Abstract
Vascular Ehlers-Danlos Syndrome (previously Ehlers-Danlos IV) is a rare autosomal dominant collagen vascular disorder caused by a 2q31 COL3A1 gene mutation encoding pro-alpha1 chain of type III collagen (in contrast to classic Ehlers-Danlos, caused by a COL5A1 mutation). The vascular type accounts for less than 4% of all Ehlers-Danlos cases and usually has a poor prognosis due to life threatening vascular ruptures and difficult, frequently unsuccessful surgical and vascular interventions. In 70% of cases, vascular rupture or dissection, gastrointestinal perforation, or organ rupture is a presenting sign. We present a case of genetically proven vascular Ehlers-Danlos with fatal recurrent retroperitoneal hemorrhages secondary to a ruptured right common iliac artery dissection in a 30-year-old male. This case highlights the need to suspect collagen vascular disorders when a young adult presents with unexplained retroperitoneal hemorrhage, even without family history of such diseases.
Keywords: Vascular type Ehlers-Danlos Syndrome, Ehlers-Danlos Syndrome, Arterial dissection, Common iliac artery dissection, Arterial rupture, Collagen vascular disease, Ectasia, Retroperitoneal hemorrhage, Axial CT, Retroperitoneal hematoma, Vascular imaging, Elastin, Iliac artery, Vascular fragility, Hypovolemic shock, Back pain, Perforation
CASE REPORT
A previously healthy 30-year-old Caucasian male presented to our emergency department (ED) with a complaint of new onset, progressive dull right flank and back pain with no radiation.
Imaging Findings
Based on the suspicion of possible urolithiasis or appendicitis, a 64 slice multi-detector helical CT of the abdomen and pelvis at 3mm slice thickness with 4mm coronal reconstructions without intravenous contrast, followed by repeat scanning with the use of intravenous contrast, was performed. The CT showed dissection of the right common iliac artery (CIA) extending into the right internal and external iliac arteries (Figure 1). The CT also showed stable dissection of the left internal iliac artery. Distal reconstitution in the right external and internal iliac arteries was noted secondary to pelvic collaterals from the left internal iliac artery. Right perinephric and retroperitoneal hematomas raised the suspicion of a rupture of the right common iliac artery dissection (Figure 2).
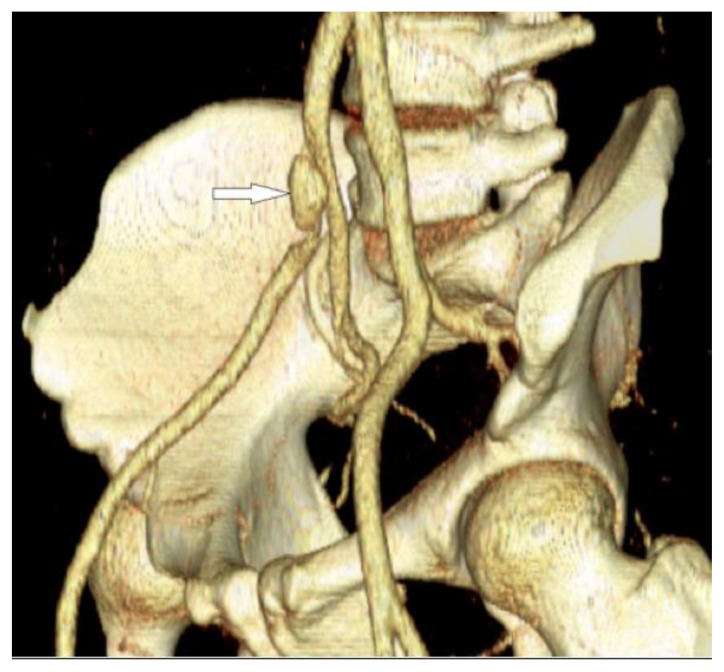
Figure 1.
30-year-old male with vascular type EDS and fatal ruptured right common iliac artery dissection. FINDINGS: An oblique 3D volume image of the pelvic bones and the iliac vessels was reconstructed from the axial contrast enhanced CT (CECT) of the pelvis. There is small (approximately 1 cm) anterior saccular aneurysmal dilatation of the distal right common iliac artery and the proximal right external iliac artery (arrow) at the level of the origin of the right internal iliac artery. This is the aneurysmally dilated false lumen. The true lumen is narrowed and compressed posteriorly. The vessels reconstitute distally through pelvic collaterals from the left internal iliac artery. TECHNIQUE: 3D volume rendered image, the original acquisition done using Siemens Sensation 64 slice multi-detector CT, 180mAs, 120KVP, 5mm axial slice thickness, 100ml of Optiray 350 intravenous contrast agent.
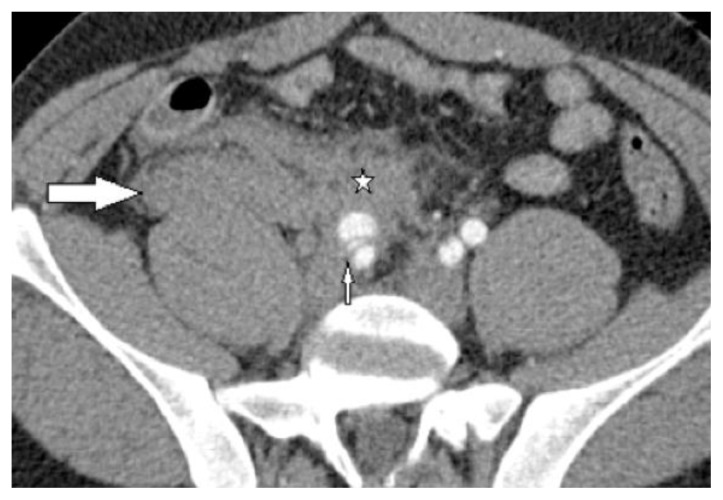
Figure 2.
30-year-old male with vascular type EDS and fatal ruptured right common iliac artery dissection. FINDINGS: Axial CECT of pelvis in the arterial phase performed when the patient initially presented to the emergency department demonstrates large right retroperitoneal hematoma (large arrow) and a large hematoma anterior to the right external iliac artery (stellate mark). A posterior dissection flap divides the proximal right external iliac artery into a larger anterior false lumen and a smaller compressed posterior true lumen (small arrow). The proximal right internal iliac artery is seen posterior to the dissected right external iliac artery. Note the loss of the normal interface between the vessel wall and the mesenteric fat by the hemorrhagic fat stranding. TECHNIQUE: Siemens Sensation 64 slice multi-detector CT, 300mAs, 120KVP, 3mm axial slice thickness, 120ml of omnipaque 360 intravenous contrast agent.
Management
The patient was taken to the operating room for emergency surgical exploration, and a ruptured dissection of the right CIA was found. The hematoma was evacuated, the right CIA was ligated and cut at its origin, and a bifemoral synthetic arterial graft was done secondary to blood vessel friability that was appreciated during surgery. Vascular Ehlers-Danlos Syndrome (EDS) was suspected. The right CIA specimen and a skin biopsy were sent for further testing. Further assessment revealed history of easy bruising, skin hyper-elasticity, and mild joint hyper-mobility, but no family history of EDS or other collagen vascular diseases. The right CIA specimen showed deficiency of collagen type III, which is very sensitive for vascular EDS. DNA testing was ordered for confirmation, and the result was positive for COL3A1 mutation, which is specific for vascular type EDS. Family members were not tested for aberrant chromosomal abnormalities.
Follow-Up
The postoperative period was complicated with poor wound healing and recurrent retroperitoneal hemorrhages (Figures 3 and 4), which were treated conservatively for fear of complicated surgical or vascular interventions. Eventually the patient went into a hypovolemic shock, and all efforts of resuscitation failed. The patient expired 7 days after the initial presentation. Autopsy confirmed the cause of death to be uncontrolled large retroperitoneal hemorrhage.
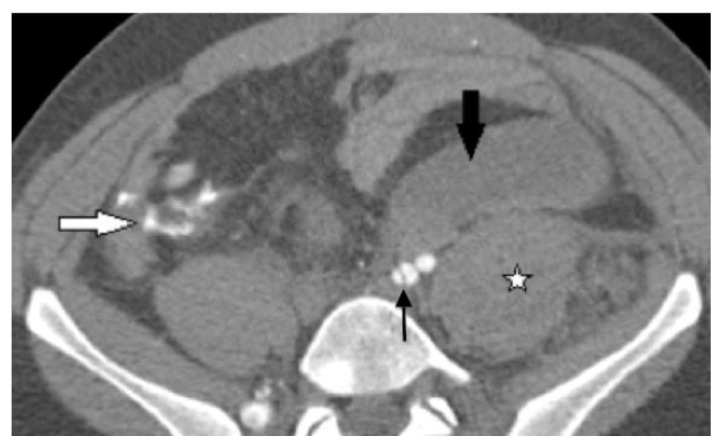
Figure 3.
30-year-old male with vascular type EDS and fatal ruptured right common iliac artery dissection. This image was taken after open resection of the aneurysm and ligation of the CIA at its origin. FINDINGS: Post surgical follow up axial CECT of the pelvis in the arterial phase demonstrates a large left retroperitoneal hematoma anterior to the left common iliac artery bifurcation and the left psoas muscle (large black arrow). A left psoas muscle hematoma (stellate mark) is seen as asymmetrically and hyper-densely enlarged in comparison to the right psoas muscle. There is a small amount of extravascular leakage of the intravenous contrast anterior to the right psoas muscle in the right lower quadrant (white arrow), which is suggestive of either vascular repair leakage or new vascular rupture after surgical excision and ligation of the ruptured right common iliac artery dissection. Note left internal iliac artery dissection (thin black arrow) and absent opacification of the right external and internal iliac arteries at the same level. TECHNIQUE: Siemens Sensation 64 slice multi-detector CT, 120KVP, 300mAs, 3mm axial slice thickness, 120ml of omnipaque 360 intravenous contrast agent.
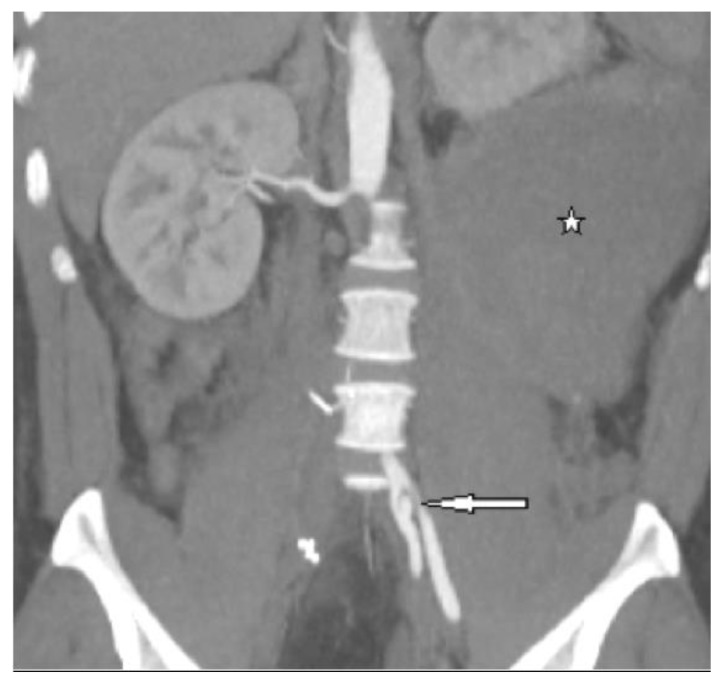
Figure 4.
30-year-old male with vascular type EDS and fatal ruptured right common iliac artery dissection. This image was taken after open resection of the aneurysm and ligation of the CIA at its origin. FINDINGS: Coronal reformation of the postoperative axial enhanced CT of the pelvis in the arterial phase demonstrates large left retroperitoneal hematoma (stellate mark) causing significant mass effect and upward displacement of the left kidney and a small lateral side wall left internal iliac artery dissection with a larger lateral false lumen and a smaller compressed medial true lumen (arrow). Note absent opacification of the surgically removed right common iliac artery and also note the left psoas intra-muscular hematoma. TECHNIQUE: Coronal 4mm slice thickness reconstruction of the abdomen and pelvis, the original acquisition was done using a Siemens Sensation 64 slice multi- detector CT, 180mAs, 120KVP, 5mm coronal slice thickness, 120ml of omnipaque 360 intravenous contrast agent.
Pathology
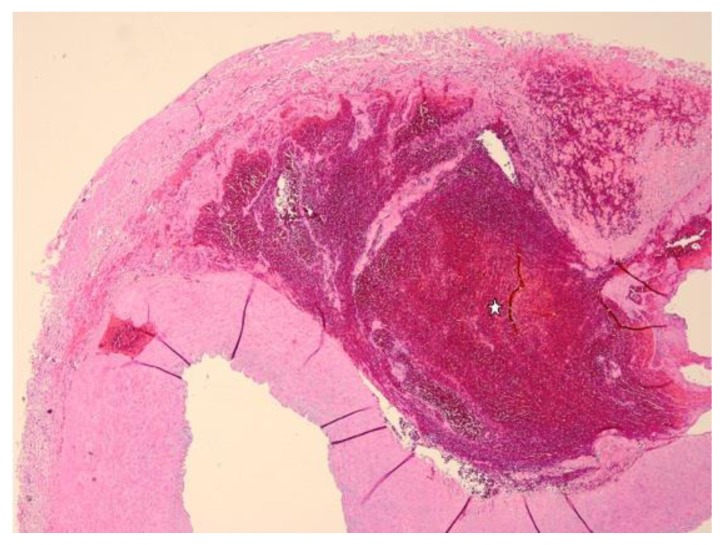
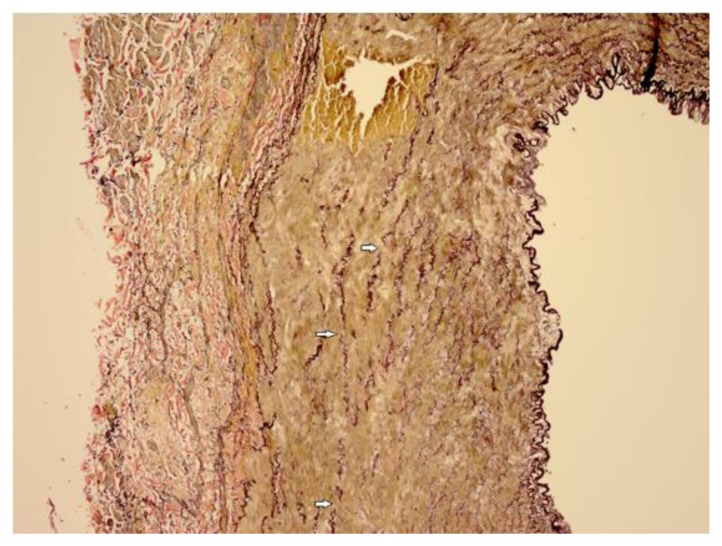
Gross examination of the resected portion of the right common iliac artery revealed an area of perforation. Microscopic examination of the H&E stained section (Figure 5) showed medial dissection of the arterial wall with hematoma formation and no significant associated inflammation. These findings were supported by elastin staining (Figure 6), which showed evidence of medial degeneration and marked elastin fragmentation. Based on these findings, it was recommended to DNA test the patient for possible Ehlers-Danlos Syndrome.
Figure 5.
30-year-old male with vascular type EDS and fatal ruptured right common iliac artery dissection. FINDINGS: High magnification (4x, objective 22) H&E (Haematoxylin eosin) stain of the surgically excised right common iliac artery demonstrates median arterial dissection and hemorrhage (stellate mark).
Figure 6.
30-year-old male with vascular type EDS and fatal ruptured right common iliac artery dissection. FINDINGS: Elastin stain (10x, objective 22) of the surgically excised right common iliac artery demonstrates fragmentation of the elastin in the vessel wall (arrows).
The results of the autopsy examination revealed spontaneous retroperitoneal hematoma on the left side with un- ruptured left internal iliac artery dissection and evidence of surgical repair of the ruptured right common iliac artery dissection. Examination of the other large vessels showed friable thin walled vessels. Collagen-vascular DNA testing confirmed Ehlers-Danlos Syndrome Type IV with a mutation in the COL3A1 gene, and the cause of death was considered to be secondary to uncontrolled spontaneous retroperitoneal hemorrhage.
DISCUSSION
Etiology and Demographics
In the early 1900s, Ehlers and Danlos described a spectrum of disorders that shared the common findings of joint hyper-mobility and skin elasticity. Since then a number of subtypes have been classified, including the vascular type of EDS, which accounts for fewer than 4% of Ehlers-Danlos cases [1,2]. This subtype has no gender predilection. Vascular EDS is caused by mutation in the COL3A1 gene locus 2q31 that encodes pro-alpha1 chain of type III collagen and is inherited in an autosomal dominant manner [3]. Deficiency of collagen type III is very sensitive for vascular EDS, but not specific. This mutation will either cause decreased or absent collagen synthesis or abnormalities in collagen secretion [4,5]. About 50% of affected individuals have inherited the COL3A1 mutation from an affected parent, and about 50% of affected individuals have a de novo disease caused by new onset mutation, as expected in our case [6]. In May of 2010, a novel point mutation at P.GLY843GLU and a rare case of left CIA rupture in classic EDS with genetically proven COL5A1 mutation were reported by Sadakata et al [7] and Borck et al [8], respectively. However, according to the literature, this is not a common mutation. Of patients with vascular EDS, 25% experience a significant medical problem by 20 years of age and more than 80% by the age of 40, leading to an average age of death of 48 years [6].
Clinical and Imaging Findings
One-fourth of individuals with vascular type EDS (EDS IV) experience a significant medical problem by 20 years of age and more than 80% by 40 years of age. The median age of death is 48 years [6]. Vascular EDS is known for causing catastrophic vascular ruptures and bleeding that more often than usual lead to death, as surgical intervention is usually risky and unsuccessful secondary to vascular fragility. Vascular dissections or ruptures, gastrointestinal perforations, or organ ruptures are the presenting symptoms in 70% of adults [6]. These young patients usually present to the ED with hemodynamic instability and undergo abdominal surgical exploration due to retroperitoneal or intraperitoneal hemorrhages if the diagnosis of EDS is not known at that time. Surgical operations with vascular resections and use of synthetic grafts are usually done secondary to vascular fragility. Due to the severe vascular fragility, interventional angiography is strongly discouraged and carries the risk for arterial tears and dissections at the site of entry of the catheter, as well as aneurysm formation due to injection pressure [6]. If vascular EDS is known at the time of presentation, CT angiography (CTA) or MR angiography (MRA) contrast can be used to search for the source of hemorrhage. Noninvasive MRA or CTA, rather than conventional angiography, can be used to survey for sites of vascular abnormalities at risk for future tears [6]. Due to the significantly improved spatial resolution of noninvasive imaging techniques in comparison to conventional angiography, which is the gold standard for vascular imaging, noninvasive imaging is now useful for both diagnosis and follow-up of vascular lesions [9].
In a cohort of 28 patients with EDS IV between 1971 and 2006, Zilocchi et al reported arterial aneurysms and dissections as the most common radiologic findings followed by arterial ectasias and occlusions [9]. They also reported in the same cohort that the vessels with the most lesions were the iliac arteries. In overall incidence of vascular abnormalities, the iliac arteries came second to visceral arteries, followed by head and neck arteries as the most common sites for vascular involvement. They also suggested that vascular EDS can be suspected on the basis of radiologic findings.
Treatment and Prognosis
Vascular EDS is considered to carry the worst prognosis secondary to the risk of life threatening arterial ruptures, and the majority of EDS with vascular ruptures or organ perforations will prove to be secondary to EDS IV. Treatment for arterial or bowel ruptures and other complications of vascular EDS can include surgery, but this is highly discouraged due to poor wound healing in such patients. Whether the fatal outcome in our case was secondary to surgical complications or the natural history of the disease is difficult to discern. Surveillance may include periodic arterial screening through digital subtraction angiography (DSA), magnetic resonance angiography (MRA), or CT angiography (CTA). DSA is not recommended because of the risk of vascular injury. Pregnant women with vascular EDS should be followed by high-risk obstetrical programs in order to prevent complications. The inherit high risks with surgery and radiologic intervention are the reasons for highlighting this case to stress on the importance, as radiologists, to have a high index of suspicion for collagen vascular diseases in similar cases and to make sure collagen vascular disease is always included in the differential diagnosis for patient presentations like the one discussed. It is also important for radiologists to have a conversation with the ordering physician or surgeon about the possibility of collagen vascular diseases.
Differential Diagnoses
When presenting signs and symptoms suggest vascular EDS, a full differential diagnosis must be considered. This includes classic and kyphoscoliotic types of EDS, idiopathic isolated arterial aneurysm, autosomal dominant polycystic kidney disease, Marfan Syndrome, and Loeys-Dietz Syndrome, which are distinguishable by x-ray, CT, and MRI findings. Compared to classic and vascular EDS, kyphoscoliotic EDS is caused by a mutation in the procollagen-lysine 1, 2-oxoglutarate 5-dioxygenase 1 (PLOD1) gene rather than COL genes. Both vascular and classic EDS can present with lumbar vertebral body scalloping due to dural ectasias on x-ray, while kyphoscoliotic EDS shows progressive scoliosis. On CT, vascular EDS presents with arterial aneurysms, dissections, occlusions, and ruptures. Classic and kyphoscoliotic EDS may show dural ectasias, but vascular ruptures are rare. All subtypes of EDS show widened cerebrospinal (CSF) spaces in the spine and dural ectasias on MRI [6].
Autosomal dominant polycystic kidney disease, caused by PKD1 and 2 gene mutations, usually produce bilaterally enlarged and cystic kidneys, along with cystic liver, pancreas, seminal vesicles, and arachnoid membranes on CT. This disease is included in the differential of EDS type IV, as both can share the common findings of intracranial aneurysms, mitral valve prolapse (MVP), and aortic root dilatations and dissections. On MRI, uncomplicated cysts will be hypointense on T1WI and hyperintense on T2WI sequences. Hemorrhagic cysts will by hyperintense on T1WI and hypointense on T2WI, however this can vary according to age of the blood in the cysts. Marfan Syndrome, caused by autosomal dominant mutation in the FBN1 gene, shows arachnodactyly, lumbar vertebral body scalloping due to dural ectasia, spondylolisthesis or spondylolysis, and pectus excavatum or carinatum that can be visualized on x-ray. CT may indicate lens dislocation, a dilated or aneurismal aorta, or dural ectasia. MRI can show wide CSF spaces and dural ectasias, similar to EDS. Loeys-Dietz Syndrome is caused by an autosomal dominant mutation in TGFBR1 and 2 genes, leading to craniofacial abnormalities and other Marfanoid physical characteristics on x-ray. CT will show vascular aneurysms, which often rupture in the first year of life [6].
Conclusion
Vascular EDS is a rare vascular collagen disorder, constituting less than 4% of all EDS cases, that is usually associated with life threatening arterial dissections and ruptures in adults. Surgical or vascular interventions, which are typically unsuccessful due to severe vascular wall friability, are risky and usually associated with poor wound healing. Such interventions are highly discouraged if the diagnosis is known at the time of presentation. In some reports, iliac arteries were the most common arteries to develop dissections and ruptures in this rare type of EDS. Vascular EDS should be suspected on basis of radiologic findings in the right clinical setting. It is extremely important for radiologists to raise the suspicion of collagen vascular disease with the ordering physician. It should also be included in the differential diagnosis for such cases.
TEACHING POINT
Vascular Ehlers-Danlos Syndrome (EDS) should be suspected in adults with unexplained vascular dissections, aneurysms, and rupture. The single vessel that is most commonly affected is the iliac artery.
Table 1.
Summary table for vascular type Ehlers-Danlos Syndrome (EDS).
| Etiology | Autosomal dominant collagen vascular disorder caused by mutation in the COL3A1 gene located on 2q31 that encodes pro-alpha1 chain of type III collagen, unlike classic EDS, which is caused by mutation in the COL5A1 gene [7]. |
| Incidence | Accounts for fewer than 4% of all EDS patients [1,2]. |
| Gender ratio | No gender predilection. |
| Age predilection | One-fourth of individuals with EDS, vascular type, experience a significant medical problem by 20 years of age and more than 80% by 40 years of age. The median age of death is 48 years [6]. |
| Risk factors | 50% are inherited as autosomal dominant and 50% are de novo mutation of the COL3A1 gene [6]. |
| Treatment | Treatment may include surgery for arterial or bowel complications/rupture; however, this is discouraged secondary to poor wound healing. Pregnant women with vascular type EDS should be followed in a high- risk obstetrical program. Surveillance may include periodic arterial screening through digital subtraction angiography (DSA), magnetic resonance angiography (MRA), or CT angiography (CTA). DSA is not recommended because of the risk of vascular injury. |
| Prognosis | Vascular EDS usually carries a poor prognosis secondary to life threatening vascular ruptures and difficult, frequently unsuccessful, surgical and vascular interventions. In 70% of cases, vascular rupture or dissection and gastrointestinal perforation or organ rupture are usually the presenting signs [6]. |
| Findings on imaging | Aneurysms and dissections are the most common radiologic findings, followed by arterial ectasias and occlusions [9]. |
Abbreviations: Ehlers-Danlos Syndrome (EDS), Magnetic resonance angiography (MRA), Computed tomography angiography (CTA), Digital subtraction angiography (DSA).
Table 2.
Differential table for Vascular Type Ehlers-Danlos Syndrome (EDS), including etiology and imaging findings for each [6].
| Etiology | X-Ray | CT | MRI | |
|---|---|---|---|---|
| EDS Vascular | Autosomal Dominant (AD), COL3A1 gene. | Lumbar vertebral body scalloping due to dural ectasias. | Arterial aneurysms, dissections, occlusions, and ruptures. | Widened cerebrospinal fluid (CSF) spaces in the spine and dural ectasias. |
| EDS Classic | AD, COL5A1 gene. | May see dural ectasias. Vascular ruptures rarely occur. | ||
| EDS Kyphoscoliotic | AR, PLOD1 gene. | |||
| Isolated arterial aneurysm | Idiopathic. | Isolated arterial aneurysm. | ||
| Polycystic kidney disease, AD | AD, PKD1 and PKD2 genes. | Bilaterally enlarged and cystic kidneys. Cysts also occur in liver, seminal vesicles, pancreas, and arachnoid membrane. Intracranial aneurysms, MVP, aortic root dilatation, and dissection. | Uncomplicated cysts will by hypointense on T1 weighted imaging (T1WI) and hyperintense on T2 weighted imaging (T2WI). Hemorrhagic cysts will be hyperintense on T1WI and hypointense on T2WI. | |
| Marfan Syndrome | AD, FBN1 gene. | Arachnodactyly, lumbar vertebral body scalloping due to dural ectasia, spondylolisthesis or spondylolysis, pectus excavatum or carinatum. | Lens dislocation and dilatation, or aneurysm of the aorta. Dural ectasias. | Widened CSF spaces in the spine and dural ectasias. |
| Loeys-Dietz Syndrome | AD, TGFBR1 and TGFBR2 genes. | Craniofacial abnormalities and Marfan-like physical presentations. | Vascular aneurysms and ruptures in the first year of life and familial aortic aneurysms. |
Abbreviations: Ehlers-Danlos Syndrome (EDS), Autosomal dominant (AD), Autosomal recessive (AR), Collagen (COL), procollagen-lysine 1, 2-oxoglutarate 5- dioxygenase 1 (PLOD1), Polycystic kidney disease gene (PKD), Fibrillin 1 (FBN1), Transforming growth factor beta receptor (TGFBR), Cerebrospinal fluid (CSF), Mitral valve prolapse (MVP), T1 weighted imaging (T1WI), T2 weighted imaging (T2WI).
ACKNOWLEDGMENTS
Dr Jens Heidenreich, MD, Neuroradiologist. University of Louisville, KY, USA
ABBREVIATIONS
- CECT
Contrast enhanced CT
- CIA
Common iliac artery
- COL3A1, COL5A1
Collagen gene type 3A1 and 5A1
- CT
Computed tomography
- CTA
Computed tomography angiography
- ED
Emergency department
- EDS
Ehlers-Danlos Syndrome
- H&E
Haematoxylin Eosin stain
- MRA
Magnetic resonance angiography
- MRI
Magnetic resonance imaging
- MVP
Mitral valve prolapse
REFERENCES
- 1.Germain DP, Herrera-Guzman Y. Vascular Ehlers-Danlos syndrome. Ann Genet. 2004;47:1–9. doi: 10.1016/j.anngen.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 2.Germain DP. Clinical and genetic features of vascular Ehlers-Danlos syndrome. Ann Vasc Surg. 2002;16:391–397. doi: 10.1007/s10016-001-0229-y. [DOI] [PubMed] [Google Scholar]
- 3.Superti-Furga A, Gugler E, Gitzelmann R, Steinman B. Ehlers-Danlos syndrome type IV: A multi-exon deletion in one of the two COL3A1 alleles affecting structures, stability, and processing of type III pro-collagen. J Biol Chem. 1988;1988;263:6226–6232. [PubMed] [Google Scholar]
- 4.Byers PH, Holbrook KA, McGillivray B, et al. Clinical and ultrastructural heterogeneity of type IV Ehlers-Danlos syndrome. Hum Genet. 1979;47:141–150. doi: 10.1007/BF00273196. [DOI] [PubMed] [Google Scholar]
- 5.Pope FM, Nicholls AC, Narcisi P, et al. Type III collagen mutations in Ehlers-Danlos syndrome type IV and other related disorders. Clin Exp Dermatol. 1988;13:285–302. doi: 10.1111/j.1365-2230.1988.tb00709.x. [DOI] [PubMed] [Google Scholar]
- 6.Pepin M, Byers P. Gene Reviews; Initial Posting: September 2, 1999. Ehlers-Danlos Syndrome, Vascular Type. Last Update: May 3, 2011. Bookshelf ID: NBK1494. [Google Scholar]
- 7.Sadakata R, Hatamochi A, Kodama K, Kaga A, Yamaguchi T, Soma T, Usui Y, Nagata M, Ohtake A, Hagiwara K, Kanazawa M. Ehlers-Danlos Syndrome Type IV, Vascular Type, Which Demonstrated a Novel Point Mutation in the COL3A1 Gene. Intern Med. 2010;49:1797–1800. doi: 10.2169/internalmedicine.49.3435. [DOI] [PubMed] [Google Scholar]
- 8.Borck G, Beighton P, Wilhelm C, Kohlhase J, Kubisch C. Arterial rupture in classic Ehlers-Danlos syndrome with COL5A1 mutation. Am J Med Genet Part A. 2010;152A:2094–2098. doi: 10.1002/ajmg.a.33541. [DOI] [PubMed] [Google Scholar]
- 9.Zilocchi M, Macedo T, Oderich G, Vrtiska T, Biondetti P, Stanson A. Vascular Ehlers-Danlos Syndrome: Imaging Findings. AJR. 2007 Sep;189(3):712–719. doi: 10.2214/AJR.07.2370. [DOI] [PubMed] [Google Scholar]