Abstract
Background
Patients with atrial fibrillation often have cardiovascular risk factors or known comorbid disease, yet the use of evidence-based primary and secondary prevention cardiac therapy among atrial fibrillation outpatients is unknown.
Methods
Using baseline data collected between June 2010 and August 2011 from 174 sites participating in ORBIT-AF, a US national registry of patients with atrial fibrillation coordinated from Durham, NC, USA, we examined professional guideline -recommended evidence-based therapy use for cardiovascular comorbid conditions and risk factors. Multivariable logistic regression was used to identify factors associated with receipt of all indicated evidence-based therapy.
Results
Among 10096 enrolled patients, 93.5% were eligible for one or more evidence-based therapy. Among those eligible, 46.6% received all indicated therapies: 62.3% received an antiplatelet agent, 72.3% received a β-blocker, 59.5% received an angiotensin converting enzyme or angiotensin receptor blocker, 15.3% received an aldosterone antagonist, 65.7% received a statin, and 58.8% received implantable cardioverter-defibrillator. A minority of patients with coronary artery disease, diabetes mellitus, heart failure, and peripheral vascular disease received all indicated therapies (25.1%, 43.2%, 42.5%, and 43.4%, respectively). A total of 52.4% of patients had controlled hypertension and 74.6% of patients with hyperlipidemia received a statin. Factors associated with non-receipt of all indicated therapies included frailty, comorbid illness, geographic region, and antiarrhythmic drug therapy.
Conclusions
The majority of eligible atrial fibrillation outpatients did not receive all guideline-recommended therapies for cardiovascular comorbid conditions and risk factors. This represents a potential opportunity to improve atrial fibrillation patients’ quality of care and outcomes.
Keywords: atrial fibrillation, evidence-based medicine, registry
Introduction
Atrial fibrillation is a growing public health concern.1,2 The lifetime risk of developing atrial fibrillation is approximately 1 in 4 among US individuals ≥ 40 years of age.3 Approximately 2.66 million US adults have been diagnosed with atrial fibrillation.4 By the year 2050, the number of patients with diagnosed atrial fibrillation will exceed 5.6 million.5 Outcomes related to atrial fibrillation, including stroke6 and death,7 may likewise increase over time.
Cardiovascular comorbidities and risk factors are common among atrial fibrillation patients and elevate the risk of atrial fibrillation-related morbidity such as stroke.8,9 In fact, coexisting conditions and risk factors account for a substantial portion10 if not the entirety11 of atrial fibrillation-related mortality. Thus, modification of cardiovascular risk in atrial fibrillation patients and treatment of comorbid conditions via the use of proven primary and secondary prevention therapeutic interventions is highly desired. However, rates of evidence-based primary and secondary cardiac prevention therapy use among atrial fibrillation outpatients are unknown. Data regarding contemporary care of these patients may provide important insights into their clinical characteristics and associated treatment patterns and thus inform future quality improvement initiatives. Using baseline data from the Outcomes Registry for Better Informed Treatment Atrial Fibrillation (ORBIT-AF), the goals of this analysis were (1) to quantify the proportion of eligible atrial fibrillation outpatients receiving guideline-directed evidence-based therapy for coronary artery disease, diabetes mellitus, heart failure, hyperlipidemia, hypertension, and peripheral vascular disease; and (2) to identify factors associated with receipt of all indicated evidence-based therapy.
Methods
Data Source
ORBIT-AF is a national, observational, community-based, ongoing registry of outpatients with atrial fibrillation. The ORBIT-AF program has been described previously.12 Baseline data collected between June 2010 and August 2011 from 174 sites were the primary dataset for this analysis. Trained personnel at participating outpatient practices, including internal medicine, cardiology, and electrophysiology clinics, abstracted data on consecutive eligible atrial fibrillation patients and submitted them to the ORBIT-AF registry via Web-enabled case report forms.
Using standard definitions, data include demographic and clinical characteristics, medical history and prior treatments, type of atrial fibrillation, pharmacologic treatment strategy, and antithrombotic therapy and monitoring. The specialties of the enrolling physician and co-treating physicians (internal medicine, neurology, cardiology, electrophysiology) in the patient’s atrial fibrillation-related care were also captured.
Study Population
Patients ≥ 18 years of age with electrocardiographically documented atrial fibrillation were enrolled. For the current analysis, 2 records with incomplete information about the use of evidence-based therapy and 653 records of patients not eligible for at least one evidence-based therapy were excluded.
Outcome Measures
The principal outcome measure was the use of evidence-based therapy among eligible patients. Eligibility for evidence-based therapy was defined according to current professional guidelines endorsed by the American College of Cardiology Foundation/American Heart Association,13–15 the American Diabetes Association,16 the National Cholesterol Education Program,17 and the National High Blood Pressure Program.18 Specifically, patients with coronary artery disease were eligible for antiplatelet therapy, a β-blocker, an angiotensin converting enzyme inhibitor (ACEI) or angiotensin receptor blocker (ARB) in the presence of diabetes mellitus or a left ventricular ejection fraction ≤ 40%, a statin, and antihypertensive therapy in the presence of previously-diagnosed hypertension or elevated blood pressure during their baseline visit (blood pressure ≥ 140/90 mm Hg or blood pressure ≥ 130/80 mm Hg among patients with diabetes mellitus or chronic kidney disease). Patients with diabetes mellitus were eligible for an ACEI/ARB if indicated, a statin, and antihypertensive therapy if indicated. Heart failure patients were eligible for a β-blocker, an ACEI/ARB if indicated, an aldosterone antagonist in the presence of New York Heart Association Class III-IV symptoms and creatinine ≤ 2.5 mg/dL among men or ≤ 2.0 mg/dL among women, antihypertensive therapy if indicated, and implantable cardioverter-defibrillator therapy in the presence of a left ventricular ejection fraction ≤ 35% and New York Heart Association Class II-III symptoms. Patients with hyperlipidemia were eligible for statin therapy in the presence of coronary artery disease, diabetes mellitus, and/or peripheral vascular disease. Hypertension treatment was defined by receipt of an antihypertensive medication, while hypertension control was defined by blood pressure < 140/90 mm Hg in the absence of diabetes mellitus or chronic kidney disease or blood pressure < 130/80 mm Hg in the presence of one or both of these comorbidities. Patients with peripheral vascular disease were eligible for antiplatelet therapy and a statin. Eligibility criteria for evidence-based therapy according to cardiovascular risk factors and comorbidities are further detailed in Supplementary Table 1.
Statistical Analysis
We compared the baseline characteristics of patients who received all evidence-based therapy to those of patients who did not receive all evidence-based therapy using χ2 tests for categorical variables and Kruskal-Wallis tests for continuous variables. We report percentages for categorical variables and medians and interquartile ranges (IQR) for continuous variables. The proportion of patients with each cardiovascular comorbidity and risk factor and corresponding use of evidence-based therapy was determined.
To identify factors associated with receipt of all indicated evidence-based therapy, we constructed a multivariable logistic regression model after stratification by the 64 combinations of 6 comorbidities (coronary artery disease, heart failure, hypertension, hyperlipidemia, peripheral vascular disease, and diabetes mellitus). Covariate associations were therefore determined within strata representing subjects with equivalent sets of comorbidities and treatment eligibilities. Strata without representation of both receipt and non-receipt of evidence-based therapy (0.8% of patients) and the stratum without comorbidities were excluded. Candidate variables were selected on the basis of prior literature and clinical experience. The initial model included variables for age, sex, race, insurance status, educational status, body mass index, heart rate, thyroid disease, obstructive sleep apnea, cognitive impairment, liver disease, alcohol abuse, cancer, osteoporosis, hip fracture, history of gastrointestinal bleeding, dialysis-dependence, anemia, frailty (a clinical syndrome in which 3 or more of the following are present: unintentional weight loss of ≥ 10 pounds, self-reported exhaustion, poor grip strength, slow walking speed, and low physical activity), chronic obstructive pulmonary disease, drug abuse (history of current, recent, or remote abuse of any controlled substance), current smoking, family history of atrial fibrillation, sinus node dysfunction or sick sinus syndrome, creatinine clearance, hemoglobin, current antiarrhythmic drug use, catheter ablation of atrial fibrillation, prior stroke or transient ischemic attack, renal insufficiency, past warfarin use, current warfarin use, contraindications to oral anticoagulant therapy, functional status, and provider specialty. Continuous variables were tested for linearity, and non-linear variables were transformed using spline functions or truncated. Using backward selection, factors for which P was ≥ 0.05 were excluded from the model. Missing covariate data (< 11%) were handled by multiple imputation using Markov Chain Monte Carlo and propensity methods. Final estimates and associated standard errors reflect the combined analysis over five imputed data sets.
P values of <0.05 were considered statistically significant, and all tests were 2-sided. Analyses were performed using SAS software version 9.3 (SAS Institute, Cary, NC). The institutional review board of the Duke University Health System and each enrolling center approved this study. The authors had full access to the data and have read and agree to the manuscript as written.
Results
Among 10096 enrolled patients, 9443 (93.5%) were eligible for one or more evidence-based therapy.Table 1 shows the baseline characteristics of the evidence-based therapy-eligible atrial fibrillation cohort. The median age was 75 (IQR 67–82) years, 57.4% were male, and 89.0% were white. A total of 4398 patients (46.6%) received all evidence-based therapy indicated for their cardiovascular comorbidities and risk factors. In comparison to patients who received all indicated evidence-based therapies, patients who did not were older (76 [IQR 69–83] years vs. 74 [IQR 66–81] years), more often male (59.0% vs. 55.5%), less frequently had private insurance (25.0% vs. 31.4%), and more frequently had Medicare or Medicaid (74.2% vs. 67.5%). They had a higher prevalence of most cardiovascular comorbidities and risk factors: 50.2% vs. 15.9% had coronary artery disease, 42.4% vs. 18.8% had diabetes mellitus, 46.0% vs. 21.7% had heart failure, and 20.8% vs. 6.8% had peripheral vascular disease. They also had a higher prevalence of most non-cardiovascular comorbidities, including anemia, cancer, cognitive impairment/dementia, chronic obstructive pulmonary disease, dialysis-dependence, frailty, history of gastrointestinal bleeding, history of hip fracture, history of stroke or transient ischemic attack, liver disease, obstructive sleep apnea, and sinus node dysfunction/sick sinus syndrome. They were more likely to have been treated with or to currently take warfarin. Further, the practices where they received their medical care were more likely to be located in the South and West.
Table 1.
Patient Characteristics*
| Characteristic | Total (n=9443) | All EBT (n=4398) | Not all EBT (n=5045) | P Value |
|---|---|---|---|---|
| Age, median (IQR), y | 75 (67–82) | 74 (66–81) | 76 (69–83) | <0.001 |
| Male, % | 57.4 | 55.5 | 59.0 | <0.001 |
| White, % | 89.0 | 89.0 | 89.0 | 0.923 |
| Insurance, % | <0.001 | |||
| Private | 28.0 | 31.4 | 25.0 | |
| Medicare or Medicaid | 71.1 | 67.5 | 74.2 | |
| Systolic blood pressure, median (IQR), mm Hg | 126 (117–138) | 128 (118–138) | 126 (115–138) | <0.001 |
| Diastolic blood pressure, median (IQR), mm Hg | 72 (66–80) | 74 (68–80) | 71 (64–80) | <0.001 |
| Heart rate, median (IQR), bpm | 70 (63–80) | 70 (62–80) | 70 (64–80) | 0.002 |
| Body mass index, median (IQR)† | 29.3 (25.4–34.3) | 29.6 (25.8–34.4) | 29.0 (25.1–34.2) | <0.001 |
| Cardiovascular comorbidities and risk factors, % | ||||
| Coronary artery disease | 34.2 | 15.9 | 50.2 | <0.001 |
| Diabetes mellitus | 31.4 | 18.8 | 42.4 | <0.001 |
| Heart failure | 34.7 | 21.7 | 46.0 | <0.001 |
| Hyperlipidemia | 74.0 | 73.7 | 74.2 | 0.541 |
| Hypertension | 98.5 | 99.5 | 97.6 | <0.001 |
| Peripheral vascular disease | 14.2 | 6.8 | 20.8 | <0.001 |
| Other medical history, % | ||||
| Anemia | 19.0 | 15.3 | 22.2 | <0.001 |
| Cancer | 24.1 | 22.8 | 25.2 | 0.005 |
| Cognitive impairment/dementia | 3.2 | 2.4 | 3.9 | <0.001 |
| COPD | 17.0 | 12.8 | 20.7 | <0.001 |
| Dialysis | 1.3 | 0.8 | 1.7 | <0.001 |
| Frailty | 6.0 | 3.9 | 7.8 | <0.001 |
| GI bleed | 9.4 | 7.8 | 10.8 | <0.001 |
| Hip fracture | 2.6 | 2.0 | 3.2 | <0.001 |
| History stroke or transient ischemic attack | 16.1 | 10.4 | 21.1 | <0.001 |
| Liver disease | 1.9 | 1.5 | 2.3 | 0.003 |
| Obstructive sleep apnea | 18.5 | 17.2 | 19.7 | 0.002 |
| Osteoporosis | 13.4 | 13.7 | 13.1 | 0.373 |
| Severe renal disease (eGFR < 30 or dialysis) | 11.1 | 10.3 | 11.8 | 0.021 |
| Sinus node dysfunction/sick sinus syndrome | 18.0 | 15.6 | 20.0 | <0.001 |
| Thyroid disease | 22.7 | 21.3 | 23.9 | 0.003 |
| Functional status | <0.001 | |||
| Living independently | 90.1 | 93.0 | 87.5 | |
| Living with assistance | 7.5 | 5.1 | 9.6 | |
| Residing in assisted living facility | 1.4 | 1.1 | 1.7 | |
| Residing in skilled nursing home | 0.4 | 0.1 | 0.6 | |
| Laboratory Data, median (IQR) | ||||
| eGFR, mL/min/1.73 m2 | 68.6 (49.2–95.4) | 73.2 (53.0–101.1) | 64.2 (46.4–90.1) | <0.001 |
| Hemoglobin | 13.5 (12.2–14.6) | 13.7 (12.5–14.8) | 13.2 (12.0–14.4) | <0.001 |
| Left ventricular ejection fraction, % | <0.001 | |||
| Normal (≥ 50%) | 69.3 | 75.2 | 64.2 | |
| Mild dysfunction (>40%, <50%) | 6.4 | 5.6 | 7.1 | |
| Moderate dysfunction (≥30% to 40%) | 9.5 | 6.1 | 12.5 | |
| Severe dysfunction (<30%) | 4.5 | 2.3 | 6.4 | |
| Current antiarrhythmic drug therapy | 28.3 | 28.2 | 28.4 | 0.791 |
| Warfarin | ||||
| History of treatment | 82.8 | 81.1 | 84.4 | <0.001 |
| Current treatment | 72.7 | 71.3 | 73.9 | 0.005 |
| Region | <0.001 | |||
| Midwest | 25.6 | 26.7 | 24.7 | |
| Northeast | 25.8 | 27.8 | 24.1 | |
| South | 34.8 | 32.8 | 36.5 | |
| West | 13.8 | 12.7 | 14.8 | |
| Provider specialty | 0.289 | |||
| Cardiology | 65.6 | 65.5 | 65.7 | |
| Electrophysiology | 14.8 | 15.3 | 14.3 | |
| Family Practice/Internal Medicine | 19.6 | 19.2 | 20.0 | |
Abbreviations: EBT, evidence-based therapy; IQR, interquartile range; eGFR, estimated glomerular filtration rate.
Data are based on patients with available data for each characteristic.
Body mass index is calculated as weight in kilograms divided by height in meters squared.
The proportion of eligible atrial fibrillation patients receiving evidence-based therapy are shown in Figure 1: 62.3% received an antiplatelet agent, 72.3% received a β-blocker, 59.5% received an ACEI/ARB, 15.3% received an aldosterone antagonist, 65.7% received a statin, 52.4% with hypertension had it controlled, 58.8% received an implantable cardioverter-defibrillator, and 46.6% received all indicated evidence-based therapies. Table 2 shows receipt of evidence-based therapy according to cardiovascular comorbidity or risk factor. A minority of patients with coronary artery disease, diabetes mellitus, heart failure, and peripheral vascular disease received all corresponding indicated evidence-based therapies (25.1%, 43.2%, 42.5%, and 43.4%, respectively). A total of 74.6% of patients with hyperlipidemia received a statin. Table 3 shows factors independently associated with receipt or lack of receipt of all evidence-based therapy in the total atrial fibrillation cohort: frailty, geographic region, prior stroke or transient ischemic attack, chronic obstructive pulmonary disease, current antiarrhythmic drug therapy, renal function, osteoporosis, and thyroid disease.
Figure 1.
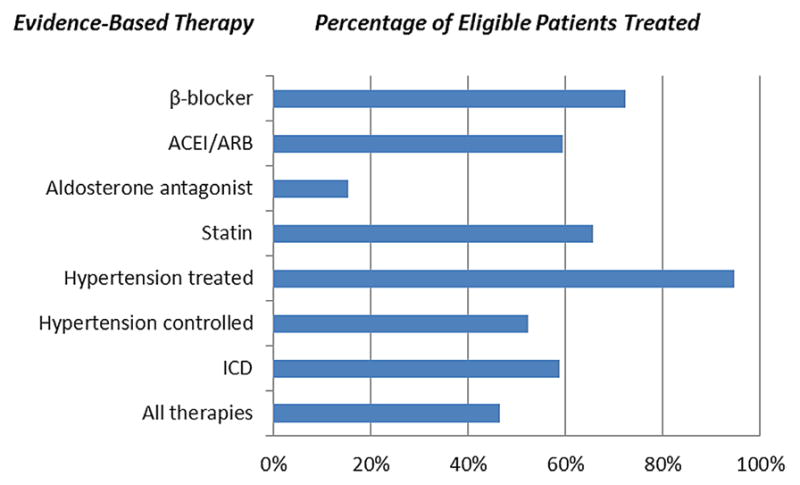
Receipt of Evidence-Based Medicine Among Eligible Patients in the Total Atrial Fibrillation Cohort
Table 2.
Receipt of Evidence-Based Therapy by Cardiovascular Comorbidity or Risk Factor
| Evidence-Based Therapy | Coronary Artery Disease (n=3,232) | Diabetes Mellitus (n=2,968) | Heart Failure (n=3,275) | Hyperlipidemia (n=4,829) | Hypertension (n=8,731) | Peripheral Vascular Disease (n=1,345) |
|---|---|---|---|---|---|---|
| Antiplatelet agent | 66.0 | - | - | - | - | 58.6 |
| β-blocker | 73.3 | - | 74.9 | - | - | - |
| ACEI/ARB | 58.1 | 61.4 | 61.7 | - | - | - |
| Aldosterone antagonist | - | - | 15.3 | - | - | - |
| Statin | 72.9 | 66.3 | - | 74.6 | - | 68.3 |
| Hypertension | ||||||
| Treated | 96.6 | 95.9 | 97.8 | - | - | - |
| Controlled | 55.4 | 44.3 | 57.7 | - | 52.4 | - |
| Implantable cardioverter-defibrillator | - | - | 58.8 | - | - | - |
| All therapies | 25.1 | 43.2 | 42.5 | 74.6 | 52.4 | 43.4 |
Data are presented as percentages of each cardiovascular comorbidity or risk factor. Analysis was confined to eligible patients according to current professional guidelines.
Table 3.
Factors Associated With Receipt of Evidence-Based Therapy Among Patients With Atrial Fibrillation*
| Factor | OR (95% CI) | P Value |
|---|---|---|
| White | 0.85 (0.72, 1.00) | 0.045 |
| Location | <0.001 | |
| West v. Northeast | 0.63 (0.53, 0.75) | <0.001 |
| South v. Northeast | 0.72 (0.63, 0.82) | <0.001 |
| Midwest v. Northeast | 0.95 (0.82, 1.09) | 0.450 |
| Heart rate < 66, per 10-bpm increase | 0.89 (0.84, 0.95) | 0.003 |
| Functional status | 0.018 | |
| Living with assistance v. Living independently | 0.74 (0.60, 0.92) | 0.005 |
| Residing in assisted living facility v. Living independently | 0.70 (0.46, 1.09) | 0.114 |
| Residing in skilled nursing home v. Living independently | 0.68 (0.25, 1.84) | 0.451 |
| Body mass index, per 2.5 kg/m2 increase | 1.02 (1.00, 1.04) | 0.022 |
| Frailty | 0.75 (0.59, 0.95) | 0.016 |
| Chronic obstructive pulmonary disease | 0.84 (0.73, 0.97) | 0.015 |
| Osteoporosis | 1.19 (1.02, 1.39) | 0.031 |
| Prior stroke or transient ischemic attack | 0.40 (0.34, 0.46) | <0.001 |
| Thyroid disease | 0.87 (0.77, 0.98) | 0.023 |
| eGFR, per 10 mL/min/1.73 m2 decrease | 0.94 (0.92, 0.97) | <0.001 |
| Current antiarrhythmic drug therapy | 0.78 (0.70, 0.88) | <0.001 |
Abbreviations: OR, odds ratio; CI, confidence interval
Listed variables were significant factors in the final logistic regression model that influenced receipt of all indicated evidence-based therapy. Variables in the initial model included age, sex, race, insurance status, educational status, body mass index, heart rate, thyroid disease, obstructive sleep apnea, cognitive impairment, liver disease, alcohol abuse, cancer, osteoporosis, hip fracture, GI bleed, dialysis-dependence, anemia, frailty, COPD, drug abuse, family history of atrial fibrillation, sinus node dysfunction or sick sinus syndrome, estimated glomerular filtration rate, current antiarrhythmic drug use, current smoking, catheter ablation of atrial fibrillation, prior stroke or transient ischemic attack, renal insufficiency, past warfarin use, current warfarin use, contraindications to oral anticoagulant therapy, functional status, and provider specialty.
Discussion
We examined the quality of care for atrial fibrillation-related cardiovascular comorbidities and risk factors in a national atrial fibrillation registry. There were three main findings from our study. First, the vast majority of atrial fibrillation outpatients were eligible for evidence-based therapy. Second, evidence-based therapies were significantly underused in eligible atrial fibrillation patients. Third, several important factors were independently associated with evidence-based therapy under use, including frailty, comorbid illness, geographic region, and antiarrhythmic drug therapy.
Mortality in atrial fibrillation patients is high, and anticoagulation may only partially reduce its occurrence.19 Emerging evidence suggests that many therapies traditionally reserved for non-atrial fibrillation conditions prevent the development of atrial fibrillation20–22 or reduce its recurrence.23–26 Treating cardiovascular comorbidities and risk factors as a means to potentially reduce atrial fibrillation-related morbidity and mortality, however, represents an often-overlooked therapeutic paradigm.27 This approach is attractive because the proportion of atrial fibrillation-related mortality attributable to coexisting conditions and risk factors is high10,11 and receipt of evidence-based therapy has been shown to reduce mortality.28,29 Despite clear professional guideline recommendations, however, one out of every two eligible patients in the current analysis did not receive one or more indicated evidence-based therapy. These gaps in care represent potential opportunities to improve atrial fibrillation patient outcomes.
Prior studies have demonstrated evidence-based therapy under use among outpatients with each of the studied cardiovascular comorbidities and risk factors.30–35 A novelty of the current analysis lies in its assessment of outpatient evidence-based therapy use in the context of atrial fibrillation. Baseline data from the Registry to Improve the Use of Evidence-Based Heart Failure Therapies in the Outpatient Setting (IMPROVE HF), provides a recent, US-based reference dataset.32 Evidence-based therapy use was suboptimal among IMPROVE HF enrollees. In comparison, however, ORBIT-AF enrollees with heart failure were even less likely to receive most evidence-based therapy (74.9% v. 87.6% received a β-blocker, 61.7% v. 79.5% received an ACEI/ARB, 15.3% v. 33.3% received an aldosterone antagonist, 97.8%, and 58.8% v. 49.1% received implantable cardioverter-defibrillator therapy). Though not accounting for case-mix or inter-practice variation, these direct comparisons nonetheless suggest that those with atrial fibrillation are even less likely to receive evidence-based therapy than their heart failure counterparts. The degree to which the presence of atrial fibrillation influences receipt of evidence-based therapy among patients with heart failure and other cardiovascular comorbidities and risk factors in the current era requires further study.
The current analysis underscores the importance of frailty in the receipt of evidence-based therapy. A geriatric syndrome of heightened vulnerability to stressors,36 frailty is associated with death and disability in patients with heart disease.37 Although the benefits of evidence-based therapy should be high in the setting of corresponding high risk, data are limited. The American Heart Association therefore has called for further study of frailty and its relation to treatment outcomes.38,39
Our finding that antiarrhythmic drug therapy may pose as a barrier to receipt of evidence-based therapy for cardiac comorbid conditions or risk factors is novel. Amiodarone receipt may preclude the use of a traditional β-blocker by virtue of its intrinsic β-blocking properties. However, clinical trial data suggest that discontinuation of a β-blocker in favor of amiodarone increases the likelihood of all-cause mortality, particularly in the post-myocardial infarction40 and heart failure41 settings. In contrast to evidence-based therapy, antiarrhythmic medications have not been shown to reduce mortality. Choosing to either initiate or continue a β-blocker rather than using antiarrhythmic medication alone is therefore preferred except in instances of evidence-based therapy intolerance or advanced symptoms necessitating antiarrhythmic use. As many patients on antiarrhythmic therapy are otherwise healthy, under appreciation of patient risk on the part of physicians prescribing antiarrhythmic agents may also play a role.
Regional variation in receipt of all indicated evidence-based therapy also offers unique insight not observed in prior United States-based, outpatient atrial fibrillation registries.42,43 Evidence-based therapy use was greatest in the Northeast, whereas treatment rates were lower in the South and West. After adjustment for demographic and clinical factors, these relationships persisted. The South and West therefore will merit close attention in future quality improvement initiatives.
Some factors associated with low evidence-based therapy use may reflect sound physician judgment. For example, the risk-benefit ratio for patients with a prior a prior hemorrhagic stroke or who are currently taking warfarin may be in favor of not using an antiplatelet agent. Patients receiving evidence-based therapy may be more likely to undergo screening for osteoporosis, leading to a surveillance bias.
Limitations
The study population was derived from practices participating in a voluntary registry and may not be fully representative of atrial fibrillation patients in the US. Data were acquired via chart review, and their accuracy is therefore dependent on completeness of initial documentation and thoroughness of subsequent abstraction. Reasons for not providing therapy in the absence of clear contraindications or other potentially important variables such as rural v. urban residence were not collected and thus could not be factored into these analyses. Low density lipoprotein levels were not available and thus eligibility for statin therapy was based on the presence of comorbidities alone. As with any observational analysis, residual unmeasured confounders may exist and impact the validity of our results.
Conclusions
In the ORBIT-AF registry, the vast majority of atrial fibrillation outpatients were eligible for primary or secondary prevention intervention. Cardiovascular comorbidities and risk factors in atrial fibrillation outpatients were often inadequately treated with guideline-recommended evidence-based therapy, underscoring opportunities to improve atrial fibrillation patients’ quality of medical care and outcomes. Further, a number of important patient and practice factors were independently associated with incomplete use of evidence-based therapy, including frailty, geographic region, prior stroke or transient ischemic attack, chronic obstructive disease, and antiarrhythmic drug use. These findings should be investigated in other atrial fibrillation populations, as they may play important roles in the careful construction of future atrial fibrillation quality improvement initiatives.
Supplementary Material
Acknowledgments
Funding Source: Ortho-McNeil Janssen Scientific Affairs, LLC
Footnotes
Potential conflicts of interest:
Paul L. Hess, MD- None
Sunghee Kim, PhD- None
Jonathan P. Piccini, MD, MHS- Johnson & Johnson, Bayer Healthcare, and Boston-Scientific, research grants; Johnson & Johnson, Forest Laboratories, and Sanofi-Aventis, consulting fees/honoraria
Larry A. Allen, MD, MHS- None
Jack E. Ansell, MD- Bristol-Myers Squibb, Pfizer, Daiichi Sankyo, Janssen, and Boehringer Ingelheim, consulting fees/honoraria
Paul Chang, MD- Johnson & Johnson, employed
JamesV. Freeman, MD, MPH- None
Bernard J. Gersh, MB, ChB, Dphil- None
Peter R. Kowey, MD- None
Kenneth W. Mahaffey, MD- AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Eli Lilly, Glaxo Smith Klein, Johnson & Johnson, Merck, Momenta Pharmaceuticals, Novartis, Portola, Pozen, Regado Biotechnologies, Sanofi-Aventis, Schering-Plough, and the Medicines Company, research grants; AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Eli Lilly, Glaxo Smith Klein, Johnson & Johnson Pharmaceutical Research and Development, Merck, Novartis, Ortho/MacNeill, Pfrizer, Polymedix, Sanofi-Aventis, Schering-Plough, consulting fees/honoraria
Laine Thomas, PhD- None
Eric D. Peterson, MD, MPH- Eli Lilly & Company and Johnson & Johnson Pharmaceutical Research and Development, research grants
Gregg C. Fonarow, MD- MedTronic, consulting fees/honoraria
Attestation: All authors had access to the data and a role in writing the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Braunwald E. Shattuck lecture- cardiovascular medicine at the turn of the millenium: Triumphs, concerns, and opportunities. N Engl J Med. 1997;337(19):1360–1369. doi: 10.1056/NEJM199711063371906. [DOI] [PubMed] [Google Scholar]
- 2.Lloyd-Jones DM, Wang TJ, Leip EP, et al. Lifetime risk for development of atrial fibrillation: The Framingham Heart Study. Circulation. 2004 Aug 31;110(9):1042–1046. doi: 10.1161/01.CIR.0000140263.20897.42. [DOI] [PubMed] [Google Scholar]
- 3.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics--2012 update: A report from the American Heart Association. Circulation. 2012 Jan 3;125(1):e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics--2011 update: A report from the American Heart Association. Circulation. 2011 Feb 1;123(4):e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults; national implications for rhythm management and stroke prevention: The AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) study. JAMA. 2001;285:2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 6.Wolf PA, Dawber TR, Thomas E, Kannel WB. Epidemiologic assessment of chronic atrial fibrillation and risk of stroke: The Framingham Heart Study. Neurology. 1978;28:973–977. doi: 10.1212/wnl.28.10.973. [DOI] [PubMed] [Google Scholar]
- 7.Benjamin EJ, Wolf PA, D’Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: The Framingham Heart Study. Circulation. 1998;98:946–952. doi: 10.1161/01.cir.98.10.946. [DOI] [PubMed] [Google Scholar]
- 8.Wang TJ, Massaro JM, Levy D, et al. A risk score for predicting stroke or death in individuals with new-onset atrial fibrillation in the community: The Framingham Heart Study. JAMA. 2003;290:1049–1056. doi: 10.1001/jama.290.8.1049. [DOI] [PubMed] [Google Scholar]
- 9.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: The Euro Heart Survey on atrial fibrillation. Chest. 2010 Feb;137(2):263–272. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 10.Conen D, Chae CU, Glynn RJ, et al. Risk of death and cardiovascular events in initially healthy women with new-onset atrial fibrillation. JAMA. 2011;305(20):2080–2087. doi: 10.1001/jama.2011.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jahangir A, Lee V, Friedman PA, et al. Long-term progression and outcomes with aging in patients with lone atrial fibrillation: A 30-year follow-up study. Circulation. 2007 Jun 19;115(24):3050–3056. doi: 10.1161/CIRCULATIONAHA.106.644484. [DOI] [PubMed] [Google Scholar]
- 12.Piccini JP, Fraulo ES, Ansell JE, et al. Outcomes registry for better informed treatment of atrial fibrillation: Rationale and design of ORBIT-AF. Am Heart J. 2011 Oct;162(4):606–612. e601. doi: 10.1016/j.ahj.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 13.Smith SC, Jr, Benjamin EJ, Bonow RO, et al. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: A guideline from the American Heart Association and American College of Cardiology Foundation. Circulation. 2011 Nov 29;124(22):2458–2473. doi: 10.1161/CIR.0b013e318235eb4d. [DOI] [PubMed] [Google Scholar]
- 14.Jessup M, Abraham WT, Casey DE, et al. 2009 focused update: ACCF/AHA guidelines for the diagnosis and management of heart failure in adults: A report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines: Developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009 Apr 14;119(14):1977–2016. doi: 10.1161/CIRCULATIONAHA.109.192064. [DOI] [PubMed] [Google Scholar]
- 15.Rooke TW, Hirsch AT, Misra S, et al. ACCF/AHA focused update of the guideline for the management of patients with peripheral artery disease (updating the 2005 guideline): A report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. Circulation. 2011 Sep 29; doi: 10.1016/j.jacc.2011.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.American Diabetes Association. Standards of medical care in diabetes--2011. Diabetes Care. 2011 Jan;34 (Suppl 1):S11–61. doi: 10.2337/dc11-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program adult treatment panel iii guidelines. Circulation. 2004 Jul 13;110(2):227–239. doi: 10.1161/01.CIR.0000133317.49796.0E. [DOI] [PubMed] [Google Scholar]
- 18.Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure: The JNC 7 report. JAMA. 2003 May 21;289(19):2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 19.Hess PL, Greiner MA, Fonarow GC, et al. Outcomes associated with warfarin use in older patients with heart failure and atrial fibrillation and a cardiovascular implantable electronic device: Findings from the ADHERE registry linked to medicare claims. Clin Cardiol. 2012 Nov;35(11):649–657. doi: 10.1002/clc.22064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nasr IA, Bouzamondo A, Hulot JS, Dubourg O, Le Heuzey JY, Lechat P. Prevention of atrial fibrillation onset by beta-blocker treatment in heart failure: A meta-analysis. Eur Heart J. 2007 Feb;28(4):457–462. doi: 10.1093/eurheartj/ehl484. [DOI] [PubMed] [Google Scholar]
- 21.Liu T, Li L, Korantzopoulos P, Liu E, Li G. Statin use and development of atrial fibrillation: A systematic review and meta-analysis of randomized clinical trials and observational studies. Int J Cardiol. 2008 May 23;126(2):160–170. doi: 10.1016/j.ijcard.2007.07.137. [DOI] [PubMed] [Google Scholar]
- 22.Schneider MP, Hua TA, Bohm M, Wachtell K, Kjeldsen SE, Schmieder RE. Prevention of atrial fibrillation by renin-angiotensin system inhibition: A meta-analysis. J Am Coll Cardiol. 2010 May 25;55(21):2299–2307. doi: 10.1016/j.jacc.2010.01.043. [DOI] [PubMed] [Google Scholar]
- 23.Ueng KC, Tsai TP, Yu WC, et al. Use of enalapril to facilitate sinus rhythm maintenance after external cardioversion of long-standing persistent atrial fibrillation. Eur Heart J. 2003;24:2090–2098. doi: 10.1016/j.ehj.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 24.Madrid AH. Use of irbesartan to maintain sinus rhythm in patients with long-lasting persistent atrial fibrillation: A prospective and randomized study. Circulation. 2002;106(3):331–336. doi: 10.1161/01.cir.0000022665.18619.83. [DOI] [PubMed] [Google Scholar]
- 25.Williams RS, deLemos JA, Dimas V, Reisch J, Hill JA, Naseem RH. Effect of spironolactone on patients with atrial fibrillation and structural heart disease. Clin Cardiol. 2011 Jul;34(7):415–419. doi: 10.1002/clc.20914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fauchier L, Pierre B, de Labriolle A, Grimard C, Zannad N, Babuty D. Antiarrhythmic effect of statin therapy and atrial fbrillation: A meta-analysis of randomized controlled trials. J Am Coll Cardiol. 2008 Feb 26;51(8):828–835. doi: 10.1016/j.jacc.2007.09.063. [DOI] [PubMed] [Google Scholar]
- 27.Fuster V, Ryden LE, Cannom DS, et al. 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: A report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. Circulation. 2011;123:e269–e367. doi: 10.1161/CIR.0b013e318214876d. [DOI] [PubMed] [Google Scholar]
- 28.Fonarow GC, Albert NM, Curtis AB, et al. Improving evidence-gased care for heart failure in outpatient cardiology practices: Primary results of the registry to Improve the Use of Evidence-Based Heart Failure Therapies in the Outpatient Setting (IMPROVE HF) Circulation. 2010 Aug 10;122(6):585–596. doi: 10.1161/CIRCULATIONAHA.109.934471. [DOI] [PubMed] [Google Scholar]
- 29.Gaede P, Lund-Anderson H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358:580–591. doi: 10.1056/NEJMoa0706245. [DOI] [PubMed] [Google Scholar]
- 30.Newby LK, LaPointe NM, Chen AY, et al. Long-term adherence to evidence-based secondary prevention therapies in coronary artery disease. Circulation. 2006 Jan 17;113(2):203–212. doi: 10.1161/CIRCULATIONAHA.105.505636. [DOI] [PubMed] [Google Scholar]
- 31.Saydah SH, Fradkin J, Cowie CC. Poor control of risk factors for vascular disease among adults with previously diagnosed diabetes. JAMA. 2004 Jan 21;291(3):335–342. doi: 10.1001/jama.291.3.335. [DOI] [PubMed] [Google Scholar]
- 32.Fonarow GC, Yancy CW, Albert NM, et al. Heart failure care in the outpatient cardiology practice setting: Findings from IMPROVE HF. Circ Heart Fail. 2008 Jul;1(2):98–106. doi: 10.1161/CIRCHEARTFAILURE.108.772228. [DOI] [PubMed] [Google Scholar]
- 33.Jackevicius CA, Mamdani M, Tu JV. Adherence with statin therapy in elderly patients with and without acute coronary syndromes. JAMA. 2002 Jul 24–31;288(4):462–467. doi: 10.1001/jama.288.4.462. [DOI] [PubMed] [Google Scholar]
- 34.Egan BM, Zhao Y, Axon RN. Us trends in prevalence, awareness, treatment, and control of hypertension, 1988–2008. JAMA. 2010 May 26;303(20):2043–2050. doi: 10.1001/jama.2010.650. [DOI] [PubMed] [Google Scholar]
- 35.Hirsch AT, Criqui MH, Treat-Jacobson D, et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001 Sep 19;286(11):1317–1324. doi: 10.1001/jama.286.11.1317. [DOI] [PubMed] [Google Scholar]
- 36.Afilalo J, Karunananthan S, Eisenberg MJ, Alexander KP, Bergman H. Role of frailty in patients with cardiovascular disease. Am J Cardiol. 2009 Jun 1;103(11):1616–1621. doi: 10.1016/j.amjcard.2009.01.375. [DOI] [PubMed] [Google Scholar]
- 37.Flint KM, Matlock DD, Lindenfeld J, Allen LA. Frailty and the selection of patients for destination therapy left ventricular assist device. Circ Heart Fail. 2012 Mar 1;5(2):286–293. doi: 10.1161/CIRCHEARTFAILURE.111.963215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alexander KP, Newby LK, Cannon CP, et al. Acute coronary care in the elderly, part i: Non-st-segment-elevation acute coronary syndromes: A scientific statement for healthcare professionals from the American Heart Association Council on Clinical Cardiology: In collaboration with the Society of Geriatric Cardiology. Circulation. 2007 May 15;115(19):2549–2569. doi: 10.1161/CIRCULATIONAHA.107.182615. [DOI] [PubMed] [Google Scholar]
- 39.Alexander KP, Newby LK, Armstrong PW, et al. Acute coronary care in the elderly, part ii: St-segment-elevation myocardial infarction: A scientific statement for healthcare professionals from the American Heart Association Council on Clinical Cardiology: In collaboration with the Society of Geriatric Cardiology. Circulation. 2007 May 15;115(19):2570–2589. doi: 10.1161/CIRCULATIONAHA.107.182616. [DOI] [PubMed] [Google Scholar]
- 40.Boutitie F, Boissel JP, Connolly SJ, et al. Amiodarone interaction with beta-blockers: Analysis of the merged EMIAT (European Myocardial Infarct Amiodarone Trial) and CAMIAT (Canadian Amiodarone Myocardial Infarction Trial) databases. The EMIAT and CAMIAT investigators. Circulation. 1999 May 4;99(17):2268–2275. doi: 10.1161/01.cir.99.17.2268. [DOI] [PubMed] [Google Scholar]
- 41.Thomas KL, Al-Khatib SM, Lokhnygina Y, et al. Amiodarone use after acute myocardial infarction complicated by heart failure and/or left ventricular dysfunction may be associated with excess mortality. Am Heart J. 2008 Jan;155(1):87–93. doi: 10.1016/j.ahj.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 42.Go AS, Hylek EM, Borowsky LH, Phillips KA, Selby JV, Singer DE. Warfarin use among ambulatory patients with nonvalvular atrial fibrillation: The AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) study. Ann Intern Med. 1999;131:927–934. doi: 10.7326/0003-4819-131-12-199912210-00004. [DOI] [PubMed] [Google Scholar]
- 43.Kowey PR, Reiffel JA, Myerburg R, et al. Warfarin and aspirin use in atrial fibrillation among practicing cardiologist (from the AFFECTS registry) Am J Cardiol. 2010 Apr 15;105(8):1130–1134. doi: 10.1016/j.amjcard.2009.11.047. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


