Abstract
Background
Whether there is an optimal time to place an implantable cardioverter-defibrillator (ICD) more than 40 days after myocardial infarction (MI) in guideline-eligible patients is unknown.
Objective
To evaluate the impact of time from MI to randomization on mortality, re-hospitalizations, and complications.
Methods
Individual data on patients enrolled in 9 primary and secondary prevention ICD trials were provided. Clinical trials were eligible for the current analysis if they enrolled patients with an MI more than 40 days prior to randomization to primary prevention ICD therapy versus usual care: MADIT-I, MUSTT, MADIT-II, and SCD-HeFT.
Results
ICD recipients died less frequently than non-recipients at 5 years across all subgroups of time from MI to randomization. In unadjusted Cox proportional hazards regression, a survival benefit was evident in most subgroups. Adjusted Bayesian Weibull survival modeling yielded hazard ratio (HR) 0.50, 95% posterior credible interval [PCI] 0.20–1.25 41–180 days after MI; HR 0.98, 95% PCI 0.37–2.37 181–365 days after MI; HR 0.22, 95% PCI 0.07–0.59 >1–2 years after MI; HR 0.42, 95% PCI 0.17–0.90 >2–5 years after MI; HR 0.55, 95% PCI 0.25–1.15 >5–10 years after MI; and HR 0.48, 95% PCI 0.20–1.02 > 10 years after MI. There was no evidence of an interaction between time from MI and all-cause mortality, re-hospitalizations, or complications.
Conclusions
In this meta-analysis, there was scant evidence that the efficacy of primary prevention ICD therapy and no evidence that the risks of re-hospitalizations or complications are dependent on time to implantation more than 40 days after MI.
Keywords: implantable cardioverter-defibrillator, sudden cardiac death, myocardial infarction, heart failure
Introduction
Some survivors of myocardial infarction (MI) are at high risk of sudden cardiac death.1 The implantable cardioverter-defibrillator (ICD) is the most effective therapy available to reduce this risk.2–4 Since risk of sudden cardiac death is highest in the first 30 days after MI and remains elevated in the first six months,5, 6 it was postulated that placement of an implantable cardioverter-defibrillator (ICD) early after MI could maximize therapeutic benefit. Counter to this postulate, the Defibrillator in Acute Myocardial Infarction Trial(DINAMIT)7 and the Immediate Risk Stratification Improves Survival(IRIS)8 trial failed to demonstrate a survival benefit of ICD placement early after MI despite a reduction in arrhythmic death. Current professional guidelines therefore mandate a 40-day waiting period prior to ICD placement after acute MI.9
Data from DINAMIT and the IRIS trial are clear: ICD placement within 40 days of a MI is not beneficial. However, whether there is an optimal time to place an ICD more than 40 days after MI remains unknown. Subgroup analyses of three clinical trials exploring the time-dependent survival benefit of ICD therapy after MI yielded conflicting results. In the Multicenter Automatic Defibrillator Implantation Trial II (MADIT-II), ICD recipients did not acquire a survival benefit compared with those who received usual care among those enrolled less than 18 months after MI. Among patients enrolled 18 months or more after MI, however, ICD therapy was associated with a survival benefit.10 By contrast, post hoc analyses of the Multicenter UnSustained Tachycardia Trial (MUSTT) and the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) found no evidence of a time-dependent benefit of ICD therapy.11, 12 A gap in evidence regarding the subacute and chronic periods after MI therefore persists.13, 14
Pooling of patient-level data from clinical trials increases the number of patients within subgroups of interest and allows for a more robust assessment of treatment effects. A collaborative consortium involving the principal investigators of 9 existing ICD therapy trials was established to explore the effectiveness of ICD therapy in various subgroups. Confined to patients enrolled in primary prevention ICD trials randomized to ICD therapy vs. usual care, the current analysis sought to assess the impact of the time from MI to randomization and corresponding ICD placement on all-cause mortality, re-hospitalizations, and complications.
Methods
Data Sources and Study Selection
Individual data on patients enrolled in 9 primary and secondary prevention ICD trials were provided. All data were previously collected as part of the primary trials and use of the de-identified dataset achieved exempt status by the Duke Institutional Review Board. Clinical trials were eligible for the current analysis if they enrolled patients with an MI more than 40 days prior to randomization to primary prevention ICD therapy versus usual care. The Antiarrhythmics Versus Implantable Defibrillators trial,15 the Cardiac Arrest Study Hamburg trial,16 the Defibrillators in Nonischemic Cardiomyopathy Treatment Evaluation trial,17 and the amiodarone arm of SCD-HeFT 4 were therefore excluded. The Coronary Artery Bypass Graft Patch trial18 was also excluded because patients enrolled in this trial are appreciably different from patients enrolled in the other 4 trials and because of the potential favorable impact of surgical revascularization on ventricular arrhythmia. The following 4 trials were included: the Multicenter Automatic Defibrillator Implantation Trial I (MADIT-I),19 MUSTT, 20 MADIT-II,3 and SCD-HeFT21(Table 1). Although ICD therapy was not randomized in MUSTT, the trial was included because the benefit of antiarrhythmic therapy guided by electrophysiological testing observed in MUSTT was due entirely to outcomes among patients who received an ICD.22 In the current analysis, MUSTT ICD recipients were considered to have received active intervention and non-recipients usual care. Patient inclusion criteria were left ventricular dysfunction due to prior MI, heart failure (New York Heart Association Class I-III), a left ventricular ejection fraction (LVEF) of ≤ 35%, and the availability of important covariates. Patients without heart failure symptoms or with New York Heart Association Class IV symptoms (53 from MADIT-II), a LVEF of > 35% (2 from MADIT-I, 108 from MUSTT, 3 from SCD-HeFT), no documented prior MI (all remaining 458 from DEFINITE, 34 from MUSTT, 913 from SCD-HeFT), an MI in the 40 days preceding randomization or whose time from randomization was unknown (15 from MADIT-I, 76 from MADIT-II, 165 from MUSTT, 50 from SCD-HeFT), or who were missing important covariates (1 from SCD-HeFT) were excluded. A total of 2,388 patients from 4 clinical trials comprised our final study population.
Table 1.
Design and Eligibility Criteria of Primary Prevention ICD Trials
| Clinical Trial | Dates of Recruitment | Participating Countries | Year of Main Publication Countries | No. of participants | Mean duration of follow-up (mos) | Eligible age range at entry (yrs) | Eligible LVEF | Factorial Comparison | Placebo Control |
|---|---|---|---|---|---|---|---|---|---|
| MADIT-I | Dec 1990 – Mar 1996 | USA, Italy, Germany | 1996 | 196 | 26.8 | 25–80 | ≤ 35% | ICD v. conventional medical therapy | No |
| MUSTT | Nov 1990 – Oct 1996 | USA, Canada | 1999 | 2202 | 39.0 | <80 | ≤ 40% | ICD or antiarrhythmic v. drugs v. placebo | No |
| MADIT-II | Jul 1997 – Nov 2001 | USA, Netherlands, Germany, Israel | 2002 | 1232 | 20.2 | >21 | ≤ 30% | ICD v. conventional medical therapy | No |
| SCD-HeFT | Sep 1997 – Jul 2001 | USA, Canada | 2005 | 2521 | 40.2 | >18 | ≤ 35% | ICD v. placebo v. amiodarone | Yes |
Data Synthesis and Analysis
Table 2 summarizes the baseline characteristics of these patients stratified by category of time from most recent MI prior to randomization. We assessed the association of each baseline characteristic with time from MI using χ2 tests and tests of equality of medians, respectively, for categorical and continuous-valued characteristics.
Table 2.
Baseline Characteristics According to Time From MI
| Characteristic | Time from MI
|
p-value | |||||
|---|---|---|---|---|---|---|---|
| 41–180 days (n=300) | 181–365 days (n=197) | >1 – 2 years (n=237) | >2 – 5 years (n=484) | >5 – 10 years (n=585) | >10 years (n=585) | ||
| Randomized to ICD, % | 42.3 | 43.7 | 48.5 | 51.9 | 49.6 | 52.5 | 0.034 |
| Age, median (IQR) | 63.5 (55, 70) | 61 (52, 68) | 61 (53, 69) | 61 (53, 69) | 65 (57, 72) | 67 (61, 73) | <0.001 |
| Male, % | 85.0 | 82.7 | 86.5 | 83.1 | 88.2 | 92.1 | <0.001 |
| Race | 0.317 | ||||||
| White | 86.6 | 81.7 | 88.2 | 86.8 | 87.7 | 88.7 | |
| Black | 10.0 | 12.2 | 8.4 | 8.7 | 8.4 | 6.3 | |
| Other | 3.3 | 6.1 | 3.4 | 4.5 | 3.9 | 5.0 | |
| Left ventricular ejection fraction, median (IQR) | 26 (20.8, 30) | 25 (20, 30) | 25 (20, 30) | 25 (20, 30) | 24 (20, 28) | 25 (20, 30) | <0.001 |
| New York Heart Association Class | 0.056 | ||||||
| I | 26.2 | 21.9 | 27.0 | 21.0 | 26.5 | 29.3 | |
| II | 46.9 | 50.3 | 47.4 | 46.4 | 45.6 | 48.2 | |
| III | 26.9 | 27.8 | 25.6 | 32.5 | 27.9 | 22.6 | |
| Serum creatinine, median (IQR) | 1.1 (1.0, 1.4) | 1.1 (0.9, 1.4) | 1.1 (0.9, 1.4) | 1.1 (1.0, 1.4) | 1.2 (1.0, 1.4) | 1.2 (1.0, 1.4) | 0.746 |
| Medical history, % | |||||||
| Atrial fibrillation | 3.8 | 6.1 | 3.7 | 6.1 | 6.4 | 8.6 | 0.649 |
| Diabetes | 34.2 | 37.7 | 30.5 | 34.7 | 31.3 | 29.1 | 0.261 |
| Hypercholesterolemia | 50.0 | 50.0 | 66.7 | 38.7 | 48.4 | 41.9 | 0.811 |
| Hypertension | 54.7 | 58.3 | 54.2 | 52.4 | 52.4 | 52.9 | 0.808 |
| Peripheral vascular disease | 12.5 | 9.5 | 18.2 | 26.3 | 14.6 | 11.1 | 0.446 |
| Percutaneous coronary intervention | 36.8 | 51.5 | 50.2 | 41.0 | 36.1 | 31.1 | <0.001 |
| Pulmonary disease | 17.4 | 22.6 | 18.5 | 19.8 | 17.9 | 20.0 | 0.945 |
| Smoking | 79.8 | 83.0 | 80.0 | 81.2 | 80.5 | 83.8 | 0.711 |
| Medication, % | |||||||
| Angiotensin converting enzyme inhibitor | 79.0 | 82.7 | 78.9 | 81.0 | 79.8 | 74.9 | 0.108 |
| β-blocker | 65.0 | 65.0 | 59.1 | 57.4 | 53.8 | 50.6 | <0.001 |
| Diuretic | 70.9 | 72.1 | 73.8 | 75.2 | 74.0 | 69.0 | 0.249 |
| Antiarrhythmic | 12.7 | 7.1 | 8.0 | 6.2 | 8.9 | 10.1 | 0.040 |
| Included Patients, n | |||||||
| MADIT-I | 32 | 21 | 11 | 38 | 41 | 36 | |
| MADIT-II | 123 | 88 | 111 | 221 | 281 | 279 | |
| MUSTT | 66 | 22 | 34 | 60 | 107 | 108 | |
| SCD-HeFT | 79 | 66 | 81 | 165 | 156 | 162 | |
IQR = interquartile range.
Data are presented as % unless otherwise indicated and are based on patients with available data.
Endpoints
We examined the impact of time from MI to randomization on all-cause mortality. Re-hospitalizations and complications were secondary endpoints. Admission to a hospital for any reason after ICD placement during each trial study period qualified as a re-hospitalization. Trial-specific complications included hypotension, syncope, bradycardia or conduction defect, pulmonary embolism, atrial fibrillation, pneumothorax, bleeding, venous thrombosis, problems with a defibrillator lead, defibrillator generator malfunction, MI, sustained ventricular tachycardia, ventricular fibrillation, shock (hemodynamic compromise), new or more advanced heart failure, postpericardiotomy syndrome, postoperative infection, renal failure, new or unanticipated drug therapy, and clinical events requiring surgical correction.
Statistical Analysis
Intervals of time from MI to randomization were chosen to balance interest in clinically relevant time points with the need for adequate sample sizes. Accordingly, patients were categorized into the following subgroups: 41–180 days, 181–365 days, >1 – 2 years, >2 – 5 years, >5 – 10 years, and >10 years. Categorical variables are presented as percentages, and continuous variables are presented as medians with 25th and 75th percentiles.
To assess the relationship between time from MI to randomization and all-cause mortality, unadjusted Cox proportional hazards regression models were fitted. Time to death was evaluated from the day of randomization. All-cause mortality event rates were summarized with Kaplan-Meier survival curves, and differences in survival between ICD recipients compared with non-recipients were assessed with logrank tests. To combine trial data and address missingness, we used Bayesian Weibull survival regression modeling.23 Missing covariate values were imputed based on their trial-specific empirical distribution. We adjusted for the following covariates: sex, race, age, left ventricular ejection fraction, New York Heart Association class, QRS duration, history of coronary artery bypass grafting, and use of cardiac medications including angiotensin converting enzyme inhibitors, β-blockers, and diuretics. Assuming random effects for trial-specific treatment effects and for parameters defining trial-specific baseline hazard functions, a model including the main effects of ICD therapy and time from MI as well as the multiplicative interaction of ICD therapy with time from MI was fitted. Statistical significance for hazard ratios was established by whether the posterior credible interval did not include the unity value.
In sensitivity analyses, time from MI to randomization was categorized according to quintiles 10, ≤ 6 months vs. > 6 months 11, and tertiles.12 We also examined the secondary endpoints of re-hospitalizations and complications with logistic regression modeling using the same groups described in the primary analysis. MUSTT was excluded in the latter analyses given a preponderance of missing values. A main effects model with continuously-valued time from MI was fitted. Analyses were performed using R version 2.15.1 and WinBUGS version 1.4.3.24
Results
Baseline characteristics
A total of 2,388 patients with a prior MI and left ventricular dysfunction due to prior MI were randomized to ICD therapy or placebo. Inspection of baseline characteristics as a function of time from MI to randomization demonstrated some differences (Table 2). Patients with longer periods from MI to randomization were older (median age 67 vs. 63.5 years), more commonly male (92.1% vs. 85.0%), less likely to have received percutaneous intervention (31.1% vs. 36.8%), and less likely to be taking a β-blocker at enrollment (50.6% vs. 65.0%). By contrast, there were no notable differences in race, LVEF, functional status by New York Heart Association classification, major comorbidities, or use of an angiotensin converting enzyme inhibitor, diuretic, or antiarrhythmic medications.
Unadjusted Outcomes
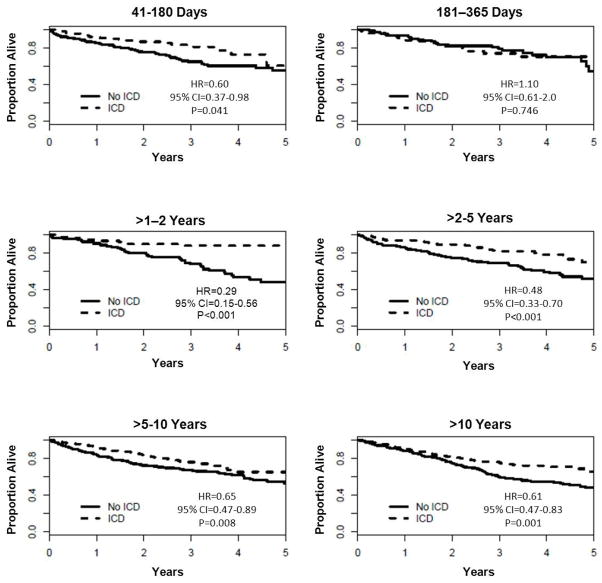
Among 2,388 patients with a history of MI, the Kaplan Meier estimate of the probability of death during follow-up was 65.1% in the 1212 patients receiving usual care as compared with 41.0% in the 1176 ICD recipients. The median duration of follow-up was 2.38 years. Patients randomized to ICD therapy in comparison to usual care were less likely to die by 5 years among all subgroups of time from MI to randomization (Table 3). Figure 1 illustrates cumulative event rates for all-cause mortality stratified according to time from MI to randomization. A survival benefit was evident by 1 year after MI and became more striking over time in most subgroups. Among patients randomized 181 to 365 days after MI, however, a survival benefit was not evident (p=0.746).
Table 3.
Outcomes According to Time From MI
| Event | Time from MI
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 41–180 days | 181–365 days | >1 – 2 years | >2 – 5 years | >5 – 10 years | >10 years | |||||||
|
| ||||||||||||
| Control (n=173) | ICD (n=127) | Control (n=111) | ICD (n=86) | Control (n=122) | ICD (n=115) | Control (n=233) | ICD (n=251) | Control (n=295) | ICD (n=290) | Control (n=278) | ICD (n=307) | |
| Death by 5 years* | 44.7 (60) | 39.3 (22) | 45.7 (26) | 29.4 (19) | 51.5 (43) | 11.9 (11) | 48.5 (76) | 29.9 (41) | 47.0 (103) | 34.9 (60) | 51.8 (113) | 34.5 (65) |
| ≥ 1 rehospitalization | 68.6 (72) | 76.5 (75) | 53.0 (44) | 71.8 (56) | 59.8 (52) | 61.6 (61) | 59.6 (106) | 71.5 (158) | 62.2 (122) | 75.8 (191) | 57.8 (107) | 75.7 (199) |
| ≥ 1 complication | 8.0 (7) | 17.4 (20) | 5.6 (4) | 13.4 (11) | 3.9 (3) | 29.8 (31) | 8.2 (12) | 20.5 (48) | 9.6 (16) | 27.9 (73) | 10.6 (17) | 27.4 (76) |
Data are presented as % (n)
Five-year Kaplan-Meier estimates (total deaths over entire follow-up period).
Figure 1.
Unadjusted Kaplan-Meier Survival Curves in ICD Recipients vs. Non-recipients According to Time From MI
A total of 1,243 (67.4%) patients were re-hospitalized one or more times and 318 (17.8%) patients experienced one or more complications. Comparison of re-hospitalizations according to time from MI to randomization showed that patients receiving ICD therapy in comparison to those who were randomized to usual care were more likely to be re-hospitalized during follow-up. Similarly, more complications were observed during the follow-up period in patients receiving an ICD versus usual care in all subgroups of time from MI to randomization (Table 3).
Adjusted Outcomes
After adjustment for baseline characteristics, the point estimates for the effect of ICD therapy in comparison to usual care on all-cause mortality estimates for most subgroups were consistent with a survival benefit (Table 4). The absence of benefit in most subgroups was not ruled out, however. Notable exceptions were among patients receiving ICD therapy >1 to 2 years after MI (hazard ratio [HR] 0.22, posterior credible interval [PCI] (0.07, 0.59) and >2 to 5 years after MI (HR 0.42, PCI 0.17, 0.90). Overall, there was no evidence of an interaction between time from MI to randomization and all-cause mortality. Likewise, there was no evidence of an interaction between time from MI and re-hospitalizations or complications.
Table 4.
Treatment Effect of ICD Therapy According to Time From MI
| Event | Trial | Time From MI
|
|||||
|---|---|---|---|---|---|---|---|
| 41–180 days (n=366) | 181–365 days (n=223) | >1 – 2 years (n=263) | >2 – 5 years (n=534) | >5 – 10 years (n=644) | >10 years (n=674) | ||
| All-cause mortality (HR, 95% PCI) | MADIT-I | 0.47 (0.23, 0.89) | 0.92 (0.42, 1.89) | 0.21 (0.09, 0.47) | 0.38 (0.21, 0.69) | 0.51 (0.28, 0.89) | 0.45 (0.24, 0.77) |
| MUSTT | 0.28 (0.13, 0.56) | 0.54 (0.23, 1.15) | 0.12 (0.05, 0.28) | 0.23 (0.12, 0.40) | 0.31 (0.16, 0.52) | 0.27 (0.14, 0.47) | |
| MADIT-II | 0.60 (0.39, 1.16) | 1.19 (0.65, 2.15) | 0.28 (0.13, 0.52) | 0.51 (0.33, 0.76) | 0.67 (0.47, 0.96) | 0.59 (0.39, 0.87) | |
| SCD-HeFT | 0.74 (0.47, 1.39) | 1.45 (0.79, 2.57) | 0.34 (0.17, 0.65) | 0.63 (0.40, 0.91) | 0.82 (0.57, 1.21) | 0.72 (0.50, 1.01) | |
| Overall | 0.50 (0.20, 1.25) | 0.98 (0.37, 2.37) | 0.22 (0.07, 0.59) | 0.42 (0.17, 0.90) | 0.55 (0.25, 1.15) | 0.48 (0.20, 1.02) | |
| Rehospitalizations (OR, 95% PCI) | MADIT-I | 1.38 (0.71, 2.93) | 1.69 (0.72, 3.61) | 0.73 (0.34, 1.57) | 1.28 (0.65, 2.36) | 1.21 (0.68, 2.32) | 1.47 (0.76, 2.56) |
| MADIT-II | 2.59 (1.50, 5.18) | 3.15 (1.55, 6.17) | 1.34 (0.77, 2.46) | 2.36 (1.54, 3.65) | 2.30 (1.53, 3.63) | 2.71 (1.77, 4.24) | |
| SCD-HeFT | 1.56 (0.90, 2.96) | 1.90 (0.94, 3.91) | 0.83 (0.44, 1.48) | 1.44 (0.90, 2.24) | 1.39 (0.90, 2.22) | 1.63 (1.04, 2.59) | |
| Overall | 1.82 (0.66, 5.18) | 2.19 (0.77, 6.91) | 0.95 (0.36, 2.55) | 1.66 (0.70, 4.23) | 1.61 (0.67, 4.39) | 1.89 (0.80, 5.05) | |
| Complications (OR, 95% PCI) | MADIT-I | 0.65 (0.29, 1.50) | 2.02 (0.57, 8.19) | 8.52 (2.33, 41.05) | 2.28 (0.98, 5.46) | 2.37 (1.09, 5.27) | 2.32 (1.01, 5.15) |
| MADIT-II | 1.10 (0.53, 2.30) | 3.54 (1.12, 12.88) | 14.44 (4.76, 63.85) | 3.89 (2.01, 8.36) | 4.11 (2.29, 7.93) | 4.04 (2.27, 7.21) | |
| SCD-HeFT | 0.56 (0.27, 1.27) | 1.83 (0.56, 7.15) | 7.47 (2.30, 33.76) | 2.00 (0.98, 4.25) | 2.14 (1.09, 4.29) | 2.09 (1.12, 3.99) | |
| Overall | 0.74 (0.25, 2.19) | 2.34 (0.55, 11.29) | 9.61 (2.19, 53.68) | 2.62 (0.92, 7.75) | 2.76 (0.91, 8.64) | 2.71 (1.01, 7.69) | |
Abbreviations: HR, hazard ratio; OR, odds ratio; PCI, posterior credible interval.
Bayesian Weibull survival regression models are adjusted for age, gender, race, New York Heart Association class, left ventricular ejection fraction, QRS duration, medication use (angiotensin converting enzyme inhibitor, β-blocker, diuretic), and coronary artery bypass grafting.
Statistical significance is established by whether posterior credible interval includes the unity value.
Sensitivity Analyses
A difference in ICD therapy effect in comparison to usual care according to MI was not detected when time from MI was categorized according to quintiles, ≤ 6 months versus > 6 months, tertiles, or analyzed as a continuous value. In a main effects model, the hazards of death related to continuously-valued time from MI was 1.01, 95% PCI (0.99, 1.02).
Discussion
The principal findings of our analysis are twofold. First, when pooling individual patient data from 4 trials to enhance estimation of treatment effects, there is scant evidence that time to implantation more than 40 days after MI impacts ICD efficacy. Second, there is no evidence that time more than 40 days after MI influences the likelihood of re-hospitalization or complication after ICD placement.
Post-MI scar formation may lead to the development of intramyocardial reentry circuits days or years after the initial insult.25 Over time, neurohormonal pathway activation causes ventricular remodeling and dysfunction in some MI survivors. Changes in ventricular architecture in turn set the stage for temporal dispersion and further reentrant arrhythmias.26, 27 Post-MI arrhythmic risk profiles therefore vary over time.28 Parallel to the evolving post-MI arrhythmic milieu concomitant with the development of heart failure, the survival benefit of ICD therapy benefit has been hypothesized to vary with time after MI.13, 14
Since adverse ventricular remodeling is associated with a greater risk of mortality,29, 30 the MADIT-II subgroup analysis10 may be consistent with the aforementioned pathophysiologic model. In this setting, the survival benefit of ICD therapy may be restricted to patients with a more remote MI.31 By contrast, subgroup analyses from MUSTT 11 and SCD-HeFT 12 suggest that ICD therapy is beneficial over all post-MI periods outside of 40 days.14 Based on adjusted point estimates, the findings of the current study appear to support the latter view. However, we cannot rule out lack of benefit from ICD therapy in most of the studied categories.
The disparate findings between the unadjusted analysis suggesting reduced ICD efficacy among those who received an ICD 181 to 365 days after MI and the adjusted Bayesian Weibull survival analysis suggesting a greater benefit from an ICD >1 to 2 years after MI merit mention. These findings reflect the inherent difference between unadjusted and adjusted analyses. In contrast to the former, the latter account for covariates that may impact the outcome.32 The reduced ICD treatment effect among patients 181–365 days after MI in the adjusted analysis may result from variability in competing causes of mortality such as recurrent MI or heart failure over time after MI. They may also be due to unmeasured factors known to have prognostic significance such as bundle branch block. That there was no evidence of an interaction between the time from MI to randomization and all-cause mortality, complications, or re-hospitalizations suggests the observation in adjusted analysis more likely represents the play of chance.
To our knowledge, our study is the first to examine whether re-hospitalizations or complications after ICD placement vary with time after MI. These events have a direct impact on quality of life and thus may influence patients’ decisions regarding ICD placement. The increased risk for re-hospitalization among ICD recipients compared to non-recipients is likely due to shocks requiring further investigation or therapy, complications after ICD placement, and shock-associated heart failure exacerbations.33, 34 The absence of evidence that the risks of re-hospitalization or complication are dependent on time from MI is reassuring.
The current analysis has important clinical implications. First, guideline-eligible post-MI patients need not wait an extended period of time prior to device placement in order to derive the maximal survival benefit. Rather, patients with a recent MI experience a survival benefit comparable to patients with a remote MI. Second, time from MI outside of 40 days should not alter patient consent or procedural planning.
Limitations
The current study was performed retrospectively; however, data were collected prospectively in the context of very well-conducted and monitored clinical trials. The factors by which we adjusted our analysis were limited to those which were common to all included trials. It is therefore possible that residual confounding from trial-specific variables we could not include such as atrial fibrillation, diabetes mellitus, bundle-branch block, and other unrecorded ones may have influenced our estimation of treatment effects. Patients with an MI within 40 days of randomization were excluded, and thus the current study neither confirms nor disproves the findings of DINAMIT or the IRIS trial. Despite pooling of individual patient data from 4 trials, the number of patients in each subgroup was still modest. Hence, the study may have been underpowered to detect small differences in ICD efficacy among subgroups. In comparison to prior subgroup analyses, however, the current study had the largest sample size and thus allowed for better characterization of patients with a recent MI. Although a prospective randomized trial could best ascertain whether there is an optimal time to place an ICD after MI, sample size considerations and corresponding costs limit the feasibility of such an endeavor.
Conclusions
In this meta-analysis of 2,388 patients in 4 clinical trials, there was scant evidence that the efficacy of primary prevention ICD therapy is dependent on time to implantation more than 40 days after MI. Similarly, there was no evidence that time more than 40 days after MI influences re-hospitalization or complication rates after ICD placement.
Acknowledgments
Sources of Funding: Primary funding was provided by the Agency for Healthcare Research and Quality (5 R01 HS018505-03). P.L.H. was funded by NIH T-32 training grant HL069749-09. The funding sources had no role in the design, analysis, or interpretation of the data or in the decision to submit the manuscript for publication.
Glossary of abbreviations
- CABG-Patch
Coronary Artery Bypass Graft Patch
- DINAMIT
Defibrillator in Acute Myocardial Infarction Trial
- HR
hazard ratio
- ICD
implantable cardioverter-defibrillator
- IRIS
Immediate Risk Stratification Improves Survival
- LVEF
left ventricular ejection fraction
- MADIT-I
Multicenter Automatic Defibrillator Implantation Trial I
- MADIT-II
Multicenter Automatic Defibrillator Implantation Trial II
- MI
myocardial infarction
- MUSTT
Multicenter UnSustained Tachyardia Trial
- OR
odds ratio
- PCI
posterior credible interval
- SCD-HeFT
Sudden Cardiac Death in Heart Failure
Footnotes
Potential Conflicts of Interest (None unless otherwise noted below):
Alan H. Kadish, MD- Sanofi-Aventis, consulting fees/honoraria
Jonathan P. Piccini, MD, MHS- Sanofi-Aventis and Forest Pharmaceuticals, consulting fees/honoraria; Johnson and Johnson, research grants
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Huikuri HV, Castellanos A, Myerburg RJ. Sudden death due to cardiac arrhythmias. N Engl J Med. 2001;345:1473–1482. doi: 10.1056/NEJMra000650. [DOI] [PubMed] [Google Scholar]
- 2.Moss AJ, Hall WJ, Cannom DS, et al. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. Multicenter automatic defibrillator implantation trial investigators. N Engl J Med. 1996;335:1933–1940. doi: 10.1056/NEJM199612263352601. [DOI] [PubMed] [Google Scholar]
- 3.Moss AJ, Zareba W, Hall J, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–883. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 4.Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–237. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 5.Solomon SD, Zelenkofske S, McMurray JJV, et al. Sudden death in patients with myocardial infarction and left ventricular dysfunction, heart failure, or both. N Engl J Med. 2005;352:2581–2588. doi: 10.1056/NEJMoa043938. [DOI] [PubMed] [Google Scholar]
- 6.Yap YG, Duong T, Bland M, et al. Temporal trends on the risk of arrhythmic vs. Non-arrhythmic deaths in high-risk patients after myocardial infarction: A combined analysis from multicenter trials. Eur Heart J. 2005;26:1385–1393. doi: 10.1093/eurheartj/ehi268. [DOI] [PubMed] [Google Scholar]
- 7.Hohnloser SH, Kuck KH, Dorian P, et al. Prophylactic use of implantable cardioverter-defibrillator after acute myocardial infarction. N Engl J Med. 2004;351:2481–2488. doi: 10.1056/NEJMoa041489. [DOI] [PubMed] [Google Scholar]
- 8.Steinbeck G, Andresen D, Seidl K, et al. Defibrillator implantation early after myocardial infarction. N Engl J Med. 2009;361:1427–1436. doi: 10.1056/NEJMoa0901889. [DOI] [PubMed] [Google Scholar]
- 9.Epstein AE, DiMarco JP, Ellenbogen KA, et al. ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: A report of the American College of Cardiology/American Heart Association task force on practice guidelines (writing committee to revise the ACC/AHA/NASPE 2002 guideline update for implantation of cardiac pacemakers and antiarrhythmia devices): Developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. Circulation. 2008;117:e350–408. doi: 10.1161/CIRCUALTIONAHA.108.189742. [DOI] [PubMed] [Google Scholar]
- 10.Wilber DJ, Zareba W, Hall WJ, et al. Time dependence of mortality risk and defibrillator benefit after myocardial infarction. Circulation. 2004;109:1082–1084. doi: 10.1161/01.CIR.0000121328.12536.07. [DOI] [PubMed] [Google Scholar]
- 11.Al-Khatib SM, Hafley G, Lee KL, Buxton AE. Relation between time from myocardial infarction to enrolment and patient outcomes in the Multicenter UnSustained Tachycardia Trial. Europace. 2010;12:1112–1118. doi: 10.1093/europace/euq116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Piccini JP, Al-Khatib SM, Hellkamp AS, et al. Mortality benefits from implantable cardioverter-defibrillator therapy are not restricted to patients with remote myocardial infarction: An analysis from the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) Heart Rhythm. 2011;8:393–400. doi: 10.1016/j.hrthm.2010.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Myerburg RJ. Implantable cardioverter-defibrillators after myocardial infarction. N Engl J Med. 2008;359:2245–2253. doi: 10.1056/NEJMra0803409. [DOI] [PubMed] [Google Scholar]
- 14.Myerburg RJ, Junttila MJ. Sudden cardiac death caused by coronary heart disease. Circulation. 2012;125:1043–1052. doi: 10.1161/CIRCULATIONAHA.111.023846. [DOI] [PubMed] [Google Scholar]
- 15.The Antiarrhythmics Versus Implantable Defibrillators Investigators. A comparison of antiarrhythmic-drug therapy with implantable defibrillators in patients resuscitated from near-fatal ventricular arrhythmias. N Engl J Med. 1997;337:1576–1583. doi: 10.1056/NEJM199711273372202. [DOI] [PubMed] [Google Scholar]
- 16.Kuck KH, Cappato R, Siebels J, Ruppel R. Randomized comparison of antiarrhythmic drug therapy with implantable defibrillators in patients resuscitated from cardiac arrest: The Cardiac Arrest Study Hamburg (CASH) Circulation. 2000;102:748–754. doi: 10.1161/01.cir.102.7.748. [DOI] [PubMed] [Google Scholar]
- 17.Kadish A, Dyer A, Daubert JP, et al. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med. 2004;350:2151–2158. doi: 10.1056/NEJMoa033088. [DOI] [PubMed] [Google Scholar]
- 18.Bigger JT., Jr Prophylactic use of implanted cardiac defibrillators in patients at high risk for ventricular arrhythmias after coronary-artery bypass graft surgery. N Engl J Med. 1997;332:1569–1575. doi: 10.1056/NEJM199711273372201. [DOI] [PubMed] [Google Scholar]
- 19.Moss AJ, Hall J, Cannom DS, et al. Improved survival with an implanted cardioverter-defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. N Engl J Med. 1996;335:1933–1940. doi: 10.1056/NEJM199612263352601. [DOI] [PubMed] [Google Scholar]
- 20.Buxton AE, Lee KL, Fisher JD, Josephson ME, Prystowsky EN, Hafley G. A randomized study of the prevention of sudden death in patients with coronary artery disease. N Engl J Med. 1999;341:1882–1890. doi: 10.1056/NEJM199912163412503. [DOI] [PubMed] [Google Scholar]
- 21.Bardy GH, Lee KL, Mark DB, et al. Amiodarone or implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–237. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 22.Lee KL, Hafley G, Fisher JD, et al. Effect of implantable defibrillators on arrhythmic events and mortality in the Multicenter UnSustained Tachycardia Trial. Circulation. 2002;106:233–238. doi: 10.1161/01.cir.0000021920.73149.c3. [DOI] [PubMed] [Google Scholar]
- 23.Ibrahim JG, Chen MH, Sinha D. Bayesian survival analysis. Springer-Verlag; 2001. [Google Scholar]
- 24.Lunn DJ, Thomas A, Best N, Spiegelhalter D. Winbugs-- a bayesian modelling framework: Concepts, structure, and extensibility. Statistics and Computing. 2000;10:325–337. [Google Scholar]
- 25.Buxton AE. Sudden death after myocardial infarction-- who needs prophylaxis, and when? N Engl J Med. 2005;352:2638–2640. doi: 10.1056/NEJMe058085. [DOI] [PubMed] [Google Scholar]
- 26.Eckardt L, Haverkamp W, Johna R, et al. Arrhythmias in heart failure: Current concepts of mechanisms and therapy. J Cardiovasc Electrophysiol. 2000;11:106–117. doi: 10.1111/j.1540-8167.2000.tb00746.x. [DOI] [PubMed] [Google Scholar]
- 27.Bunch TJ, Hohnloser SH, Gersh BJ. Mechanisms of sudden cardiac death in myocardial infarction survivors: Insights from the randomized trials of implantable cardioverter-defibrillators. Circulation. 2007;115:2451–2457. doi: 10.1161/CIRCULATIONAHA.106.683235. [DOI] [PubMed] [Google Scholar]
- 28.Piccini JP, Zhang M, Pieper K, et al. Predictors of sudden cardiac death change with time after myocardial infarction: Results from the valiant trial. Eur Heart J. 2010;31:211–221. doi: 10.1093/eurheartj/ehp425. [DOI] [PubMed] [Google Scholar]
- 29.Pfeffer MA, Braunwald E, Moye LA, et al. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 1990;327:669–677. doi: 10.1056/NEJM199209033271001. [DOI] [PubMed] [Google Scholar]
- 30.Pfeffer MA, Braunwald E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation. 1990;81:1161–1172. doi: 10.1161/01.cir.81.4.1161. [DOI] [PubMed] [Google Scholar]
- 31.Tung R, Zimetbaum P, Josephson ME. A critical appraisal of implantable cardioverter-defibrillator therapy for the prevention of sudden cardiac death. J Am Coll Cardiol. 2008;52:1111–1121. doi: 10.1016/j.jacc.2008.05.058. [DOI] [PubMed] [Google Scholar]
- 32.Christensen R, Johnson W, Branscum A, Hanson T. Bayesian ideas and data analysis: An introduction for scientists and statisticians. CRC Press; 2011. Basic Concepts of Regression; pp. 161–178. [Google Scholar]
- 33.Moss AJ, Greenberg H, Case RB, et al. Long-term clinical course of patients after termination of ventricular tachyarrhythmia by an implanted defibrillator. Circulation. 2004;110:3760–3765. doi: 10.1161/01.CIR.0000150390.04704.B7. [DOI] [PubMed] [Google Scholar]
- 34.Goldenberg I, Moss AJ, Hall WJ, et al. Causes and consequences of heart failure after prophylactic implantation of a defibrillator in the Multicenter Automatic Defibrillator Implantation Trial II. Circulation. 2006;113:2810–2817. doi: 10.1161/CIRCULATIONAHA.105.577262. [DOI] [PubMed] [Google Scholar]