Abstract
Uncontrolled continued exposure to oxidative stress is a precursor to many chronic diseases including cancer, diabetes, degenerative disorders and cardiovascular diseases. Of the many known mediators of oxidative stress, reactive oxygen species (ROS) and advanced glycation end products (AGEs) are the most studied. In the present review, we have summarized current data on the origin of circulating AGEs, discussed issues associated with reliable assessment of its steady state level, and changes in its level with age and select metabolic diseases. Lastly, we have made recommendations about life style changes that may decrease AGEs burden to promote healthy aging.
Keywords: Aging, glycation, oxidative stress, reactive nitrogen species, reactive oxygen species
All living organisms are exposed to oxidative stress (OS) throughout the life cycle. Oxidative stress expresses itself by generation of reactive oxygen species (ROS), reactive nitrogen species (RNS) and other highly reactive mediators such as advanced glycation end products (AGEs) that modify macromolecules essential to life processes resulting in altered and abnormal physiology [1]. However, the body is also equipped with an anti-oxidant system to protect it from excessive oxidant insult. It is the breakdown of this balance that leads to undesired health outcomes. While excessive uncontrolled experience with oxidative stress is detrimental to health [1–3], low levels of ROS may compromise protection rendered by apoptosis [4,5], phagocytosis [1] and detoxification reactions [1]. The concept of relationship between oxidation, modification of essential macromolecules, and disease is summarized in Figure 1. Sustained elevated oxidative state increases risk for chronic diseases (cancer, cardiovascular diseases, cataracts and degenerative disorders) resulting from damage to cell constituents (DNA, proteins, lipids, etc.) and cell structures [1]. In contrast, inappropriately low oxidative state results in compromised apoptosis and phagocytosis hence, decreased protection from certain cancers and infections [1]. Although antioxidants may counterbalance the ill effects of high oxidant state, these antioxidants may prove detrimental due to further decrease in oxidant-dependent protective mechanisms [6].
Figure 1.
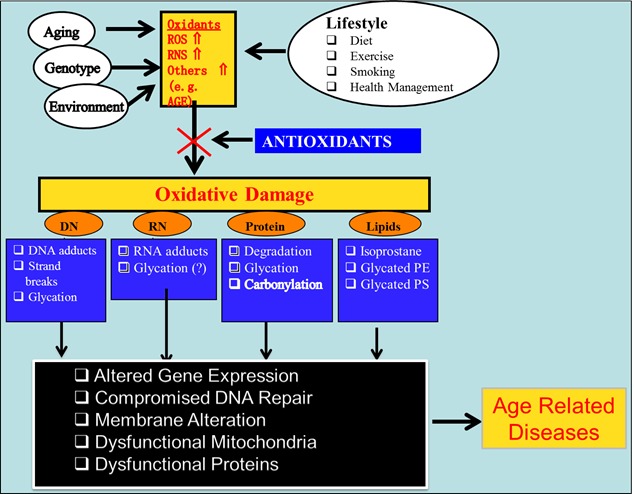
Relationship between oxidation, modification of essential macromolecules and age-related diseases.
Advanced glycation end products (AGEs) belong to the group of other highly reactive mediators that alter essential macromolecules in cell. The purpose of this review is to focus on how lifestyle changes can control generation of AGEs and thus risk for chronic diseases.
Origin of Advanced Glycation End Products (AGEs)
Advanced glycation end products (AGEs) constitute a diverse group of compounds formed when glucose or other reducing sugars such as galactose and fructose react with amino acids, nucleotide bases or fatty acids, forming glycated molecules [7–10]. Protein glycation, commonly known as Maillard reaction is non-enzymatic reaction that was first described by Louis Maillard [11]. The Maillard reaction includes a series of slow non-enzymatic reactions between carbonyl group of reducing sugars such as glucose, fructose, or pentose and the amino groups of proteins that may take several days to several weeks. These slow non-enzymatic reactions include coupling between carbonyl group from reducing sugar and amino group from proteins to yield an unstable Schiff’s base followed by generation of stable Amadori product and glycation of proteins. The chemistry and process of glycation is complex and has been extensively reviewed elsewhere [12, 13].
High levels of AGEs may elicit undesired health consequences through one or more of the following mechanisms. First, certain AGEs such as pentosidine are capable to form crosslinks with adjacent proteins that may stiffen previously flexible or elastic tissues [14]. Second, certain AGEs devoid of crosslinking ability can glycate proteins and change their functions [15]. Third, glycated proteins or smaller glycated peptides may serve as a ligand for the receptor for AGE (RAGE) that is coupled to inflammatory pathway [16].
Cellular Targets of AGEs and Its Consequences
Receptors for advanced glycation end products (RAGEs) belong to a member of the immunoglobulin superfamily of receptors [17]. RAGE, an approximately 45-kDa protein, has an extracellular component consisting of a variable (V) immunoglobulin-like domain followed by two constant domains (type C and C’) [18]. It has a single transmembrane domain followed by a cytosolic tail [18]. The N-terminus of the V domain is the ligand-binding site, and the cytosolic tail is essential for RAGE-induced intracellular signaling [19]. In addition to full-length RAGE, truncated forms have also been reported resulting from alternative mRNA splicing or proteolytic cleavage from the cell surface [2]. One such variant, which lacks both the cytosolic and the transmembrane domains and thus is secreted extra-cellularly, is known as the soluble receptor for advanced glycation end products (sRAGE) [18,19]. Because sRAGE retains its ability to bind circulating ligands, it may play an important role in attenuating the adverse effects associated with RAGE signaling [18].
RAGE is a pattern recognition receptor [20,21] and has a large repertoire of ligands, making it crucial to the etiology of a variety of chronic disorders generally associated with cellular stress and inflammation. Some of these ligands include: advanced glycation end products (AGEs), amyloid-β peptide (which accumulates in Alzheimer’s disease), amyloid A (which accumulates in systemic amyloidosis), S100/calgranulins, a family of closely related calcium-binding polypeptides that accumulate extracellularly at sites of chronic inflammation, DNA-binding protein HMGB1 (amphoterin), which is released by cells undergoing apoptosis, and surface molecules on bacteria and leukocytes (macrophage-1 antigen or Mac-1) [17–21].
RAGE is widely expressed in a variety of tissues (heart, lung, skeletal muscle, and vessel wall) and cell types (monocytes/macrophages and lymphocytes) [17]. The activation of RAGE by its ligands (including AGEs) results in induction of oxidative stress and expression of pro-inflammatory cytokines and chemokines.
AGE Burden in Normal Aging and Under Select Metabolic Diseases
The steady state level of circulating serum AGEs is the function of a delicate balance between endogenous production, exogenous intake and renal and enzymatic clearance. Several enzymes (glyoxalase I, II and carbonyl reductase) and a receptor (AGER1) have been shown to participate in a detoxification and counterregulation of the prooxidant effects of AGEs [22,23]. In addition, renal excretion of excess AGEs also plays an important role under physiological conditions. Therefore, a disruption in the balance between endogenous production, exogenous intake, and renal clearance has been suggested to be responsible for changes in its steady-state levels with aging as well as in pathological situations [23,24].
What role these excursions in AGEs may have in causation and/or progression of any diseased condition remains speculative. There are animal studies implicating AGEs in disease processes in a manner consistent with cause-effect relationship. For example, normal rats receiving an AGEs-rich diet for six weeks exhibited dose- and time-dependent increase in proteinuria [32]. In another study, a 6-month continuous consumption of high AGEs diet resulted in higher levels of fasting glucose, insulin and serum AGEs, as well as increased AGEs localization in ovarian tissue of rats [33]. These observations are further supported by a series of reports from the laboratory of Dr. Vlassara suggesting improvement in insulin sensitivity [29], prevention of diabetic nephropathy [30] and prevention of autoimmune diabetes in NOD mice [31]. However, the relevance of these observations from animal studies to human can only be suggestive.
Human studies dealing with relationships between circulating AGEs and markers of chronic diseases (e.g., insulin sensitivity, lipids, and fasting glucose) can be subdivided largely into two groups. First, those that compare associations between levels of AGEs and markers of chronic diseases in control and diseased subjects. While results of such studies provide some insight into relationships between levels of AGEs and markers of chronic disease, it may not be possible to discern a cause-effect relationship [35]. The second group of studies includes population studies on healthy humans examining relationships between AGEs and markers of chronic diseases as well as intervention studies. While these studies are fewer, they yield valuable results. For example, Vlassara and her co-workers examined correlation between AGEs levels, markers of inflammation and oxidative stress in 172 healthy volunteers [25]. The results showed a significant correlation between AGEs and insulin sensitivity (r = 0.247, p =. 044) [25]. In a separate study, the same group conducted a cross-sectional study with 2-yr follow-up of 325 healthy adults and 66 chronic kidney disease (CKD-3) patients, a subset of which received a reduced AGE or regular diet [22]. The results show reduction in AGEs, oxidant stress, receptor for AGE, and TNF-alpha in normal and CKD-3 patients after the low-AGE diet, independently of age [22].
The accumulation of AGEs in tissues may contribute to increased OS, and as a final result, impair organ function [34]. Indeed, within the complex association between OS and aging ovarian follicles, AGEs may have an important role as they accumulate over the lifespan [26]. More recent work suggests a mechanism for this in that detoxification of an AGEs precursor is significantly diminished in older mice, allowing AGEs to increase [27]. Additionally, slowly diminishing renal function with age [28] could affect the ability to excrete AGEs. Uribarri et al. suggest this as a possible explanation as to why they found high AGE levels in the older group, even though the intake of dietary AGEs by the older age group was reduced [25]. More evidence is needed, but these results suggest the important role of dietary and circulating AGEs in chronic degenerative diseases, which are more prevalent in the elderly. In conclusion, levels of circulating AGEs in healthy as well as diseased individuals may have implications for health outcomes.
Reducing AGEs Burden Through Lifestyle Changes
The difficulties that investigators have experienced in establishing a conclusive relationship between circulating/tissue AGE and healthy aging and chronic disease morbidity are multiple. The first and foremost is the nature AGEs and their measurements. The term AGEs include a plethora of glycated molecules (proteins, carbohydrates and nucleic acids) of different sizes. The measurement of AGEs, however, generally utilizes enzyme-linked immunosorbent assay (ELISA) designed to recognize a very specific epitope on the molecule. This can certainly result in underestimation of total as well as specific epitopes for any AGE. Furthermore. The glycated epitope on a macromolecule may be buried in such way to escape detection by ELISA. Secondly, there is no reliable data on absorption, metabolism, pharmacokinetics and metabolism of any specific member of AGEs family. Lastly, whether it is endogenous (cell or tissue resident), or exogenous AGEs responsible for pro-oxidative inflammatory role is not established. Furthermore, we do not know whether exogenous AGEs (some or all) will be internalized by cells or not. Despite the aforementioned limitations, the existing data indeed suggest the importance of AGEs in healthy aging and chronic disease morbidity.
Our lifestyle is a product of the way we live, where we live and who we are. This includes, our genetics and ethnicity, environment, diet, extent of use of legal drugs (tobacco and alcohol) and professions we practice. Although data on all possible determinants of circulating AGEs do not exist, we have enough empirical data to recommend specific changes.
Food-derived AGEs seem to be easy target for reduction of AGEs intake. There are many excellent reviews [36, 37] on this subject that outline strategies for reducing AGEs in food. Briefly, AGEs form as food browns during cooking, primarily when foods high in protein or fat are subjected to high temperatures. Cooking at a higher temperature for a shorter period of time creates more AGEs than cooking at lower temperatures for longer periods of time. Also, exposure to dry heat produces more AGEs than cooking in liquid. Thus, broiling, frying, or grilling of meats creates more AGEs than boiling, poaching, or stewing. For example, a chicken breast broiled for 15 minutes contains more than five times as much AGEs as the same food boiled for one hour.
Cigarette smoking appears to be another source of AGEs. It is known that conditions of curing tobacco lead to formation of AGEs [38, 39]. Cerami et al. have reported the presence of AGEs in aqueous extracts of tobacco as well as tobacco smoke [38]. Furthermore, AGE-apolipoprotein B and serum AGE levels in cigarette smokers were significantly higher than those in nonsmokers [38]. In support Nicoll reported that in smokers, tobacco-derived AGEs accumulates on plasma low density lipoprotein (LDL), structural proteins present within the vascular wall, and the lens proteins of the eye [39].
Recipe for Reducing Advanced Glycation End Products Burden
While much needs to be learned about nature of different species of AGEs and their formation and fate of exogenous AGEs in vivo, there are certain practical ways to reduce load of AGEs from exogenous sources. These can be summarized as Cook right, eat right and live right. A meal rich in complex carbohydrates, fresh fruits and vegetables with moderate intake of meat will significantly lower daily AGEs intake. Meats should be cooked by boiling, poaching, or stewing rather than broiling, frying, or grilling. Foods to be avoided should include those that may be high in sugar, high in fat and cooked by frying or grilling. Smoking must be avoided at any cost since it is not only sources of ready made AGEs, but it also promotes AGE formation in vivo.
It is apparent from the data presented here that AGEs consist of a variety of chemically distinct glycated molecules of different sizes. The formation of these molecules is generally associated with diminution in biologic functions of affected molecules as well as structural changes in cell architecture. Much needs to be learned about the nature of different species of AGEs, their synthesis, metabolism, distribution and cellular response, if we were to design logical interventions.
References
- [1].Salganik RI. Apoptosis and other protective mechanisms in cancer patients and the human population. J Am Coll Nutr. 2001;20:464S–472S. doi: 10.1080/07315724.2001.10719185. [DOI] [PubMed] [Google Scholar]
- [2].Halliwell B, Cutteridge JMC. Free radicals in biology and medicine. Oxford: Oxford University Press; 1969. [Google Scholar]
- [3].Aims BN, Shinegava MK, Hagen TM. Oxidants, antioxidants and the degenerative diseases. Proc Natl Acad Sci USA. 1993;90:7915–7922. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kerr JFR, Winterfold CM, Harmon BV. Apoptosis, its significance in cancer and cancer therapy. Cancer. 1994;73:2013–2026. doi: 10.1002/1097-0142(19940415)73:8<2013::aid-cncr2820730802>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- [5].Blackstone NW, Green DR. The evolution of a mechanism of cell suicide. BioEssays. 1999;21:84–88. doi: 10.1002/(SICI)1521-1878(199901)21:1<84::AID-BIES11>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- [6].Ghosh MK, Mukhopadhyay M, Chatterjee IB. NADPH-initiated P450-dependent free-iron-dependent microsomal lipid peroxidation: specific prevention by ascorbic acid. Mol Cell Biochem. 1997;166:35–44. doi: 10.1023/a:1006841228483. [DOI] [PubMed] [Google Scholar]
- [7].Barlovic DP, Soro-Paavonen A, Jandeleit-Dahm KA. RAGE biology, atherosclerosis and diabetes. Clin Science. 2011;121:43–55. doi: 10.1042/CS20100501. [DOI] [PubMed] [Google Scholar]
- [8].Kalousová M, Zima T, Tesař V, Dusilová-Sulková S, Jan Škrha J. Advanced glycooxidation end products in chronic disease—clinical chemistry and genetic background. Mutation Res. 2005;579:37–46. doi: 10.1016/j.mrfmmm.2005.03.024. [DOI] [PubMed] [Google Scholar]
- [9].Semba RD, Bandinelli S, Sun K, Guralnik JM, Ferrucci L. Relationship of an advanced glycation end product, plasma carboxymethyl-lysine, with slow walking speed in older adults: the InCHIANTI study. Eur J Appl Physiol. 2010;108:109–115. doi: 10.1007/s00421-009-1192-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Uribarri J, Woodruff S, Goodman S, Cai W, Chen X, Pyzik R, Yong A, Striker GE, Vlassara H. Advanced glycation end products in foods and a practical guide to their reduction in the diet. J of the Am Diet Assoc. 2010;110:911–916. doi: 10.1016/j.jada.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Maillard LC. Action des acides amines sur les sucres: formation des melanoidines par voie methodique. C R Acad Sci (Paris) 1912;154:66–68. [Google Scholar]
- [12].Luevano-Contreras C, Chapman-Novakofski K. Dietary Advanced Glycation End Products and Aging. Nutrients. 2010;2:1247–1265. doi: 10.3390/nu2121247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Prasad A, Bekker P, Tsimikas S. Advanced glycation end products and diabetic cardiovascular disease. Cardiol Rev. 2012;20:177–183. doi: 10.1097/CRD.0b013e318244e57c. [DOI] [PubMed] [Google Scholar]
- [14].Willett TL, Kandel R, De Croos JNA, Avery NC, MGrynpas MD. Enhanced levels of nonenzymatic glycation and pentosidine crosslinking in spontaneous osteoarthritis progression. Osteoarthritis Cartilage. 2012;20:7736–744. doi: 10.1016/j.joca.2012.03.012. [DOI] [PubMed] [Google Scholar]
- [15].Ulrich P, Cerami A. Protein glycation, diabetes, and aging. Recent Prog Horm Res. 2001;56:1–21. doi: 10.1210/rp.56.1.1. [DOI] [PubMed] [Google Scholar]
- [16].Sparvero LJ, Asafu-Adjei D, Kang R, Tang D, Amin N, Im J, Rutledge R, Lin B, Amoscato AA, Zeh HJ, Lotze MT. RAGE (Receptor for Advanced Glycation Endproducts), RAGE Ligands, and their role in Cancer and Inflammation. J Transl Med. 2009;17:7–17. doi: 10.1186/1479-5876-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ramasamy R, Vannucci SJ, Shi Du Yan S, Herold K, Fang Yan S, Schmidt AM. Advanced glycation end products and RAGE: a common thread in aging, diabetes, neurodegeneration, and inflammation. Glycobiology. 2005;15(7):16R–28R. doi: 10.1093/glycob/cwi053. [DOI] [PubMed] [Google Scholar]
- [18].Raucci A, Cugusi S, Antonelli A, Barabino SM, Monti L, Bierhaus A, Reiss K, Saftig P, Bianchi ME. A soluble form of the receptor for advanced glycation endproducts (RAGE) is produced by proteolytic cleavage of the membrane-bound form by the sheddase a disintegrin and metalloprotease 10 (ADAM10) FASEB J. 2008;22:3716–3727. doi: 10.1096/fj.08-109033. [DOI] [PubMed] [Google Scholar]
- [19].Kalea AZ, Schmidt AM, Hudson BI. Alternative splicing of RAGE: roles in biology and disease. Frontiers in Bioscience. 2011;l16:2756–2770. doi: 10.2741/3884. [DOI] [PubMed] [Google Scholar]
- [20].Ramasamy R, Yan S-F, Schmidt AM. Advanced glycation endproducts: from precursors to RAGE: round and round we go. Amino Acids. 2012;42(4):1151–1161. doi: 10.1007/s00726-010-0773-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Fritz G. RAGE a single receptor fits multiple ligands. Trends in Biochem Sci. 2011;36(12):625–632. doi: 10.1016/j.tibs.2011.08.008. [DOI] [PubMed] [Google Scholar]
- [22].Vlassara H, Cai W, Goodman S, Pyzik R, Yong A, Chen X, Zhu L, Neade T, Beeri M, Silverman JM, Ferrucci L, Tansman L, Striker GE, Uribarri J. Protection against loss of innate defenses in adulthood by low advanced glycation end products (AGE) intake: Role of the antiinflammatory AGE receptor-1. J Clin Endocrinol Metab. 2009;94:4483–4491. doi: 10.1210/jc.2009-0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Vlassara H, Striker G. Glycotoxins in the diet promote diabetes and diabetic complications. Curr Diab Rep. 2007;7:235–241. doi: 10.1007/s11892-007-0037-z. [DOI] [PubMed] [Google Scholar]
- [24].Peppa M, Uribarri J, Vlassara H. Aging and glycoxidant stress. Hormones (Athens) 2008;7:123–132. doi: 10.1007/BF03401503. [DOI] [PubMed] [Google Scholar]
- [25].Uribarri J, Cai W, Peppa M, Goodman S, Ferrucci L, Striker G, Vlassara H. Circulating glycotoxins and dietary advanced glycation endproducts: two links to inflammatory response, oxidative stress, and aging. J Gerontol A Biol Sci Med Sci. 2007;62:427–433. doi: 10.1093/gerona/62.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Tatone C, Amicarelli F, Carbone MC, Monteleone P, Caserta D, Marci R, Artini PG, Piomboni P, Focarelli R. Cellular and molecular aspects of ovarian follicle ageing. Hum Reprod Update. 2008;14:131–142. doi: 10.1093/humupd/dmm048. [DOI] [PubMed] [Google Scholar]
- [27].Tatone C, Carbone MC, Campanella G, Festuccia C, Artini PG, Talesa V, Focarelli R, Amicarelli F. Female reproductive dysfunction during ageing: role of methylglyoxal in the formation of advanced glycation endproducts in ovaries of reproductively-aged mice. J Biol Regul Homeost Agents. 2010;24:63–72. [PubMed] [Google Scholar]
- [28].Lindeman RD. Overview: Renal physiology and pathophysiology of aging. Am J Kidney Dis. 1990;16:275–282. doi: 10.1016/s0272-6386(12)80002-3. [DOI] [PubMed] [Google Scholar]
- [29].Hofmann SM, Dong HJ, Li Z, Cai W, Altomonte J, Thung SN, Zeng F, Fisher EA, Vlassara H. Improved insulin sensitivity is associated with restricted intake of dietary glycoxidation products in the db/db mouse. Diabetes. 2002;51:2082–2089. doi: 10.2337/diabetes.51.7.2082. [DOI] [PubMed] [Google Scholar]
- [30].Zheng F, He C, Cai W, Hattori M, Steffes M, Vlassara H. Prevention of diabetic nephropathy in mice by a diet low in glycoxidation products. Diabetes Metab Res Rev. 2002;18:224–237. doi: 10.1002/dmrr.283. [DOI] [PubMed] [Google Scholar]
- [31].Peppa M, He C, Hattori M, McEvoy R, Zheng F, Vlassara H. Fetal or neonatal low–glycotoxin environment prevents autoimmune diabetes in NOD mice. Diabetes. 2003;52:1441–1448. doi: 10.2337/diabetes.52.6.1441. [DOI] [PubMed] [Google Scholar]
- [32].Sebekova K, Hofmann T, Boor P, Sebekova K, Jr, Ulicna O, Erbersdobler HF, Baynes JW, Thorpe SR, Heidland A, Somoza V. Renal effects of oral maillard reaction product load in the form of bread crusts in healthy and subtotally nephrectomized rats. Ann N Y Acad Sci. 2005;1043:482–491. doi: 10.1196/annals.1333.055. [DOI] [PubMed] [Google Scholar]
- [33].Diamanti-Kandarakis E, Piperi C, Korkolopoulou P, Kandaraki E, Levidou G, Papalois A, Patsouris E, Papavassiliou AG. Accumulation of dietary glycotoxins in the reproductive system of normal female rats. J Mol Med. 2007;85:1413–1420. doi: 10.1007/s00109-007-0246-6. [DOI] [PubMed] [Google Scholar]
- [34].Bengmark S. Impact of nutrition on ageing and disease. Curr Opin Clin Nutr Metab Care. 2006;9:2–7. doi: 10.1097/01.mco.0000171129.29278.26. [DOI] [PubMed] [Google Scholar]
- [35].Davis K, Prasad C, Juma S, Vijayagopal P, Imrhan V. Advanced Glycation End Products, Inflammation, and Chronic Metabolic Diseases: Links in a Chain? Critical Reviews in Nutrition and Food Science. 2013. (In Press). [DOI] [PubMed]
- [36].Goldberg T, Cai W, Peppa M, Dardaine V, Baliga BS, Uribarri J, Vlassara H. Advanced glycoxidation end products in commonly consumed foods. J Am Diet Assoc. 2004;104:1287–1291. doi: 10.1016/j.jada.2004.05.214. [DOI] [PubMed] [Google Scholar]
- [37].Uribarri J, Woodruff S, Goodman S, Cai W, Chen X, Pyzik R, Yong A, Striker GE, Vlassara H. Advanced glycation end products in foods and a practical guide to their reduction in the diet. J Am Diet Assoc. 2010;110:911–16. doi: 10.1016/j.jada.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Nicholl ID, Bucala R. Advanced glycation endproducts and cigarette smoking. Cell Mol Biol (Noisy-le-grand) 1998;44(7):1025–33. [PubMed] [Google Scholar]
- [39].Cerami C, Founds H, Nicholl I, Mitsuhashi T, Giordano D, Vanpatten S, Lee A, Al-Abed Y, Vlassara H, Bucala R, Cerami A. Tobacco smoke is a source of toxic reactive glycation products. Proc Natl Acad Sci U S A. 1997;94(25):13915–20. doi: 10.1073/pnas.94.25.13915. [DOI] [PMC free article] [PubMed] [Google Scholar]


