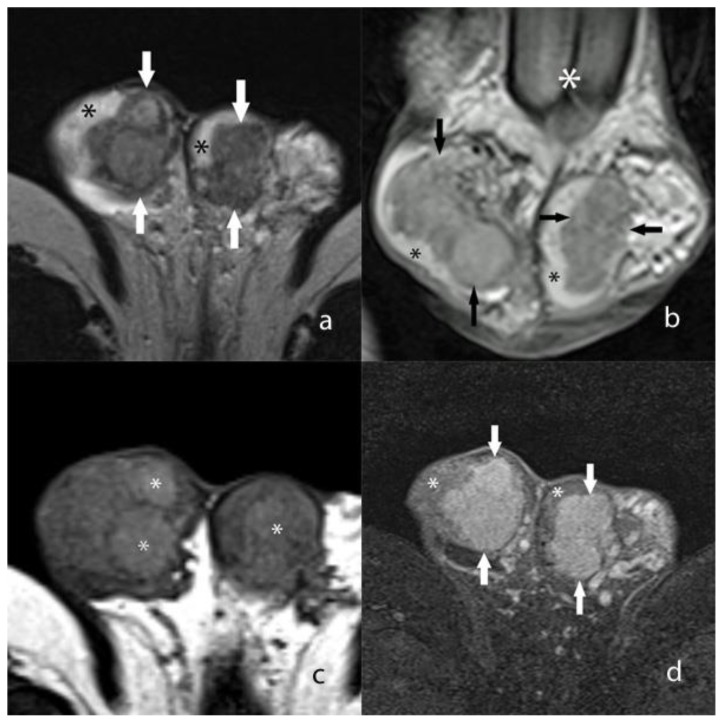
A CT scan of the abdomen was subsequently performed in order to further evaluate the adrenal enlargement that was discovered on the testicular ultrasound. The CT scan revealed diffuse, irregular enlargement of both adrenal glands (Fig. 2). However, both adrenal glands maintained an adreniform shape. An MRI of the scrotum was likewise performed for further characterization of the testicular masses which revealed multiple, predominantly coalescent lesions throughout both testes. The lesions were well visualized on T2 weighted images as mildly heterogeneous masses that were hypointense relative to the background testicular parenchyma (Fig. 3). On unenhanced T1 weighted images, the masses appeared heterogeneous with a few discrete hyperintense foci, although the margins of the masses were very poorly visualized (Fig. 3). However, the masses demonstrated avid, uniform enhancement with discrete margins that were readily visualized on T1 images following intravenous gadolinium administration (Fig. 3). Overall, the margins of the masses were optimally depicted on MR when compared to the prior US. Additionally, the MR demonstrated more extensive involvement (>90% of both testes) of the background parenchyma when compared to the US.
Figure 3.
27 year old male with testicular adrenal rest tumors.
a) Axial T2 weighted MR image shows heterogeneous, coalescent masses (arrows) that occupy the majority of the testes. A small portion of the uninvolved hyperintense background testicular parenchyma is seen (*). Technique: 1.5 Tesla MRI (Siemens Avanto) TR/TE 5750/86, 5 mm slice thickness, non-contrast.
b) Coronal T2 weighted MR image shows heterogeneous, coalescent masses (arrows) that are hypointense relative to the background testicular parenchyma (black asterisk). The base of the penis is seen on this image (white asterisk). Technique: 1.5 Tesla MRI (Siemens Avanto) TR/TE 5750/86, 5 mm slice thickness, non-contrast.
c) Unenhanced axial T1 weighted MR image without fat saturation shows diffuse heterogeneity throughout both testes which contain discrete hyperintense foci (*). However, the outer tumor margins are not well visualized. Technique: 1.5 Tesla MRI (Siemens Avanto) TR/TE 514/17, 5 mm slice thickness, non-contrast.
d) Axial T1 weighted MR image with fat saturation following intravenous gadolinium administration shows avidly enhancing masses (arrows) within both testes. A small portion of uninvolved testicular parenchyma is seen (*). Technique: 1.5 Tesla MRI (Siemens Avanto) TR/TE 514/15 with fat saturation, 3 mm slice thickness following 2 ml/kg Multihance, late arterial phase.
Due to the unremitting, debilitating pain in his right testis, his urologist recommended a right orchiectomy for pain alleviation, in spite of the fact that a diagnosis of testicular adrenal tumors was strongly suspected based on imaging findings. Upon pathologic review, the intratesticular lesions were initially interpreted as Leydig cell tumors. However, further review at an outside facility was requested, which concluded that these lesions were most consistent with testicular tumors of the adrenogenital syndrome. Unfortunately, the patient’s reported right groin pain did not regress following right orchiectomy. He was subsequently referred to a neurosurgeon for evaluation of a possible radiculopathy or peripheral neuropathy as a source of pain, but was eventually lost to follow up.
DISCUSSION
Etiology and demographics
Congenital adrenal hyperplasia (CAH) is an autosomal recessive genetic disorder that is caused by a deficient 21-?- hydroxylase (CYP21) enzyme in more than 90% of cases [1]. The incidence of 21-?-hydroxylase congenital adrenal hyperplasia detectable in childhood is roughly 1 in 15,000 births [2]. The CYP21 enzyme is essential for the normal production of cortisol and aldosterone. Individuals with this disorder have low levels of both glucocorticoids and mineralocorticoids, which leads to excess adrenocorticotropin (ACTH) secretion by the pituitary gland. Excess levels of ACTH result in adrenal hyperplasia as overstimulated adrenal glands produce large amounts of steroids that have little glucocorticoid or mineralocorticoid activity [3]. Impaired cortisol and aldosterone production may result in an Addisonian crisis with salt wasting and dehydration in the neonate [1]. An overproduction of adrenal androgens often leads to prenatal virilization of the female external genitalia.
Testicular lesions in the setting of CAH were first described by Wilkins et al in 1940 [4]. These lesions are commonly referred to as testicular adrenal rest tumors (TART) in the endocrinology and radiology literature or testicular tumors of the adrenogenital syndrome in the pathology literature [5]. It has been hypothesized that testicular adrenal rest tumors arise from aberrant adrenal cells that descend with the testes during embryogenesis. [6] Testicular adrenal rest tumors are benign, and are frequently multiple and bilateral. The tumors are most prevalent in younger adult males with a peak incidence between 20–40 years of age [7].
Clinical and imaging findings
The diagnosis of severe CAH is generally established by neonatal screening programs. However, the diagnosis of CAH in individuals with milder forms of the disease may not be established until late childhood, adolescence or even early adulthood. Ectopic adrenal rest tissue can be found in up to 50% of normal neonates [8]. The site of ectopic adrenal tissue can occur within the retroperitoneum, broad ligament, ovaries, inguinal region and testes. In normal individuals, this ectopic tissue becomes atrophic during normal development, and persists in only about 1% of individuals. However, in patients with CAH, testicular adrenal rest tumors are much more frequent, with a widely variable prevalence of up to 94% [7]. Testicular adrenal rest tumors can be difficult to detect on physical exam due to their close proximity to the rete testis. Lesions smaller than 2 cm are generally not apparent by palpation [6].
Imaging plays a vital role in the detection and surveillance of testicular adrenal rest tumors. Ultrasound (US) is generally considered the first line imaging modality in the detection and surveillance of testicular adrenal rest tumors. Advantages of US include relatively low cost and widespread availability. The true incidence of sonographically detectable testicular adrenal rest tissue is unknown, since most boys with CAH are not routinely screened with scrotal sonography. In one large study, Avila et al reported an incidence of testicular adrenal rest tumors in 12 (29%) of 42 male CAH patients that participated in a research protocol from 1981 to 1996 [5]. The majority of these lesions are sharply marginated and hypoechoic, although larger lesions (> 2 cm) may have regions of centrally increased echogenicity [9]. Heterogeneous lesions may have posterior calcifications, a hyperechoic rim or internal spokelike areas of echogenicity [10]. Investigators generally agree that the majority of individuals with testicular adrenal rest tumors have bilateral testicular involvement [9]. These lesions may vary significantly in size, with Delfino et al reporting diameters ranging from 4 to 38 mm in one recent larger series [7]. The vast majority of testicular adrenal rest tumors are located adjacent to the mediastinum testis, although larger lesions may not be confined to this region as in this particular instance. [9]. On color Doppler, these lesions may vary from predominantly hypovascular to normovascular, but may be infrequently hypervascular [9, 10].
The role of magnetic resonance (MR) imaging in the evaluation of testicular adrenal rest tumors has not been thoroughly validated. One comparative study determined that ultrasound is as sensitive as MR in the detection of testicular adrenal rest tumors, and is therefore the modality of choice given the ready accessibility and lower cost [11]. However, some investigators have suggested that the margins of these lesions may be better defined at MR. Specifically, MR may be superior in differentiating between multiple small, discrete lesions and a larger, solitary multilobular lesion. Therefore, when precise mapping regarding the size and extent of disease is required (prior to testis sparing surgery), MR is often preferred to US [12]. Testicular adrenal rest tumors are usually isointense relative to the normal background testicular parenchyma on T1-weighted sequences, but can occasionally be T1 hyperintense in lesions that contain a higher concentration of lipid. [13] These lesions are uniformly hypointense relative to the normal testis on T2-weighted sequences [9, 14].
Functional radioisotope imaging has been used with variable success for the evaluation of primary adrenal masses. In particular, 131I-6-β-iodomethyl-19-norcholesterol (NP-59) has been used to evaluate disorders involving the adrenal cortex [15]. However, this agent is not FDA approved for use in the United States. To the best of our knowledge, there is scant published data on the role of adrenal scintigraphy in the evaluation of testicular adrenal rest tumors.
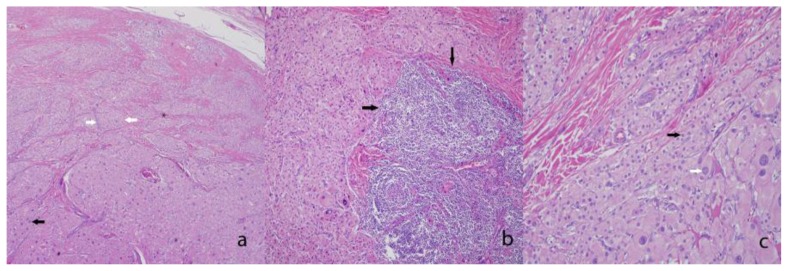
Histologically, testicular tumors of the adrenogenital syndrome are commonly mistaken for Leydig cell tumors or nodular Leydig cell hyperplasia [16]. Both tumors demonstrate a proliferation of polygonal cells with abundant granular, eosinophilic cytoplasm. A few helpful discriminating features that are more common to testicular tumors of the adrenogenital syndrome include a greater degree of nuclear pleomorphism (endocrine atypia), low mitotic activity, extensive fibrosis separating the nests of cells, associated lymphoid aggregates, and lipochrome pigment [17]. The tumor in this case demonstrated all of the aforementioned features to a large degree, with the exception of identifiable lipochrome pigment (Fig. 4). Another helpful feature is the absence of Reinke crystals (a frequent finding in Leydig cell tumors) in testicular tumors of the adrenogenital syndrome. Immunohistochemically, synaptophysin (a marker of neuroendocrine differentiation) shows strong reactivity in testicular tumor of the adrenogenital syndrome and is rarely positive in Leydig cell tumors [17]. Inhibin-alpha, an often utilized positive stain in Leydig cell tumors, will also be positive in testicular tumor of the adrenogenital syndrome [18].
Figure 4.
27 year old male with testicular adrenal rest tumors.
a) Low power image showing a proliferation of nested neoplastic cells (white arrows) with abundant eosinophilic cytoplasm, endocrine atypia (black arrow), and with collagenous bands dividing the tumor nests (*). (Hematoxylin and eosin stained section, original magnification ×40).
b) Medium power image showing a lymphoid aggregate associated with the tumor (black arrows). (Hematoxylin and eosin stained section, original magnification ×100.)
c) High power view of the neoplastic cells with their abundant granular cytoplasm, and round nuclei, features resembling adrenal cortical tissue (black arrow). Again, note the extensive neuroendocrine atypia (white arrow). (Hematoxylin and eosin stained section, original magnification ×400.)
Treatment and prognosis
Testicular adrenal rest tumors may regress with administration of exogenous glucocorticoids in doses that are high enough to suppress endogenous ACTH levels [19]. However, these lesions may increase in size and number when exogenous hormone therapy is inadequate. Most importantly, untreated testicular adrenal rest tumors may compress and distort normal background testicular parenchyma which can result in infertility. Hormone replacement (glucocorticoid and mineralocorticoid ) therapy is often employed as a first line treatment. Individuals with chronic testicular pain that are refractory to hormone therapy may elect to undergo partial vs total orchiectomy [12].
Differential diagnosis
Unfortunately, imaging findings of testicular adrenal rest tumors are nonspecific, and frequently overlap with other testicular malignancies such as primary germ cell tumors or gonadal (sex cord) tumors in a young adult male. However, primary testicular tumors are overwhelmingly unilateral and fewer in number than testicular germ cell tumors [14]. Epidermoid cysts often present as avascular, hypoechoic intratesticular masses that may mimic adrenal rest tumors. These lesions frequently contain characteristic concentric or lamellar echogenic rings that aid in differentiating these lesions from testicular adrenal rest tumors [14].
Given the fact that testicular adrenal rest tumors are generally bilateral, processes that involve both testis should be considered more strongly in the differential. Leydig’s cell hyperplasia presents with multiple discrete, hypoechoic lesions that are frequently bilateral and are virtually indistinguishable from testicular adrenal rest tumors. However, endocrine abnormalities such as elevated serum testosterone, luteinizing hormone or hCG are invariably seen [14]. Sarcoidosis may likewise present as multiple bilateral hypoechoic masses, but is much more prevalent in the African-American population [14]. Other hypoechoic intratesticular lesions include lymphoma and metastases, although both conditions occur in older males, and are therefore not generally included in the differential for a hypoechoic testicular mass in the younger male. If the diagnosis of testicular adrenal rest tumors cannot be established by imaging, testicular vein sampling can be performed that will often show elevated cortisol levels compared with peripheral blood levels [20].
Conclusion
Testicular imaging is essential in the workup of males with congenital adrenal hyperplasia, and should be initially performed at early puberty. The presence of testicular adrenal rest tumors in these individuals is significant, since their presence is suggestive of suboptimal hormone replacement therapy. Additionally, untreated nodules of adrenal rests may expand and destroy the testicular parenchyma, resulting in low testosterone production and infertility. Ultrasound is recommended as the imaging modality of choice in the detection and surveillance of these lesions, while MR may be useful for certain individuals requiring highly accurate preoperative assessment. Most importantly, clinical radiologists should be aware of this benign disease entity in the setting of CAH in order to prevent unnecessary biopsies and orchiectomies.
TEACHING POINT
Testicular adrenal rest tumors are frequently seen in males with congenital adrenal hyperplasia, and are often misdiagnosed as primary testicular germ cell tumors or other more common benign testicular lesions. These benign tumors generally present as multiple, bilateral hypoechoic masses at ultrasound, which serves as the primary imaging modality of choice.
Table 1.
Summary table for testicular adrenal rest tumors
| Etiology | Abnormal intratesticular adrenaloid tissue deposition which occurs in males with congenital adrenal hyperplasia due to excess ACTH stimulation. |
|
| |
| Incidence | May occur in up to 94% of individuals with poorly controlled congenital adrenal hyperplasia (prevalence highly variable depending on the degree of treatment compliance per individual) |
|
| |
| Gender ratio | Exclusively male |
|
| |
| Age predilection | 20–40 years |
|
| |
| Risk factors | Poorly controlled individuals with congenital adrenal hyperplasia |
|
| |
| Treatment | Hormone replacement (glucocorticoid and mineralocorticoid) therapy is often employed as a first line treatment. Individuals with chronic testicular pain that are refractory to hormone therapy may elect to undergo partial vs total orchiectomy. |
|
| |
| Prognosis | Testicular adrenal rest tumors are benign lesions that often regress with adequate hormone replacement therapy. However, infertility and chronic testicular pain may occur in individuals that do not respond to hormone replacement. |
|
| |
| Imaging findings | |
| Ultrasound | Ultrasound is considered the modality of choice due to low cost and widespread availability. Testicular adrenal rest tumors generally present as hypoechoic intratesticular masses of variable size that are generally hypovascular on color Doppler interrogation. |
| MRI | MRI is occasionally employed for further characterization of testicular adrenal tumors. Tumoral margins are optimally depicted by MR, which may be essential in preoperative assessment. These lesions are generally isointense to mildly hyperintense on T1 weighted images, and hypointense on T2 weighted images. |
Table 2.
Differential table for testicular adrenal rest tumors
| Pathology | Ultrasound | MRI |
|---|---|---|
| Germ cell tumor | Discrete or multifocal hypoechoic or mixed echogenicity testicular masses, +/− vascularity. May show cystic areas or necrosis. | Isointense on T1-weighted images and hypointense on T2-weighted images with variable enhancement. |
| Gonadal stromal tumor | Discrete masses of variable echogenicity. Typically solid, but may show cystic areas, hemorrhage, or necrosis. Usually indistinguishable from germ cell tumors. | Isointense on T1-weighted images and hypointense on T2-weighted images with avid enhancement. |
| Lymphoma | Poorly marginated, hypoechoic infiltrative masses. Frequently bilateral. | Poorly marginated T1 and T2-weighted hypointense masses with low-level enhancement. |
| Metastatic disease | Solid, hypoechoic masses. Frequently bilateral. | Sharply marginated masses, T1-weighted variable, T2-weighted hypointense with variable enhancement. |
| Epidermoid cyst | Discrete hypoechoic masses with prominent internal concentric rings. No internal flow. | Sharply marginated, hypointense on both T1 and T2-weighted images. |
| Leydig cell hyperplasia | Multiple small, predominantly hypoechoic masses that are invariably bilateral. | Isointense on T1-weighted images with avid enhancement. Hypointense on T2-weighted images. |
| Sarcoidosis | Multiple small hypoechoic masses, usually bilateral. Much more common in the African-American population. | Variable signal on T1-weighted images with moderate enhancement, hypointense on T2-weighted images. |
ABBREVIATIONS
- ACTH
adrenocorticotropin hormone
- CAH
congenital adrenal hyperplasia
- CT
computed tomography
- MR
magnetic resonance
- TART
testicular adrenal rest tumor
- US
ultrasound
REFERENCES
- 1.Speiser PW, White PC. Congenital adrenal hyperplasia. The New England journal of medicine. 2003;349(8):776–788. doi: 10.1056/NEJMra021561. [DOI] [PubMed] [Google Scholar]
- 2.Therrell BL, Jr, Berenbaum SA, Manter-Kapanke V, Simmank J, Korman K, Prentice L, Gonzalez J, Gunn S. Results of screening 1.9 million Texas newborns for 21-hydroxylase-deficient congenital adrenal hyperplasia. Pediatrics. 1998;101(4 Pt 1):583–590. doi: 10.1542/peds.101.4.583. [DOI] [PubMed] [Google Scholar]
- 3.Auchus RJ. Management of the adult with congenital adrenal hyperplasia. International journal of pediatric endocrinology. 2010;2010:614107. doi: 10.1155/2010/614107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilkins L. Macrogenitosomia precox associated with hyperplasia of the androgenic tissue of the adrenal and death from corticoadrenal insufficiency. Endocrinology. 1940;26:385–395. [Google Scholar]
- 5.Avila NA, Shawker TS, Jones JV, Cutler GB, Jr, Merke DP. Testicular adrenal rest tissue in congenital adrenal hyperplasia: serial sonographic and clinical findings. AJR American journal of roentgenology. 1999;172(5):1235–1238. doi: 10.2214/ajr.172.5.10227495. [DOI] [PubMed] [Google Scholar]
- 6.Claahsen-van der Grinten HL, Hermus AR, Otten BJ. Testicular adrenal rest tumours in congenital adrenal hyperplasia. International journal of pediatric endocrinology. 2009;2009:624823. doi: 10.1155/2009/624823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delfino M, Elia J, Imbrogno N, Argese N, Mazzilli R, Toscano V, Mazzilli F. Testicular adrenal rest tumors in patients with congenital adrenal hyperplasia: prevalence and sonographic, hormonal, and seminal characteristics. Journal of ultrasound in medicine : official journal of the American Institute of Ultrasound in Medicine. 2012;31(3):383–388. doi: 10.7863/jum.2012.31.3.383. [DOI] [PubMed] [Google Scholar]
- 8.Anderson JR, Ross AH. Ectopic adrenal tissue in adults. Postgraduate medical journal. 1980;56(661):806–808. doi: 10.1136/pgmj.56.661.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stikkelbroeck NM, Suliman HM, Otten BJ, Hermus AR, Blickman JG, Jager GJ. Testicular adrenal rest tumours in postpubertal males with congenital adrenal hyperplasia: sonographic and MR features. European radiology. 2003;13(7):1597–1603. doi: 10.1007/s00330-002-1786-3. [DOI] [PubMed] [Google Scholar]
- 10.Avila NA, Premkumar A, Shawker TH, Jones JV, Laue L, Cutler GB. Testicular adrenal rest tissue in congenital adrenal hyperplasia: findings at Gray-scale and color Doppler US. Radiology. 1996;198(1):99–104. doi: 10.1148/radiology.198.1.8539414. [DOI] [PubMed] [Google Scholar]
- 11.Avila NA, Premkumar A, Merke DP. Testicular adrenal rest tissue in congenital adrenal hyperplasia: comparison of MR imaging and sonographic findings. AJR American journal of roentgenology. 1999;172(4):1003–1006. doi: 10.2214/ajr.172.4.10587136. [DOI] [PubMed] [Google Scholar]
- 12.Walker BR, Skoog SJ, Winslow BH, Canning DA, Tank ES. Testis sparing surgery for steroid unresponsive testicular tumors of the adrenogenital syndrome. The Journal of urology. 1997;157(4):1460–1463. [PubMed] [Google Scholar]
- 13.Nagamine WH, Mehta SV, Vade A. Testicular adrenal rest tumors in a patient with congenital adrenal hyperplasia: sonographic and magnetic resonance imaging findings. Journal of ultrasound in medicine : official journal of the American Institute of Ultrasound in Medicine. 2005;24(12):1717–1720. doi: 10.7863/jum.2005.24.12.1717. [DOI] [PubMed] [Google Scholar]
- 14.Woodward PJ, Sohaey R, O’Donoghue MJ, Green DE. From the archives of the AFIP: tumors and tumorlike lesions of the testis: radiologic-pathologic correlation. Radiographics : a review publication of the Radiological Society of North America, Inc. 2002;22(1):189–216. doi: 10.1148/radiographics.22.1.g02ja14189. [DOI] [PubMed] [Google Scholar]
- 15.Avram AM, Fig LM, Gross MD. Adrenal gland scintigraphy. Seminars in nuclear medicine. 2006;36(3):212–227. doi: 10.1053/j.semnuclmed.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 16.Rosai J. Rosai and Ackerman Surgical Pathology. 10 edn. Elsevier Mosby; 2011. [Google Scholar]
- 17.Ashley RA, McGee SM, Isotaolo PA, Kramer SA, Cheville JC. Clinical and pathological features associated with the testicular tumor of the adrenogenital syndrome. The Journal of urology. 2007;177(2):546–549. doi: 10.1016/j.juro.2006.09.041. discussion 549. [DOI] [PubMed] [Google Scholar]
- 18.Emerson RE, Ulbright TM. The use of immunohistochemistry in the differential diagnosis of tumors of the testis and paratestis. Seminars in diagnostic pathology. 2005;22(1):33–50. doi: 10.1053/j.semdp.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 19.Vanzulli A, DelMaschio A, Paesano P, Braggion F, Livieri C, Angeli E, Tomasi G, Gatti C, Severi F, Chiumello G. Testicular masses in association with adrenogenital syndrome: US findings. Radiology. 1992;183(2):425–429. doi: 10.1148/radiology.183.2.1561344. [DOI] [PubMed] [Google Scholar]
- 20.Shawker TH, Doppman JL, Choyke PL, Feuerstein IM, Nieman LK. Intratesticular masses associated with abnormally functioning adrenal glands. Journal of clinical ultrasound : JCU. 1992;20(1):51–58. doi: 10.1002/jcu.1870200110. [DOI] [PubMed] [Google Scholar]