Abstract
The purpose of the present study was to identify the changes in the levels of proinflammatory cytokines like IL-1β, IL-6 and TNF-α in peripheral circulation in Alzheimer’s disease (AD) subjects and to correlate these with associated depression and cognitive deficit. Fifty five AD subjects and thirty seven age and sex matched controls were included in the study. The AD patients were grouped as AD with depression (n= 31) and AD without depression (n= 24). The serum levels of IL-1β, IL-6 and TNF-α were determined by immunoassay by commercially available kits. The serum levels of IL-6 and TNF-α were elevated in AD patients with depression compared to control (p<0.001) or AD without depression (p<0.001). The serum level of IL-1β was higher in AD patients with or without depression as compared to controls. Furthermore, a strong inverse correlation was observed between the MMSE scores and serum levels of IL-6 or TNF-α in AD subjects with depression. The study highlights the important role of peripheral IL-6 and TNF-α in AD associated depression and cognitive deficits.
Keywords: Alzheimer’s disease, IL-6, IL-1β, TNF-α, Depression, Cognitive deficit
Alzheimer’s disease (AD) is the most common form of dementia above the age group of 65 years, and the sporadic variety of the disease accounts for the majority of AD patients [1]. The disease is diagnosed clinically as probable AD based on neuropsychiatric evaluation of clinical features, and an insidious failure of memory with multiple cognitive deficits is the key feature [2]. However, the clinical features of the disease also include various mood alterations like depression [3]. The molecular mechanisms of AD associated cognitive deficits and depression is likely to be complex, but one link could be through the alterations in peripheral immune system. There are numerous reports of altered levels of various cytokines in peripheral circulation in AD subjects [4–6]. Despite many variations in such reports, a general pattern of rise in the serum levels of several proinflammatory cytokines including IL-6, IL-1β and TNF-α has been noticed in AD patients [6,7]. On the other hand, there is accumulating evidence of alterations in peripheral immune system with increased circulating levels of proinflammatory cytokines in major depressive disorders [8–10]. The circulating proinflammatory cytokines like IL-6 and TNF-α are thought to promote the depressive disorder by affecting CNS functions in multiple ways [9–12]. Likewise, cognitive functions is known to be affected by peripheral inflammatory response with elevated levels of circulating proinflammatory cytokines both in human beings and experimental models [8,9,13]. It will be, therefore, interesting to explore how the changes in the levels of proinflammatory cytokines in peripheral circulation impact the cognitive deficit and mood changes like depression associated with AD. This is particularly important because the pathways of communication between the peripheral immune system and the brain involving neural mechanisms and chemical mediators have been more or less clearly defined, and extensive information is also available on cytokine signaling within the brain that affects cognition, mood and behavior [9,12,14–16]. The present case-control study, therefore, seeks to correlate the serum levels of the proinflammatory cytokines in AD subjects with the degree of cognitive decline and the presence of depression in this disease condition.
MATERIALS AND METHODS
This case control study comprises of 55 AD cases recruited from the ‘Dementia clinic’ of Bangur Institute of Neurosciences, which is associated with our institute [Institute of Post Graduate Medical Education & Research (IPGME&R)]. Age and sex matched 37 volunteers (controls) were recruited from the relatives of various patients visiting the out-patient departments of IPGME&R after detailed clinical examination and routine biochemical tests. A complete neuropsychiatrical evaluation was done to rule out any cognitive impairment of control subjects. The exclusion criteria in AD and control groups included overt cardiovascular disease, diabetes, cancer, chronic kidney disease, chronic infection and any other associated neurological disease. The diagnosis of probable AD was based on neuropsychiatrical parameters from DSM-IV (Diagnostic and Statistical Manual of Mental Disorders) criteria and relevant MRI (Magnetic resonance imaging) findings. The battery of neuropsychiatrical examinations also included the determination of MMSE scores (out of 30) through a series of questions and tests to check the patient’s memory, attention, orientation, registration and reasoning. Despite some limitations, MMSE score is a good indicator of the severity of the disease. A maximum score of 30 is attainable by a person without any neuropsychological impairment, while severe cases of AD have MMSE scores less than 10 and moderate cases between 10 – 20. The cases were further divided into two groups: AD without features of depression (n = 24) and AD with depression (n = 31) diagnosed as per DSM-IV criteria. The informed consent was taken from all the controls and close relatives of AD patients. The study was cleared by the Institutional Human Ethics Committee of IPGME&R which follows the Helsinki guidelines.
Venous blood samples (4 ml) were collected aseptically from both the control and AD subjects, and several aliquots of the serum samples were stored at −20°C. IL-1β, IL-6, TNF-α were assayed by solid-phase sandwich ELISA using commercial kits from Raybiotech, USA using the manufacturer’s protocol. For the immunoassay of cytokines in serum, anti-human IL-1β antibody or anti-human IL-6 antibody or anti-human TNF-α antibody coated microtitre plates were used to capture IL-1β or IL-6 or TNF-α respectively from the standards or the samples. The detecting antibody was biotinylated anti-human IL-1β antibody or biotinylated anti-human IL-6 antibody or biotinylated anti-human TNF-α antibody, while streptavidin-HRP (horse radish peroxidase) conjugate was employed with TMB (tetramethylbenzidine) as the substrate for the colour development. The routine biochemical tests were performed for both the controls and cases. The tests included fasting plasma glucose (Glucose oxidase method) and non-fasting levels of serum urea (urease method), creatinine (Jaffe’s method), total cholesterol (cholesterol oxidase method), total bilirubin and enzymes for liver functions (alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase) measured by commercial kits in a clinical auto analyzer (Randox, Daytona) with appropriate internal quality control.
The statistical analysis of different parameters in 3 groups was performed by means of one way analysis of variance (ANOVA). This analysis was followed by post-hoc comparisons between the groups (Tukey’s test). Pearson correlation coefficient was used to find out the correlation between two variables. A value of p < 0.05 was considered as statistically significant. For statistical analysis, Graph Pad prism software (version5, 2007, Sandiego, California, USA) was used.
RESULTS
The demographic profile of the subjects under study is depicted in Table 1. There was no statistically significant difference in age (years), male: female ratio and BMI among the 3 groups Table 1. The routine biochemical parameters (not shown) were also within normal limits and in conformity with our exclusion criteria in all 3 groups of subjects except for serum total cholesterol. The serum total cholesterol (Mean ± SD) was slightly elevated in AD subjects without depression (189.5±25.84) compared to control (170.4±14.98) which was statistically significant (p < 0.01), but no significant difference was observed between the control and AD with depression (176.7±19.30). The results in Fig. 1 show that the serum levels of IL-6 and TNF-α were significantly higher (p<0.001) in AD subjects with depression compared to control or AD without depression (Fig.1). There was nearly a two-fold increase in the mean serum level of IL-6 or TNF-α in AD subjects with depression compared to control (Fig. 1). On the other hand, there was a less marked, but statistically significant (p < 0.001), rise in IL-1β serum level in AD subjects with depression compared to control (Fig. 1). Further, in the case of IL-1β a statistically significant (p < 0.001) rise in the serum level was noticed also in AD patients without depression compared to control (Fig. 1). There was no noticeable difference in serum IL-1β levels between two subgroups of AD subjects (Fig.1). The results presented in Fig. 2 depict the statistical correlation between MMSE scores and the serum levels of cytokines. This statistical analysis has been performed in order to find the correlation between the severity of the disease and the extent of alterations in the serum cytokine levels. A statistically significant and strong inverse correlation was seen between MMSE scores and serum TNF-α (r = − 0.62, p = 0.0002) or serum IL-6 (r = − 0.51, p = 0.003) levels in AD subjects with depression (Fig.2). However, no statistically significant correlation was observed between the serum levels of IL-1β and MMSE scores (r = 0.24, p = 0.175) in AD subjects with depression (Fig. 2). In AD subjects without depression, no correlation was seen between MMSE scores and the serum levels of IL-6, TNF-α or IL-1β (Fig.2).
Table 1.
Demographic profile of control, AD subjects with or without depression
| Control (n = 37) | AD with depression (n = 31) | AD without depression (n = 24) | |
|---|---|---|---|
| Age (in years) | 66.46 ± 4.24 | 68.58 ± 6.86 | 68.42 ± 7.03 |
| Sex (M/F) | 19/18 | 16/15 | 13/11 |
| BMI (kg/m2) | 23.61 ± 1.65 | 21.94 ± 1.94 | 22.68 ± 2.35 |
| MMSE | 29.16 ± 0.72 | 10.90 ± 5.02 | 13.88 ± 7.46 |
The age, BMI and MMSE scores are presented as the Means ±SD. The number of subjects in each group is given in the parenthesis.
Fig. 1.
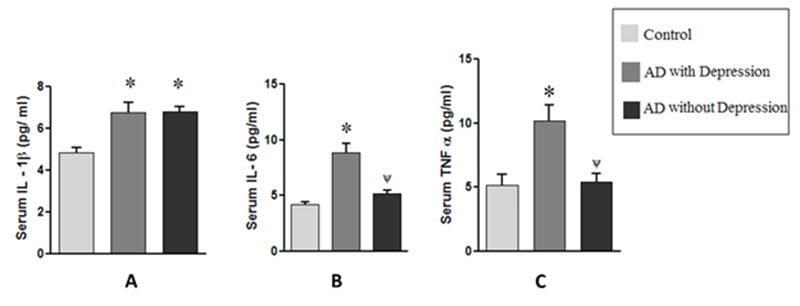
Serum levels of (A) IL-1β, (B) IL-6 and (C) TNF-α in AD subjects with and without depression. The values are expressed as the means ± SEM for the number of cases (n) in each group of subjects. Statistical comparisons were made by one way Anova followed by post-hoc analysis as described in the methods. *p< 0.001 vs. control; Ψp< 0.001 vs AD with depression.
Fig. 2.
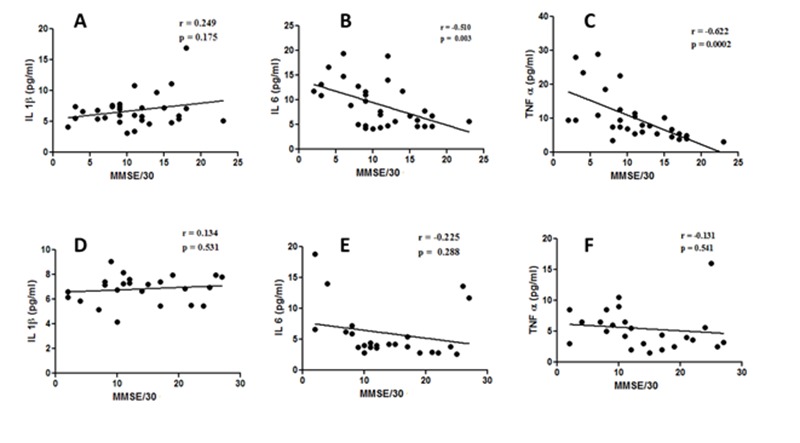
XY scatter plots between MMSE scores and serum levels of IL-1β (A) or IL-6 (B) or TNF-α (C) in AD subjects with depression or serum levels of IL-1β (D) or IL-6 (E) or TNF-α (F) in AD subjects without depression. The degree and nature of correlation between the MMSE score and the serum parameter in AD patients is given by the value of r (correlation coefficient) as explained in the methods. A value of p < 0.05 was considered as statistically significant.
DISCUSSION
The inflammatory reaction by activated microglia and astrocytes is an important element of AD pathology as evidenced from post-mortem analysis of AD brains and studies in animal models [17,18,19]. This inflammatory response is presumably triggered by soluble amyloid beta peptide or amyloid fibrils leading to an activation of microglia especially in the vicinity of neuritic plaques with the typical morphological changes, appearance of proinflammatory surface markers(CD 36, CD 40, CD 45), production of interleukins, interferons, chemokines, components of complement system and reactive oxygen species [18,19]. The proinflammatory cytokines like IL-1β, IL-6, IL-8 and TNF-α released by the reactive microglia have been strongly implicated with neurodegeneration and AD pathology, although the recent studies have also indicated the beneficial effects of microglial in this disease condition through the alternative activation state and release of anti-inflammatory cytokines [17, 18, 19]. Apart from the detailed studies of the inflammatory changes in AD brain, there also exists a surfeit of studies showing altered levels of proinflammatory cytokines in peripheral circulation in AD subjects [4–7,20,21]. Despite the variability in the reported results, a systematic meta-analysis has shown a general rise in the levels of several proinflammatory cytokines including IL-1β, IL-6 and TNF-α [7]. The reasons for the variability in the results may be many, but apparently one important factor could be the inclusion or exclusion of co-morbidities like obesity, diabetes, chronic systemic inflammation etc. in the study subjects which can affect the serum proinflammatory cytokine levels. Likewise, the analysis of CSF for alterations in the levels of cytokines has also produced variable results, and further no clear correlation between the serum and CSF levels of proinflammatory cytokines has been established [7, 20, 21]. Thus, the relationship between the inflammatory state of the AD brain and the elevated levels of IL-6, IL-1β and TNF-α in peripheral circulation is not apparent at present. Nevertheless the elevated levels of peripheral proinflammatory cytokines have important biological implications in AD in view of our recent understanding of peripheral immune system to brain communication affecting mood, cognition and behaviour. In contrast to most other studies we have shown clearly that the serum levels of IL-6 and TNF-α are increased significantly only in AD subjects with depression compared to control or AD without depression and this observation has several implications. Firstly, it indicates that the variability in the reports related to altered levels of IL-6 and TNF-α in serum of AD subjects in the existing literature may be accounted partly by the fact that in many studies the depression associated with AD has not been taken in to account. More importantly, our results indicate an activation of peripheral immune system in AD subjects with depression. The altered affective and cognitive functions of the brain as a result of the activation of peripheral immune system with elevated levels of circulating proinflammatory cytokines have been identified through a large number of clinical and experimental studies [16, 22, 23]. The mechanisms include stimulation of peripheral afferent vagal nerves by the proinflammatory cytokines, direct entry of the cytokines in the brain through saturable transport system or passively through deficient blood brain barrier (BBB) at Organum Vasculosum of Lamina Terminalis (OVLT), and the generation of diffusible messengers like prostaglandins and NO at the cerebral vasculature [12,14,16,22]. These different mechanisms represent the communication pathway between the central nervous system and the peripheral immune system, and through this pathway the peripheral immune system can activate microglia in the brain with enhanced production of cytokines [12,16, 22]. The proinflammatory cytokines and their receptors are present widely within the brain, and the cytokine effect involves many different regions including hippocampus, prefrontal cortex, amygdala and hypothalamus [9,12,15,23]. The proinflammatory cytokines function as neuromodulators and alter synaptic plasticity, synaptic scaling and neurigenesis, central neurotransmitter activity, activity of hypothalamic - pituitary -adrenal axis and the expression of neurotrophic factor like BDNF [9,12,14–16]. Thus, during systemic infection or inflammation the activation of the peripheral immune system may lead to enhanced activity of central cytokines affecting behavior, mood and cognition [9,11,12]. On the other hand, there are numerous reports which show that major depressive disorders (MDD) are associated with the raised levels of proinflammatory cytokines in peripheral circulation, presumably because the psychological and physical stress that trigger the depressive illness also activates the peripheral immune system leading to increased release of these cytokines in circulation [8,24,25]. The communication between the peripheral immune system and the brain as explained above makes it plausible that the raised levels of the proinflammatory cytokines in the circulation reinforce the depressive illness [9,10]. In the context of the present study, therefore, it can be surmised that the higher serum levels of IL-6 and TNF-α in some AD patients confer on them the clinical features of depression (Fig. 1). An alternative possibility may also be envisaged. Depression is long considered as a predisposing factor for AD [26–28]. The chronically raised levels of circulating cytokines in patients of depressive illness can activate brain microglia through aforementioned mechanisms to produce elevated levels of proinflammatory cytokines within CNS which in turn can trigger the amyloid pathology in AD brain. It is well established through experimental studies that the cytokines like IL-6, IL-1 and TNF-α can increase Aβ accumulation in the brain by several mechanisms and also trigger other neurodegenerative processes in the brain [29–31].
From whatever already stated about the functions of central cytokines and the pathway of communication between the peripheral immune system and the CNS, it is hardly surprising to observe a good correlation between the severity of cognitive decline and the level of IL-6 or TNF-α in AD patients with depression (Fig.2). In fact, the role of central cytokines in cognition has strong experimental support at the cellular level, and the peripheral inflammation affecting cognition is well documented [11, 13, 14, 15, 16]. Despite the pro-inflammatory cytokine IL-1β having a similar role to that of IL-6 and TNF-α in the communication between the peripheral immune system and the CNS as documented in many studies, it is surprising that no clear association is seen between the serum levels of IL-1β and the AD related depression or cognitive deficit in this study (Fig. 1 and Fig. 2).
In summary, our study has highlighted that the activation of peripheral immune system in some, but not all, AD patients has important implications in the associated mood disorder and cognitive deficit, and the circulating IL-6 and TNF-α may play a crucial role in this process. Conceptually it is possible that a similar mechanism through raised levels of peripheral pro-inflammatory cytokines will be operative in non-AD types of dementia with or without depression, but the reports are lacking in the literature in this regard. It is also important to point out several important limitations of the present report. Firstly, the sample size is not very high, especially when comparing the data between AD groups with or without depression. This has prevented us from further sub-grouping the AD patients according to severity of the disease (say, based on MMSE scores) and then reanalyzing the data. Moreover, the standard deviations in serum levels of cytokines in both the control and AD subjects in our study are very high, but this problem is apparent in most of the published reports of altered cytokines in AD [4–7]. The reasons for such a wide scatter in the serum cytokine levels are not clear, but a large-scale population based study with standardized assay methods could provide some clues to the problem. Another limitation of the study is the confounding effect of the drug history of the patient. Apart from the cholinesterase inhibitor (donepezil) and anti-excitotoxicity agent (memantine), the patients were additionally given antihypertensives (ACE or angiotensin-converting enzyme inhibitors and calcium channel blockers), statins and mixtures of B vitamins for variable periods. Moreover, the AD patients with depression were all under the treatment with Selective serotonin reuptake inhibitors (SSRIs) for 3 – 6 months at the time of collection of blood for cytokine assay. Notwithstanding these limitations, the data are interesting, and in particular the role of IL-6 and TNF-α in AD related depression and cognitive deficits subjects should be further explored in longitudinal studies as also in animal models of AD. Furthermore, it will be interesting to find if antidepressant drugs or drugs reducing proinflammatory cytokines in the circulation can alter the clinical progression of the disease in AD subjects.
Acknowledgments
The work was supported in part by a grant to Prof. Sasanka Chakrabarti from the Department of Science and Technology, Govt. of India, New Delhi (Sanction No.SR/SO/HS-78/2008).We acknowledge ‘The West Bengal University of Health Sciences’ and the Director, IPGME&R for help and encouragement.
Footnotes
Conflicts of Interest
The authors declare no conflict of interest.
References
- [1].Qiu C, Fratiglioni L. Epidemiology of dementia. In: Mcnamara P, editor. Dementia Treatments and Developments. ABC-CLIO; 2011. pp. 1–33. [Google Scholar]
- [2].McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Starkstein SE, Mizrahi R. Depression in Alzheimer’s disease. Expert Rev Neurother. 2006;6:887–895. doi: 10.1586/14737175.6.6.887. [DOI] [PubMed] [Google Scholar]
- [4].Alvarez XA, Franco A, Fernandez-Novoa L, Cacabelos R. Blood levels of histamine, IL-1β, and TNF-α in patients with mild to moderate alzheimer disease. Mol Chem Neuropathol. 1996;29:237–252. doi: 10.1007/BF02815005. [DOI] [PubMed] [Google Scholar]
- [5].Gezen-Ak D, Dursun E, Hanagasi H, Bilgiç B, Lohman E, Araz OS, et al. BDNF, TNFα, HSP90, CFH, and IL-10 serum levels in patients with early or late onset Alzheimer’s disease or mild cognitive impairment. J Alzheimers Dis. 2013;37:185–195. doi: 10.3233/JAD-130497. [DOI] [PubMed] [Google Scholar]
- [6].Humpel C, Hochstrasser T. Cerebrospinal fluid and blood biomarkers in Alzheimer’s disease. World J Psychiatry. 2011;1:8–18. doi: 10.5498/wjp.v1.i1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Swardfager W, Lanctot K, Rothenburg L, Wong A, Capell J, Hermann NA. Meta-analysis of cytokines in Alzheimer’s disease. Biol Psychiatry. 2010;68:930–941. doi: 10.1016/j.biopsych.2010.06.012. [DOI] [PubMed] [Google Scholar]
- [8].Leonard BE. Inflammation, depression and dementia: are they connected? Neurochem Res. 2007;32:1749–1756. doi: 10.1007/s11064-007-9385-y. [DOI] [PubMed] [Google Scholar]
- [9].Anisman H. Cascading effects of stressors and inflammatory immune system activation: implications for major depressive disorder. J Psychiatry Neurosci. 2009;34:4–20. [PMC free article] [PubMed] [Google Scholar]
- [10].Khairova RA, Machado-Vieira R, Du J, Manji HK. A potential role for pro-inflammatory cytokines in regulating synaptic plasticity in major depressive disorder. Int J Neuropsycho pharmacol. 2009;12:561–578. doi: 10.1017/S1461145709009924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kronfol Z, Remick DG. Cytokines and the brain: implications for clinical psychiatry. Am J Psychiatry. 2000;157:683–694. doi: 10.1176/appi.ajp.157.5.683. [DOI] [PubMed] [Google Scholar]
- [12].Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Terrando N, Monaco C, Ma D, Foxwell BM, Feldmann M, Maze M. Tumor necrosis factor-alpha triggers a cytokine cascade yielding postoperative cognitive decline. Proc Natl Acad Sci U S A. 2010;107:20518–20522. doi: 10.1073/pnas.1014557107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wilson CJ, Finch CE, Cohen HJ. Cytokines and cognition – The case for head–to-toe inflammatory paradigm. J Am Geriatr Soc. 2002;50:2041–2056. doi: 10.1046/j.1532-5415.2002.50619.x. [DOI] [PubMed] [Google Scholar]
- [15].McAfoose J, Baune BT. Evidence for a cytokine model of cognitive function. Neurosci Biobehav Rev. 2009;33:355–366. doi: 10.1016/j.neubiorev.2008.10.005. [DOI] [PubMed] [Google Scholar]
- [16].Lim A, Krajina K, Marsland AL. Peripheral inflammation and cognitive aging. Mod Trends Pharmacopsychiatry. 2013;28:175–187. doi: 10.1159/000346362. [DOI] [PubMed] [Google Scholar]
- [17].Cameron B, Landreth GE. Inflammation, microglia, and Alzheimer’s disease. Neurobiol Dis. 2010;37:503–509. doi: 10.1016/j.nbd.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Weisman D, Hakimian E, Ho GJ. Interleukins, Inflammation, and Mechanisms of Alzheimer’s Disease. Vitam Horm. 2006;74:505–530. doi: 10.1016/S0083-6729(06)74020-1. [DOI] [PubMed] [Google Scholar]
- [19].Weitz TM, Town T. Microglia in Alzheimer’s Disease: It’s All About Context. Int J Alzheimers Dis. 2012;2012:314185. doi: 10.1155/2012/314185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Mrak RE, Griffin WS. Potential inflammatory biomarkers in Alzheimer’s disease. J Alzheimer’s Dis. 2005;8:369–375. doi: 10.3233/jad-2005-8406. [DOI] [PubMed] [Google Scholar]
- [21].Lee KS, Chung JH, Choi TK, Suh SY, Oh BH, Hong CH. Peripheral cytokines and chemokines in Alzheimer’s disease. Dement Geriatr Cogn Disord. 2009;28:281–287. doi: 10.1159/000245156. [DOI] [PubMed] [Google Scholar]
- [22].Barrientos RM, Frank MG, Watkins LR, Maier SF. Memory Impairments in Healthy Aging: Role of Aging-Induced Microglial Sensitization. Aging Dis. 2010;1:212–231. [PMC free article] [PubMed] [Google Scholar]
- [23].Maier SF. Bi-directional immune-brain communication: implications for understanding stress, pain, and cognition. Brain Behav Immun. 2003;17:69–85. doi: 10.1016/s0889-1591(03)00032-1. [DOI] [PubMed] [Google Scholar]
- [24].Makhija K, Karunakaran S. The role of inflammatory cytokines on the aetiopathogenesis of depression. Aust N Z J Psychiatry. 2013;47:828–839. doi: 10.1177/0004867413488220. [DOI] [PubMed] [Google Scholar]
- [25].Cyranowski JM, Marsland AL, Bromberger JT, Whiteside TL, Chang Y, Matthews KA. Depressive symptoms and production of proinflammatory cytokines by peripheral blood mononuclear cells stimulated in vitro. Brain Behav Immun. 2007;21:229–237. doi: 10.1016/j.bbi.2006.07.005. [DOI] [PubMed] [Google Scholar]
- [26].Green RC, Cupples LA, Kurz A, Auerbach S, Go R, Sadovnick D, et al. Depression as a risk factor for Alzheimer disease - The MIRAGE study. Arch Neurol. 2003;60:753–759. doi: 10.1001/archneur.60.5.753. [DOI] [PubMed] [Google Scholar]
- [27].Steffens DC, Plassman BL, Helms MJ, Welsh-Bohmer KA, Saunders AM, Breitner JCS. A twin study of late-onset depression and apolipoprotein E ɛ4 as risk factors for Alzheimer’s disease. Biol Psychiatry. 1997;41:851–856. doi: 10.1016/S0006-3223(96)00247-8. [DOI] [PubMed] [Google Scholar]
- [28].Devanand DP, Sano M, Tang MX, Taylor S, Gurland BJ, Wilder D, Yakov S, Mayeux R. Depressed mood and the incidence of Alzheimer’s disease in the elderly living in the community. Arch Gen Psychiatry. 1996;53:175–182. doi: 10.1001/archpsyc.1996.01830020093011. [DOI] [PubMed] [Google Scholar]
- [29].Cacquevel M, Lebeurrier N, Cheenne S, Vivien D. Cytokines in neuroinflammation and Alzheimer’s disease. Curr Drug Targets. 2004;5:529–534. doi: 10.2174/1389450043345308. [DOI] [PubMed] [Google Scholar]
- [30].Blasko I, Marx F, Steiner E, Hartmann T, Grubeck-Loebenstein B. TNF alpha plus IFN gamma induce the production of Alzheimer beta-amyloid peptides, and decrease the secretion of APPs. FASEB J. 1999;13:63–68. doi: 10.1096/fasebj.13.1.63. [DOI] [PubMed] [Google Scholar]
- [31].Smith JA, Das A, Ray SK, Banika NL. Role of pro-inflammatory cytokines released from microglia in neurodegenerative diseases. Brain Research Bulletin. 2010;87:10–20. doi: 10.1016/j.brainresbull.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]


