Abstract
Objectives:
To eliminate microorganisms that are responsible for pulpal and periapical infections and to prevent reinfection of the root canal system an effective chemomechanical preparation by irrigants with sustained antimicrobial activity is beneficial. Hereby, we evaluated the residual antibacterial activity of MTAD after canal obturation at different time intervals.
Materials and Methods:
A total of 120 human single-canalled anterior teeth were selected. The root canals were instrumented to a standardized apical size. Among all, 90 teeth received final irrigation with MTAD and were divided into three groups according to their obturation materials; i.e. gutta-percha/AH26, Resilon/RealSeal SE and positive controls. All these groups were divided into three 1-, 3- and 6-week time interval subgroups. Thirty teeth as negative control had no final irrigation with MTAD, but were obturated with gutta-percha/AH26 or Resilon/RealSealSE. Dentin powder was prepared after 1, 3 and 6 weeks. Dentin powder was exposed to Enterococcus faecalis for 24h and then cultured. Colony Forming Unit (CFU) was counted.
Results:
Residual antimicrobial activity of MTAD in the teeth obturated with guttapercha/AH26 was significantly higher than the teeth obturated with Resilon/RealSeal SE (p<0.001). It also showed a time dependent decrease in MTAD antimicrobial activity for all groups. The highest antimicrobial activity of MTAD was found in the 1-week positive control and 1-week gutta-percha/AH26 specimens. The lowest antimicrobial activity of MTAD was found in 6-week Resilon/RealSeal SE samples and then the negative controls.
Conclusion:
MTAD had antimicrobial activity even at the sixth week, although it had a time-dependent decrease. Resilon/Epiphany SE significantly decreased antimicrobial activity of MTAD at all time points.
Keywords: MTAD, Antimicrobial Agents, Resilon Sealer, Epiphany Sealer, Gutta-Percha, AH26 Sealer
INTRODUCTION
Microorganisms in the root canal system play an integral part in pulpal and periapical disease. The purpose of endodontic treatment is to eliminate these microorganisms and to prevent canal reinfection [1]. These microorganisms are present in dentinal tubules, isthmuses and lateral canals, so an effective chemomechanical preparation by means of antimicrobial irrigants is needed. For eradication of the remaining microorganisms, selection of an irrigant with substantivity is beneficial [2]. In addition, a root filling material with antimicrobial activity and good sealing ability can prevent reinfection of the root canal [3].
MTAD is a mixture of 3% doxycycline, 4.25% citric acid and 0.5% Tween 80. It has antimicrobial activity against different microbes such as Enterococcus faecalis, the most common pathogen in persistent endodontic infections [4]. The antimicrobial properties of MTAD are attributed to doxycycline's antibiotic properties and exerting disturbance in bacterial cell wall by citric acid and Tween 80 [5]. It has sustained antimicrobial effect because of the high affinity of doxycycline to dentin [6]. MTAD can remove the smear layer with minimal changes on dentin structure [7]. Sustained antimicrobial activity of irrigants in unobturated teeth has been reported by some authors [8, 9]. For instance, a half-life as long as 3-weeks has been reported for doxycycline [8]. Another study evaluated the residual antimicrobial activity of BioPure MTAD in unobturated dentin tubes for 4 weeks and showed that the substantivity of MTAD was significantly higher than that of chlorhexidine and sodium hypochlorite [9]. There are different obturation materials such as gutta-percha/AH26 or Resilon/RealSeal SE. Root filling materials may affect doxycycline’s bonding to dentin and reduce its substantivity and antimicrobial activity overtime [10, 11]. A recent study conducted by Bolhari et al. showed that doxycycline was present in the canal at 1, 3 and 6 weeks after canal obturation with guttapercha/AH26 and Resilon/RealSeal SE [12]. Following the mentioned study, this study was designed to evaluate MTAD’s residual antibacterial activity in filled root canals after different time intervals.
MATHERIALS AND METHODS
Tooth preparation:
One-hundred and twenty extracted single-rooted human teeth without caries were selected for this study. The teeth were stored in 5.25% NaOCl for 30 minutes. Straight and angulated radiographs were taken to determine the root canal anatomy. Teeth with more than one canal or calcified root canals were excluded. Access cavities were prepared and the working lengths were determined. Root canals were prepared to the apical size #40 by Mtwo rotary instruments (VDW, Munich, Germany) according to the manufacturer’s instructions. All canals were irrigated between each file with 2 ml of sterile saline. Apical foramens were sealed with wax to prevent bacterial leakage.
The root surface of all teeth were covered with two layers of nail polish and dried. Final rinse with MTAD was done for ninety teeth according to the manufacturer’s instructions, so that a 2-minute rinse with 3 ml of 1.3% NaOCl was initially performed, then a brief rinse with 1 ml of MTAD (DentsplyTulsa, Tulsa, OK) was done and the irrigant was left in place for 5 minutes, finally a 1-minute flush with 4 ml of MTAD was carried out to finalize the irrigation. Canals were dried with paper points (Ariadent, Iran) and then randomly divided into six experimental groups (n=10), and three positive control groups (n=10). Experimental groups 1, 3 and 5 were obturated with guttapercha (Gapadent Co., Ltd., Korea) /AH26 (Dentsply, DeTrey, Germany) using the lateral compaction technique. Samples were restored with Coltosol (AriaDent, Iran). Experimental groups 2, 4 and 6 were obturated with Resilon/RealSeal SE (Pentron Clinical Technologies, USA) using the lateral compaction technique and light-cured for 40 seconds. Teeth were restored with Coltosol. Samples in groups 1 and 2, 3 and 4, and 5 and 6 were incubated at 37°C and 100% humidity for 1, 3 and 6 weeks, respectively.
Teeth that were irrigated with MTAD, but not obturated with gutta-percha/AH26 or Resilon/RealSeal SE were used as positive controls (10 teeth for each time point). MTAD solution alone was used as positive control too. The teeth that were not irrigated with MTAD, but obturated with gutta-percha/AH26 or Resilon/RealSeal SE were used as negative controls (10 teeth for each time point).
Dentin powder preparation:
Specimens were sterilized by gamma radiation (40 k gray) (ISO standard, 11137). At the designed time points, the teeth were split longitudinally by a high-speed diamond bur and a spatula. Under aseptic conditions, root filling material was removed and dentin powder was prepared from the middle thirds of the roots by using a #7 low-speed round bur. Dentin powder was prepared from a 3-millimeter-long area 2 mm apical to the CEJ corresponding with the diameter of the bur by which the sample was taken.
The dentin powder of each specimen was collected in a sterile eppendrof tube (1.5ml) and microbiological tests were carried out.
Microbiological procedure:
Lyophilized E. faecalis (ATCC 25922, obtained from Rayen Biotechnology Co. Ltd., Tehran, Iran) were rehydrated in brain heart infusion (BHI) broth (Merck, Darmstadt, Germany) and incubated in an aerobic atmosphere at 37 °C for 24h.
Fresh BHI bacterial cultures in the logarithmic growth phase (6–7h old) were adjusted to a concentration of 108 CFU (colony-forming units)/mL as verified by both spectrophotometry (OD600:0.4–0.5) (Biophotometer, Tokyo, Japan) and colony counting. The exact density (CFU/mL) of each suspension was verified on BHI plates. Dentin powders were prepared as previously described. In a typical assay, freshly prepared dentin powder was mixed with 500μL of bacterial suspension.
In addition, tubes containing 500 μL of the bacterial suspension without dentin powder were served as controls. The tubes were then incubated at 37°C under aerobic conditions and then measured after 24 hours. Bacterial cultures were diluted serially 10-fold by transferring 50 μL aliquots of the inocula into tubes containing 450 μL of BHI broth. 100 μL of bacterial culture from each tube was subcultured onto BHI plates, and bacterial growth and concentration was assessed. Throughout the experiments, cultures were checked for contamination by blind cultures on BHI plates. Residual antimicrobial activity was calculated by this formula:
(a=numbers of E. faecalis in prepared bacterial suspension (CFU/mL), b= E. faecalis numbers that remained after exposure to each sample (CFU/mL). Statistical analysis was performed using Mann-Whitney and Kruskal-Wallis test. In all analyses, the confidence level was set at p < 0.05.
RESULTS
The mean percentage of residual antimicrobial activity for control and test groups is shown in Table 1. In positive controls, the highest residual antimicrobial activity of MTAD was detected after 1, 3 and 6 weeks. In negative controls, antimicrobial activity of guttapercha/AH26 was higher than Resilon/RealSeal SE. In gutta-percha/AH26 group, the difference between group 1 with groups 3 and 5 was considered significant (p<0.05 and p<0.001), but there was no significant difference between groups 3 and 5 (p>0.05). Antimicrobial activity decreased gradually in a time-dependent manner and it was still considerable over 6 weeks. In Resilon/RealSeal SE group, the difference between groups 2 and 6 was significant (p<0.05), but there was no significant difference between groups 2 and 4 as well as groups 4 and 6 (p>0.05).
Table 1.
Mean Residual Antimicrobial Activity (%) and Standard Deviation at Different Time Points
| Study Groups |
Residual Antimicrobial Activity Percentage (%) at Different Time Points
|
||
|---|---|---|---|
| 1 week (Mean±SD) | 3 week (Mean±SD) | 6 week (Mean±SD) | |
| Gutta-Percha/AH26 | Group 1 98.93±0.78* |
(Group 3) 90.3±7.82* |
(Group 5) 76.08±17.70* |
| Resilon/RealSeal SE | (Group 2) 76.22±18.25 |
(Group 4) 71.87±15.04 |
(Group 6) 47.17±28.10 |
| Positive Control | 99.27±0.53 | 92.54±3.69 | 81.35±6.57 |
| MTAD Solution (Positive Control) | 99.99±0.002 | _ | _ |
| Negative Control (Gutta-Percha/AH26) | 20.87±6.14 | 16.43±5.73 | 12.26±3.51 |
| Negative Control (Resilon/RealSeal SE) | 16.51±8.36 | 5.51±2.18 | 1.64±0.98 |
Significantly higher antimicrobial activity in all time intervals; p<0.001
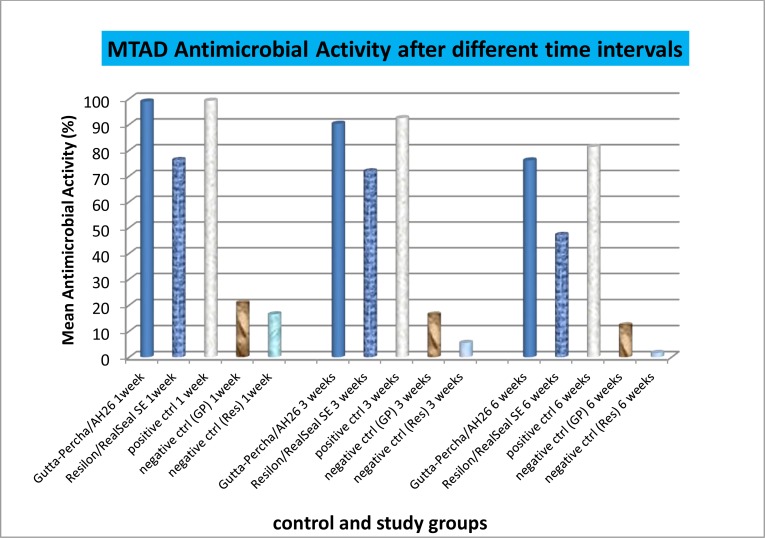
Antimicrobial activity decreased in a time-dependent manner, but its reduction was faster than that of the gutta-percha/AH26 group. Comparision between different groups is shown in figure 1.
Fig 1.
Mean residual antimicrobial activity in experimental and control groups
DISCUSSION
This study indicated that MTAD sustained its antimicrobial activity up to the sixth week, although it had a time-dependent decrease.
In addition, Resilon/RealSeal SE significantly decreased the residual antimicrobial activity of MTAD. Facultative bacteria such as E. faecalis have an important role in persistent infection of endodontically treated teeth. This bacterial strain can penetrate deeply into dentinal tubules and resist common endodontic irrigants [13]. Microorganisms that remain after canal instrumentation significantly increase failure in endodontic treatment. On the other hand, bacterial leakage through temporary restorations and root filling materials may contaminate the root canal system, so an antimicrobial irrigant with substantivity is beneficial in eradicating bacteria after canal obturation [14, 15]. MTAD is composed of three constituents that act synergistically against bacteria. Doxycycline is a bacteriostatic antibiotic used in high concentration in MTAD mixture [16]. Rasimick et al. reported an approximate three-week half-life for doxycycline in the unobturated tooth [8].
Tetracyclines are able to bind with cations in the tooth structure such as Ca2+ and Mg2+ and this can explain MTAD substantivity and its affinity to dentin [5]. Citric acid is a demineralizing agent that might cause cell wall damage by removing divalent cations [17, 18]. Tween 80 decreases the surface tension of MTAD and increases the penetration of MTAD to the dentinal tubules [19]. On the other hand, in a study carried out by Pappen et al., Tween 80 had a neutral or negative impact on the antimicrobial activity of MTAD [20].
In a study conducted by Torabinejad et al., MTAD was effective in killing E. faecalis even upon 1:200 dilution; but NaOCl ceased to exert its antibacterial activity beyond 1:32 dilution [6]. In another study, Rasimick et al. determined the antimicrobial activity of MTAD after exposure to simulated bacterial leakage. They reported that MTAD sustained its antimicrobial activity for more than 72 hours in unobturated canals [21]. The difference between the results of this study and the results we obtained may be due to the used bacterial leakage method and the unobturated canals. Another study performed by Rasimick et al. reported that the stability of doxycycline in unobturated canals was as long as 3 weeks [8]. On the other hand, Bolhari et al. evaluated doxycycline concentration in obturated root canals and concluded that it was present until 6 weeks after obturation, although it has a time-dependent decrease [12]. Their results were in accordance with our study. Khademi et al. showed that MTAD had an antimicrobial activity even at the fourth week and NaOCl had no substantivity [22]. Based on their results, the antimicrobial activity of MTAD reduced overtime, which was consistent with our study. Giardino et al. compared the antimicrobial efficacy of 5.25% NaOCl, MTAD, and Tetraclean against E. faecalis biofilm and found that only 5.25% NaOCl could completely remove the biofilm [23]. They concluded that although MTAD had substantivity, it is not as effective as NaOCl in the eradication of E. faecalis biofilms. More long-term studies regarding the efficacy of MTAD on E. faecalis biofilms are recommended.Various potential inactivators such as dentin, serum proteins, hydroxyapatite and collagen may affect the antimicrobial activity of endodontic irrigants [24]. In addition, other factors such as root filling materials might alter the sustained antimicrobial activity of MTAD [25]. Resilon/RealSeal SE is a new generation of Resilon/Epiphany system.
It consists of a self-adhesive resin-based sealer and Resilon. Self-etch primer, which is added to decrease binding errors, may decrease the environment pH [26]. Since it has been shown that acidic pH levels may affect the antimicrobial activity of the irrigants by altering the diffusion rate of its components [10, 11], this fact may explain the rapid reduction of antimicrobial activity in Resilon/RealSealSE groups. Epoxy resin sealers such as AH26 have acceptable physical properties, binding ability to dentin and apical sealing ability [27].
It has been reported that gutta percha/AH plus had better adhesion and less bacterial leakage than Resilon/Epiphany SE [28]. AH26 binds to amino groups of exposed collagen by its open epoxide rings [29]. Furthermore, this sealer has the ability to bind with amino groups of doxycycline through its epoxide rings [30]. These facts may explain the higher stability of MTAD and its antimicrobial activity in canals obturated with gutta percha /AH26 in our study. Generally, even though the residual antimicrobial activity of MTAD in Resilon/Epiphany SE groups was significantly decreased compared to that of MTAD in gutta percha/AH26, it has been well-documented that a successful outcome may be achieved with a high quality root canal therapy and effective coronal seal [31, 32]. Therefore, more clinical studies are required to determine the effect of the irrigant’s substantivity and root canal materials on the success or failure of the treatment.
CONCLUSION
Within the limitations of this study, MTAD sustained its antimicrobial activity even at the sixth week, although it showed a time-dependent decrease. Resilon/Epiphany SE significantly decreased the residual antimicrobial activity of MTAD at all time intervals.
Acknowledgments
This study was supported by Tehran University of Medical Sciences, Tehran, Iran (grant no: 91-01-69-15657)
REFERENCES
- 1.Poggio C, Colombo M, Scribante A, Sforza D, Bianchi S. In vitro antibacterial activity of different endodontic irrigants. Dent Traumatol. 2012 Jun;28(3):205–9. doi: 10.1111/j.1600-9657.2011.01074.x. [DOI] [PubMed] [Google Scholar]
- 2.Baca P, Junco P, Arias-Moliz MT, González-Rodríguez MP, Ferrer-Luque CM. Residual and antimicrobial activity of final irrigation protocols on Enterococcus faecalis Biofilm in dentin. J Endod. 2011 Mar;37(3):363–6. doi: 10.1016/j.joen.2010.11.036. [DOI] [PubMed] [Google Scholar]
- 3.Nawal R, Parande M, Sehgal R, Naik A, Rao N. A comparative evaluation of antimicrobial efficacy and flow properties for Epiphany, Guttaflow and AH-Plus sealer. Int Endod J. 2011 Apr;44(4):307–13. doi: 10.1111/j.1365-2591.2010.01829.x. [DOI] [PubMed] [Google Scholar]
- 4.Singla MG, Garg A, Gupta S. MTAD in endodontics: an update review. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011 Sep;112(3):e70–6. doi: 10.1016/j.tripleo.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 5.Sapadin AN, Fleischmajer R. Tetracyclines: nonantibiotic properties and their clinical implications. J Am Acad Dermatol. 2006 Feb;54(2):258–65. doi: 10.1016/j.jaad.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 6.Torabinejad M, Shabahang S, Aprecio RM, Kettering JD. The antimicrobial effect of MTAD: an in vitro investigation. J Endod. 2003 Jun;29(6):400–3. doi: 10.1097/00004770-200306000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Qian W, Shen Y, Haapasalo M. Quantitative analysis of the effect of irrigant solution sequences on dentin erosion. J Endod. 2011 Oct;37(10):1437–41. doi: 10.1016/j.joen.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 8.Rasimick BJ, Wan J, Musikant BL, Deutsch AS. Stability of Doxycycline and Chlorhexidine Absorbed on Root Canal Dentin. J Endod. 2010 Mar;36(3):489–92. doi: 10.1016/j.joen.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Mohammadi Z, Shahriari S. Residual antibacterial activity of chlorhexidine and MTAD in human root dentin in vitro. J Oral Sci. 2008 Mar;50(1):63–7. doi: 10.2334/josnusd.50.63. [DOI] [PubMed] [Google Scholar]
- 10.Williams A, Lemke L. Foye’s Principles of Medicinal Chemistry Fifth Edition Antiviral Agents and protease inhibitors. Philadelphia: Lippincott Williams & Wilkins; 2002. [Google Scholar]
- 11.Khalil SA, Daabis NA, Naggar VF, Motawi MM. The in vitro adsorption of some antibiotics on antacids. Pharmazie. 1976;31(2):105–9. [PubMed] [Google Scholar]
- 12.Bolhari B, Meraji N, Nosrat A, Hassani S. Stability of Doxycycline Absorbed on Root Canal Dentin After Obturation with Gutta-Percha/AH26 and Resilon/RealSeal at Different Time Intervals. J Dent (Tehran) 2013;10:4. [PMC free article] [PubMed] [Google Scholar]
- 13.Delgado R, Gasparoto TH, Sipert CR, Pinheiro CR, Moraes IG, Garcia RB, et al. Antimicrobial effects of calcium hydroxide and chlorhexidine on Enterococcus faecalis. J Endod. 2010 Aug;36(8):1389–93. doi: 10.1016/j.joen.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 14.Basmadjian-Charles CL, Farge P, Bourgeois DM, Lebrun T. Factors influencing the long-term results of endodontic treatment: a review of the literature. Int Dent J. 2002 Apr;52(2):81–6. doi: 10.1111/j.1875-595x.2002.tb00605.x. [DOI] [PubMed] [Google Scholar]
- 15.Shipper G, Teixeira FB, Arnold RR, Trope M. Periapical inflammation after coronal microbial inoculation of dog roots filled with gutta-percha or Resilon. J Endod. 2005 Feb;31(2):91–6. doi: 10.1097/01.don.0000140569.33867.bf. [DOI] [PubMed] [Google Scholar]
- 16.Shabahang S, Pouresmail M, Torabinejad M. In vitro antimicrobial efficacy of MTAD and sodium hypochlorite. J Endod. 2003 Jul;29(7):450–2. doi: 10.1097/00004770-200307000-00006. [DOI] [PubMed] [Google Scholar]
- 17.González-López S, Camejo-Aguilar D, Sanchez-Sanchez P, Bolaños-Carmona V. Effect of CHX on the decalcifying effect of 10% citric acid, 20% citric acid, or 17% EDTA. J Endod. 2006 Aug;32(8):781–4. doi: 10.1016/j.joen.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 18.Eswaranandam S, Hettiarachchy N, Johnson M. Antimicrobial Activity of Citric, Lactic, Malic, or Tartaric Acids and Nisin-incorporated Soy Protein Film Against Listeria monocytogenes, Escherichia coli O157: H7, and Salmonella gaminara. J Food Sci. 2006;69(3):79–84. [Google Scholar]
- 19.Stojicic S, Shen Y, Qian W, Johnson B, Haapasalo M. Antibacterial and smear layer removal ability of a novel irrigant, QMiX. Int Endod J. 2012 Apr;45(4):363–71. doi: 10.1111/j.1365-2591.2011.01985.x. [DOI] [PubMed] [Google Scholar]
- 20.Pappen F, Shen Y, Qian W, Leonardo M, Giardino L, Haapasalo M. In vitro antibacterial action of Tetraclean, MTAD and five experimental irrigation solutions. Int Endod J. 2010 Jun;43(6):528–35. doi: 10.1111/j.1365-2591.2010.01712.x. [DOI] [PubMed] [Google Scholar]
- 21.Rasimick BJ, Shah RP, Musikant BL, Deutsch AS. Bacterial colonisation of root canal dentine previously treated with endodontic irrigants. Aust Endod J. 2010 Aug;36(2):70–3. doi: 10.1111/j.1747-4477.2009.00193.x. [DOI] [PubMed] [Google Scholar]
- 22.Mohammadi Z, Havaee A. Evaluation of the antibacterial substantivity of several intra-canal agents. Aust Endod J. 2006 Dec;32(3):112–5. doi: 10.1111/j.1747-4477.2006.00033.x. [DOI] [PubMed] [Google Scholar]
- 23.Giardino L, Ambu E, Savoldi E, Rimondini R, Cassanelli C, Debbia EA. Comparative evaluation of antimicrobial efficacy of sodium hypochlorite, MTAD, and Tetraclean against Enterococcus faecalis biofilm. J Endod. 2007 Jul;33(7):852–5. doi: 10.1016/j.joen.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 24.Haapasalo M, Qian W, Portenier I, Waltimo T. Effects of dentin on the antimicrobial properties of endodontic medicaments. J Endod. 2007 Aug;33(8):917–25. doi: 10.1016/j.joen.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 25.Portenier I, Waltimo T, Ørstavik D, Haapasalo M. Killing of Enterococcus faecalis by MTAD and Chlorhexidine Digluconate with or without Cetrimide in the Presence or Absence of Dentine Powder or BSA. J Endod. 2006 Feb;32(2):138–41. doi: 10.1016/j.joen.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 26.Shokouhinejad N, Sabeti M, Gorjestani H, Saghiri MA, Lotfi M, Hoseini A. Penetration of Epiphany, Epiphany self-etch, and AH Plus into dentinal tubules: a scanning electron microscopy study. J Endod. 2011 Sep;37(9):1316–9. doi: 10.1016/j.joen.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 27.Hashem AA, Ghoneim AG, Lutfy RA, Fouda MY. The effect of different irrigating solutions on bond strength of two root canal-filling systems. J Endod. 2009 Apr;35(4):537–40. doi: 10.1016/j.joen.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 28.Oliveira A, Tanomaru J, Faria-Junior N, Tanomaru-Filho M. Bacterial leakage in root canals filled with conventional and MTA-based sealers. Int Endod J. 2011 Apr;44(4):370–5. doi: 10.1111/j.1365-2591.2011.01852.x. [DOI] [PubMed] [Google Scholar]
- 29.Amin SA, Seyam RS, El-Samman MA. The effect of prior calcium hydroxide intracanal placement on the bond strength of two calcium silicate-based and an epoxy resin-based endodontic sealer. J Endod. 2012 May;38(5):696–9. doi: 10.1016/j.joen.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 30.Lee KW, Williams MC, Camps JJ, Pashley DH. Adhesion of endodontic sealers to dentin and gutta-percha. J Endod. 2002 Oct;28(10):684–8. doi: 10.1097/00004770-200210000-00002. [DOI] [PubMed] [Google Scholar]
- 31.Gillen BM, Looney SW, Gu LS, Loushine BA, Weller RN, Loushine RJ, et al. Impact of the quality of coronal restoration versus the quality of root canal fillings on success of root canal treatment: a systematic review and meta-analysis. J Endod. 2011 Jul;37(7):895–902. doi: 10.1016/j.joen.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ng YL, Mann V, Gulabivala K. A prospective study of the factors affecting outcomes of nonsurgical root canal treatment: part 1: periapical health. Int Endod J. 2011 Jul;44(7):583–609. doi: 10.1111/j.1365-2591.2011.01872.x. [DOI] [PubMed] [Google Scholar]