Abstract
Animal nutrition is profoundly influenced by the gut microbiota, but knowledge of the scope and core mechanisms of the underlying animal–microbiota interactions is fragmentary. To investigate the nutritional traits shaped by the gut microbiota of Drosophila, we determined the microbiota-dependent response of multiple metabolic and performance indices to systematically varied diet composition. Diet-dependent differences between Drosophila bearing its unmanipulated microbiota (conventional flies) and experimentally deprived of its microbiota (axenic flies) revealed evidence for: microbial sparing of dietary B vitamins, especially riboflavin, on low-yeast diets; microbial promotion of protein nutrition, particularly in females; and microbiota-mediated suppression of lipid/carbohydrate storage, especially on high sugar diets. The microbiota also sets the relationship between energy storage and body mass, indicative of microbial modulation of the host signaling networks that coordinate metabolism with body size. This analysis identifies the multiple impacts of the microbiota on the metabolism of Drosophila, and demonstrates that the significance of these different interactions varies with diet composition and host sex.
KEY WORDS: B vitamins, Drosophila, Gut microbiota, Hyperlipidemia, Protein nutrition, Symbiosis
INTRODUCTION
The resident gut microbiota plays a pivotal role in animal nutrition (Flint et al., 2012; Karasov and Douglas, 2013). These microorganisms engage with animal acquisition and allocation of nutrients, the two key processes that shape the nutrition of an animal, in multiple ways. They can consume ingested nutrients or provide supplementary nutrients to the host; they can alter feeding and nutrient assimilation rates; and they can modify nutrient allocation patterns of the host by modulating the nutrient-sensing and -signaling pathways of the animal host (Bäckhed et al., 2004; Caricilli and Saad, 2013; Goodman et al., 2009; Vijay-Kumar et al., 2010). Multiple studies indicate that the resident microorganisms generally promote animal nutrition, although the nutritional benefit can vary with diet, composition of the microbiota and animal genotype (Benson et al., 2010; Kau et al., 2011; Parks et al., 2013; Smith et al., 2013). Mismatch between the microbiota and animal results in poor host health, a condition known as dysbiosis (Nicholson et al., 2012; Stecher et al., 2013).
Much of current understanding of the nutritional significance of the microbiota in animals comes from comparisons between untreated animals bearing the microbiota (conventional animals) and animals deprived of their microbiota (germ-free/axenic animals) (Gordon and Pesti, 1971; Smith et al., 2007; Yi and Li, 2012). Most of these studies are conducted on a single diet, or a few diets in which either a single nutritional component or total nutrient concentration is altered. However, inclusive information on the nutritional significance of microorganisms to their animal host can only be obtained from comparative analyses using diets of systematically varied composition. For example, disproportionately low performance of axenic animals, relative to conventional animals, on certain diets can be attributed to microbial production of nutrients that are deficient in the diet and/or microbial consumption of dietary nutrients in excess; and the performance of axenic animals can be superior to that of conventional animals where the microbiota depresses host access to nutrients in short dietary supply. Parallel analysis of the host metabolic composition, especially major classes of macronutrients (protein, lipid, etc.), provides additional insight into the impact of the microbiota on the nutrient allocation patterns of the host.
This study on the microbiota-dependent response of animals to diet was conducted on Drosophila melanogaster, which is well suited to large experimental designs involving extensive dietary manipulations. The gut microbiota, which is dominated by members of the Acetobacteraceae and Lactobacillales (Chandler et al., 2011; Wong et al., 2011; Wong et al., 2013), has substantive effects on Drosophila (Broderick and Lemaitre, 2012; Erkosar et al., 2013). Axenic Drosophila display extended larval development time and, in some studies, depressed adult mass and total lifespan (Bakula, 1969; Brummel et al., 2004; Ren et al., 2007; Ridley et al., 2012; Shin et al., 2011; Storelli et al., 2011). Elimination or perturbation of the microbiota can also result in altered metabolic indices, including elevated lipid and carbohydrate levels, together with reduced basal metabolic rates (Ridley et al., 2012; Shin et al., 2011). Furthermore, there is evidence that the Drosophila nutrient signaling pathways, especially insulin/TOR signaling, are sensitive to the microbiota, but the mechanistic detail is uncertain (Shin et al., 2011; Storelli et al., 2011).
The specific aim of this study was to determine the complement of nutritional interactions between Drosophila melanogaster and its microbiota by comparing the performance and metabolic indices of conventional and axenic flies on diets of systematically varied composition. Our analysis reveals that the microbiota enables Drosophila to utilize low-nutrient/unbalanced diets by sparing the requirement for dietary B vitamins, promotes protein nutrition, and suppresses energy (lipid/carbohydrate) storage, especially on high sugar diets.
RESULTS
Drosophila performance
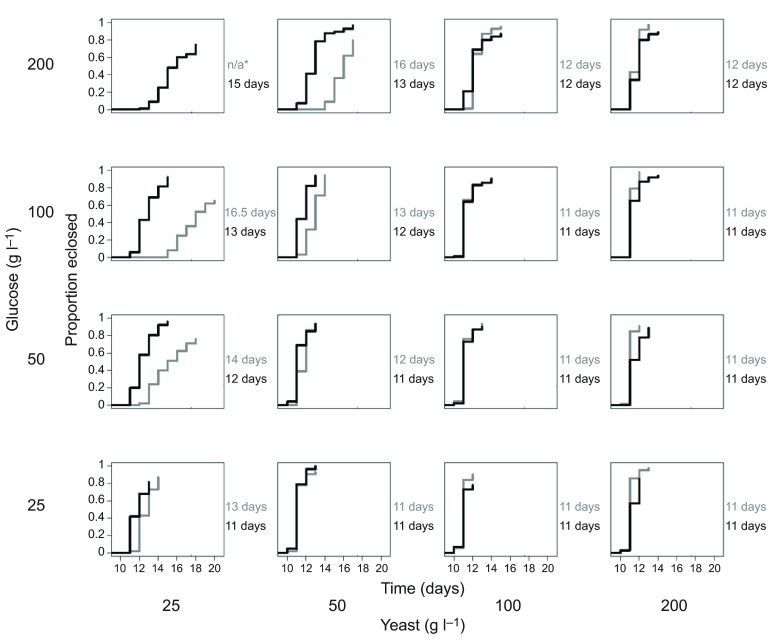
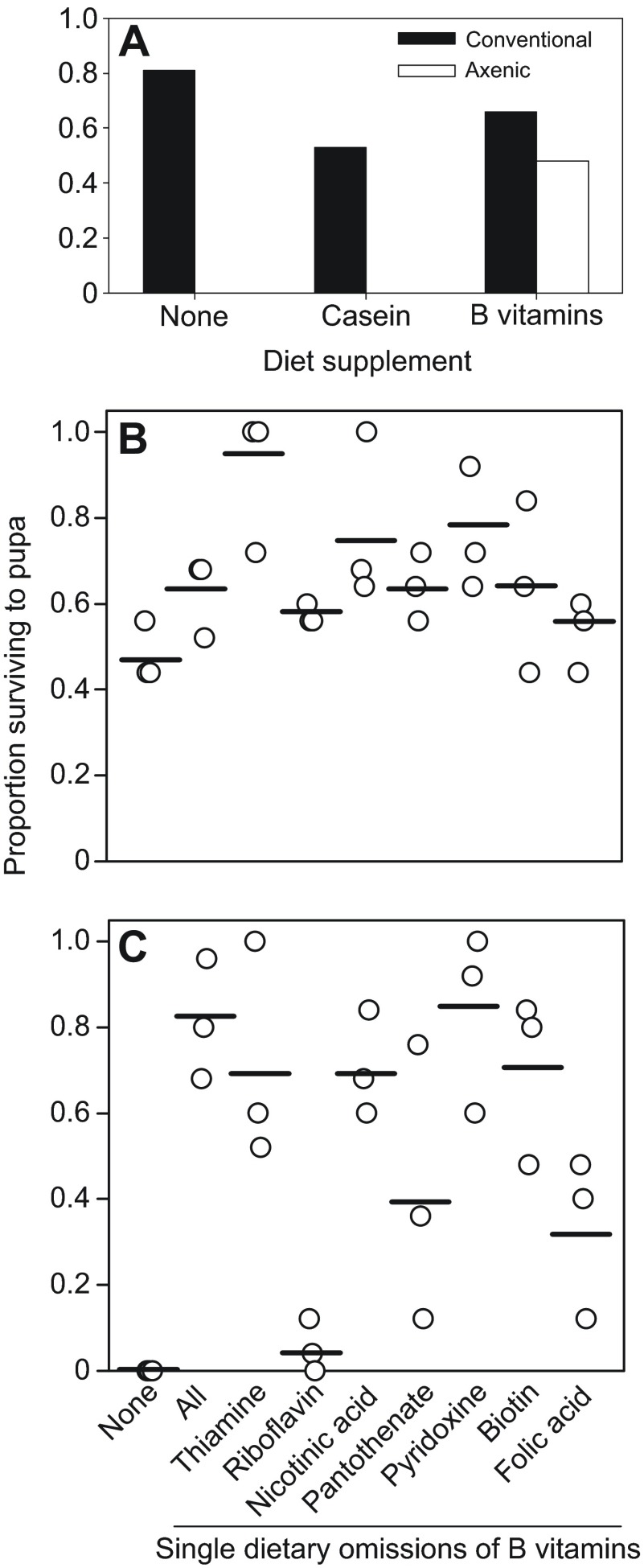
The first experiments quantified the survival and development time to adulthood of axenic and conventional Drosophila on 16 diets containing glucose and yeast, both at concentrations systematically varied over an eightfold range (25–200 g l−1). Pre-adult mortality of conventional Drosophila was ≤35% on all 16 diets, but axenic Drosophila displayed high mortality on diets of low yeast/high glucose content, all dying as larvae on the diet containing 25 g yeast and 200 g glucose l−1 (Fig. 1). The critical dietary determinant of development time for axenic and conventional flies was yeast:glucose ratio (P<0.0001), but the strength of this effect was reduced in conventional flies relative to axenic flies (P=0.03) (supplementary material Table S1A). We investigated the basis of the mortality of axenic Drosophila on the diet containing 25 g yeast and 200 g glucose l−1. We hypothesized that the microbiota may spare the Drosophila requirement for certain yeast constituents, particularly protein and B vitamins, which are especially important for Drosophila development (Blatch et al., 2010; Sang, 1962). Fig. 2A shows that the conventional and axenic Drosophila responded differently to the dietary supplements of casein protein and B vitamins. In four replicate trials, all the axenic Drosophila died before pupation on the diets with no supplement or with casein supplement, but 59±4.4% (mean ± s.e.) of the axenic Drosophila on the B vitamin supplemented diet survived to pupae. For the conventional treatment, >50% of the Drosophila developed to pupation on the three test diets, and the development time of conventional Drosophila was significantly accelerated from a median of 15 days on the diet without supplement to 13 days on the casein-supplemented diet (Mann–Whitney U: P<0.05) but not the vitamin-supplemented diet.
Fig. 1.
Development time of Drosophila. Cumulative proportion of eclosed conventional (black) and axenic (gray) adults (pooled data for five replicate vials of 20 eggs), with median development time to adulthood (right) for each of 16 diets; *n/a: no axenic Drosophila survived to adulthood. The statistical analysis is displayed in supplementary material Table S1A.
Fig. 2.
Performance of Drosophila on diet supplemented with B vitamins. Survival to pupation or adulthood of replicate sets of 25 eggs on diets containing 25 g yeast l−1 and 200 g glucose l−1 with supplements as indicated. (A) Survival of conventional and axenic larvae to pupation on diet with casein protein or B vitamin supplement. (B,C) Survival (four replicates) to pupation of conventional (B) and axenic (C) Drosophila on diets with no B vitamin supplement (none), all seven B vitamins (all), and each vitamin individually omitted.
To identify the specific dietary B vitamin(s) that promoted survival of axenic Drosophila to pupation, the Drosophila were reared on diets with the vitamin supplement from which each B vitamin was individually omitted (Fig. 2B,C). The proportion of individuals surviving to pupation varied significantly with diet for axenic Drosophila (Kruskall–Wallis: H8=19.46, P=0.013) but not conventional Drosophila (H8=15.41, P>0.05). Post hoc analysis revealed that survival of axenic Drosophila was significantly depressed on two diets, relative to the diet with all B vitamins: the diet with no vitamin supplement and the diet lacking riboflavin (P<0.05). These data suggest that the microbiota spares the requirement of conventional Drosophila for dietary riboflavin.
Nutritional indices of Drosophila
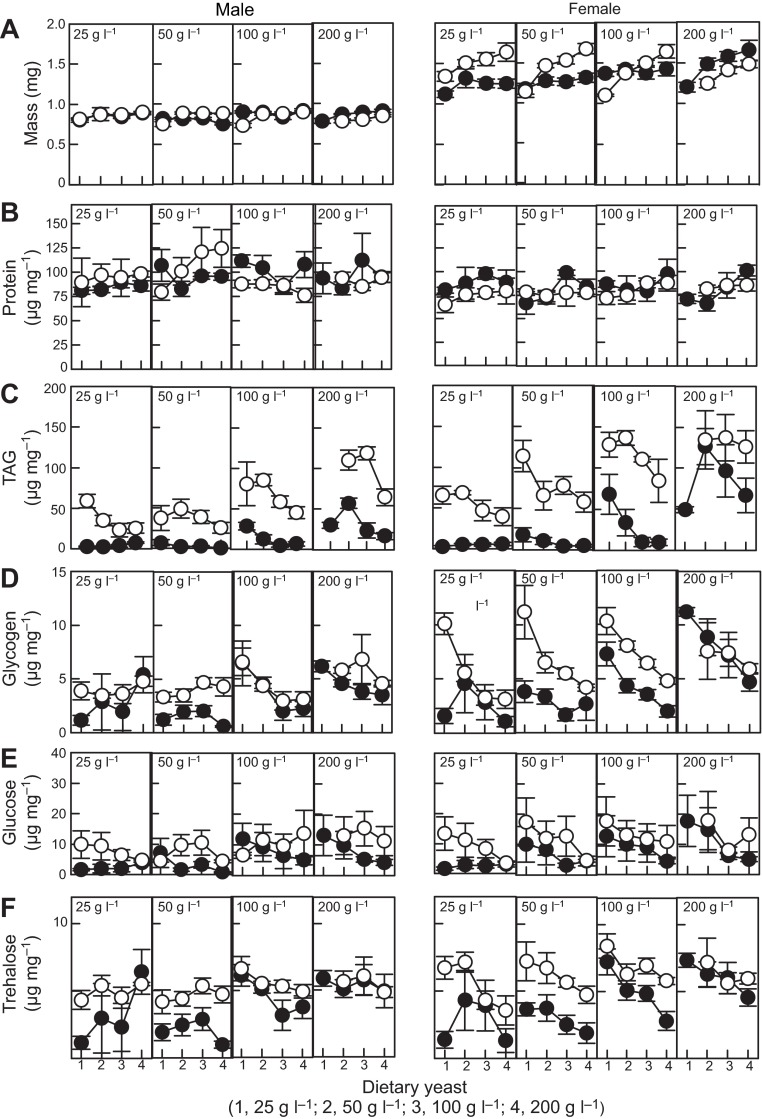
Overall, the adult body masses of conventional and axenic Drosophila were comparable (Fig. 3A), but the two sexes differed in their response to diet composition (supplementary material Table S1B). For females, dietary yeast promoted the mass of axenic flies more than that of conventional flies. The mass of males was more robust to variation in diet composition, but axenic males on low yeast diets were generally small.
Fig. 3.
Mass and nutritional indices of 5 day old adult Drosophila. The diets contain 25–200 g glucose l−1 (concentration provided in each plot) and 25–200 g yeast l−1 (labeled 1–4 on x-axis). Filled circles, conventional flies; open circles, axenic flies; values are means ± s.e. (five replicates). TAG, triglyceride. The statistical analysis is displayed in supplementary material Table S1B.
The impact of eliminating the microbiota on protein content differed between the two sexes (Fig. 3B; supplementary material Table S1B). For females, protein content varied significantly with dietary yeast and was significantly reduced in axenic flies, on average by 7% relative to conventional flies, but the depressed protein content of axenic males was evident only on high glucose diets. These data suggest that the microbiota spares the demand of adult Drosophila for dietary protein, with the microbiota effect more pervasive across the diets in females than in males.
The four indices of energy storage (Fig. 3C–F) were positively correlated, with broadly similar results in the univariate analyses (supplementary material Table S1B), and so they were grouped together in a multivariate analysis (Table 1), which provides an aggregate measure of energy metabolism. For both sexes, energy storage was significantly elevated by elimination of the microbiota and high dietary glucose. For males, no interaction between microbiota and diet was evident, but for females the effect of the microbiota on energy storage was shaped by the interaction between dietary glucose and yeast.
Table 1.
Multivariate analysis of energy storage indices of 5 day old adult Drosophila
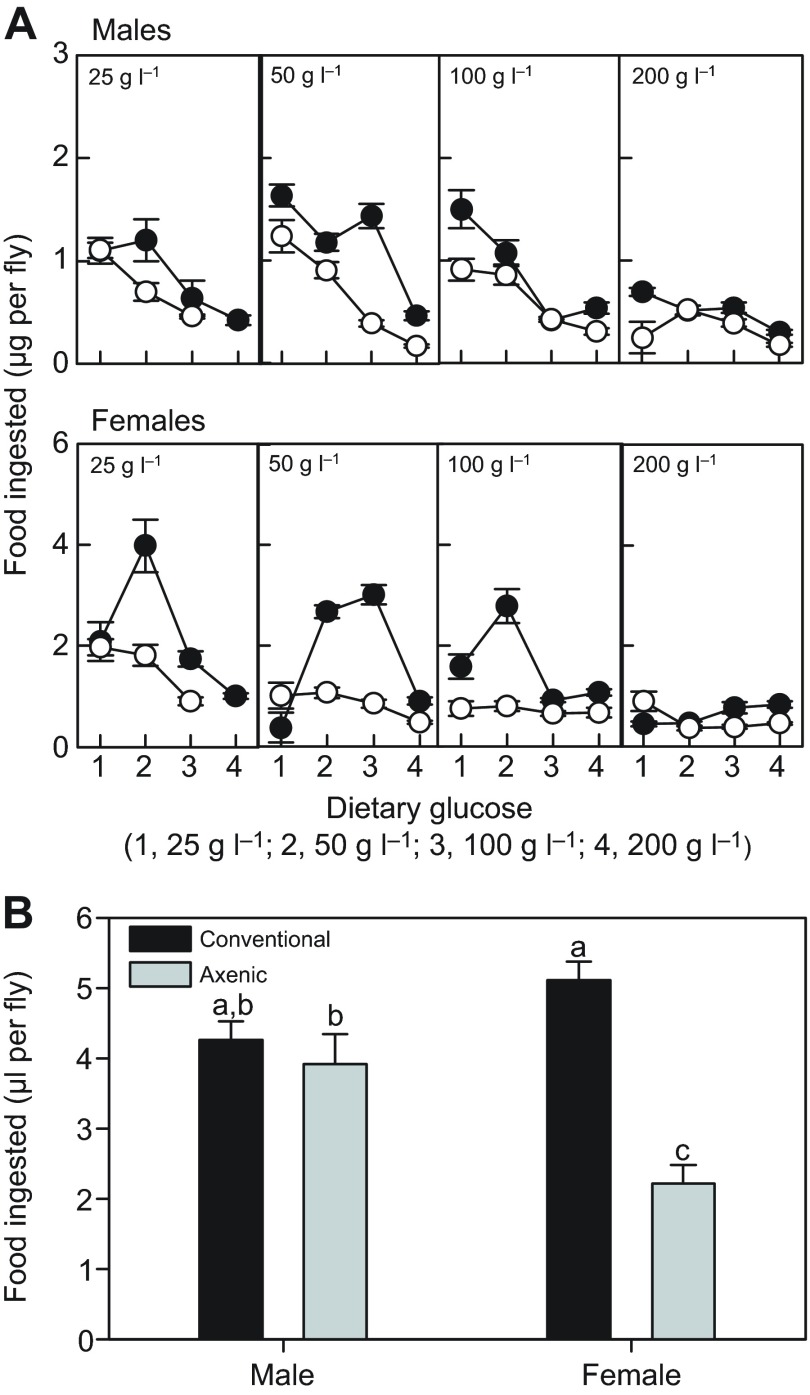
We hypothesized that the elevated energy storage in axenic flies was linked to higher feeding rates. Contrary to expectation, axenic flies consumed significantly less food than conventional flies (supplementary material Table S1B), on average reduced by 30% in males and 48% in females in feeding trials of 30 min duration (Fig. 4A). Food consumption also varied with diet composition, with flies generally displaying increased food intake on diets of intermediate and low glucose content, as reported previously (Lee et al., 2008). In complementary feeding trials conducted over 48 h, mean food consumption was reduced in axenic flies, relative to conventional flies, and the effect was significant for females (Fig. 4B), demonstrating that the reduced feeding by axenic flies in the 30 min trials was not compensated by an extended duration of feeding over the daily cycle. We concluded that the increased energy storage in axenic flies could not be attributed to hyperphagia.
Fig. 4.
Food consumption by adult Drosophila. (A) Mass of food consumed over 30 min by flies reared on diets containing 25–200 g yeast l−1 (concentration provided in each plot) and 25–200 g glucose l−1 (labeled 1–4 on x-axis). Filled circles, conventional flies; open circles, axenic flies; values are means ± s.e. The statistical analysis is displayed in supplementary material Table S1B. (B) Volume of liquid food consumed from capillary tubes over 48 h. Values are means ± s.e. (N=18). ANOVA: microbiota F1,68=27.15, P<0.001; sex F1,68=1.87, P>0.05; interaction F1,68=16.83, P<0.001. Different letters denote statistically significant differences by Tukey's post hoc test.
The interactive effects of diet and microbiota on Drosophila nutrition were investigated further by two sets of principal components analysis (PCA). The first PCA focused on energy storage. These indices covaried with mass in conventional flies, but the relationship was reversed in axenic flies (Fig. 5A–D). This difference arises from the greater responsiveness of the mass of axenic flies compared with the mass of conventional flies to dietary yeast, and of their energy storage to dietary glucose, with the result that diets of low yeast/high glucose content yielded small axenic flies with disproportionately high energy storage.
Fig. 5.
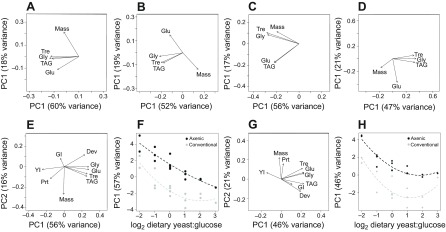
Global analysis of energy storage and nutritional responses of conventional and axenic Drosophila. (A–D) Principal components analysis (PCA) loading scores of energy storage indices for first and second axes (PC1 and PC2) with percentage variance in parentheses, for (A) conventional males, (B) axenic males, (C) conventional females and (D) axenic females. (E–H) PCA analysis of all performance and metabolic responses. (E) Loading scores for PC1 and PC2 for males. (F) Relationship between dietary yeast:glucose ratio and male PC1. The regression equations are: male conventional: y=−2.536–0.616x+1.969x2, male axenic y=1.268–6.128x+3.237x2, with ANOVA: diet F2,24=34.29, P<0.001; microbiota F1,24=63.99, P<0.001; interaction F2,24=0.706, P>0.05. (G) Loading scores for PC1 and PC2 for females. (H) Relationship between dietary yeast:glucose ratio and female PC1. The regression equations are: female conventional y=−2.940+1.071x+2.643x2, female axenic y=1.470–8.309x+0.9478x2, with ANOVA: diet F2,24=49.98, P<0.001; microbiota F1,24=98.84, P<0.001; interaction F2,24=1.016, P>0.05. Dev, development time to adulthood; Glu, glucose content; GI, dietary glucose ingested; Gly, glycogen content; Prt, protein content; TAG, triglyceride content; Tre, trehalose content; Mass, body mass; YI, dietary yeast ingested.
In the second analysis, the average values of all measured traits in conventional and axenic flies were compressed by PCA to investigate the global patterns in performance and nutritional responses to diet (Fig. 5E–H). Here, PC1 was strongly positively loaded by development time and energy storage, and negatively loaded by yeast ingested, for both sexes; and the PC1 values for the 15 diets were significantly negatively related to dietary yeast:glucose ratio, with elevated intercepts for axenic flies relative to conventional flies (see Fig. 5F,H). The relationship was robust to removal of yeast and glucose ingestion from the PCA (data not shown), indicating that it is not an artefact of autocorrelation between dietary nutrient content and amount of food ingested. This analysis reveals that the microbiota interacts with diets of high yeast:glucose content, to favor rapid host development at the expense of deposition of energy reserves.
DISCUSSION
It is widely recognized that the relationship between animals and their gut microbiota involves multiple interactions that vary with composition of the microbiota, host genotype and environmental factors, especially diet (Faith et al., 2011; Human Microbiome Project Consortium, 2012; Kau et al., 2011; Turnbaugh et al., 2009). This study focused on the consequences of these interactions for host nutrition, by investigating the combined effects of diet and elimination of the microbiota on the performance and nutrient allocation of Drosophila. A key result was that the gut microbiota is either beneficial (promotes host performance) or benign (no discernible effect on host performance) but not deleterious to Drosophila reared on the full range of diets tested. The implications are twofold. First, host and microbiota do not compete for dietary nutrients, which would be indicated by the superior performance of axenic Drosophila on certain diets. This suggests that the various diet-derived nutrients are either not utilized by both host and microbiota, or are in sufficient abundance that their consumption by microbiota does not limit host performance. Second, Drosophila is not dependent on its microbiota for normal physiological function, which would be revealed as the superior performance of conventional Drosophila on all diets. Instead, the microbiota particularly promoted Drosophila performance on diets of low or unbalanced nutrient content, indicating that the association has a nutritional basis. Our analysis implicated the microbiota in B vitamin nutrition, protein nutrition and energy storage, and we discuss these three nutritional processes below.
On the most unbalanced diet tested (25 g yeast and 200 g glucose l−1), the microbiota spared the Drosophila requirement for the B vitamin riboflavin. Our results differ from previous reports implicating the microbiota in folic acid provisioning (Blatch et al., 2010; Piper et al., 2013), and the discrepancies may reflect detailed differences in the diets used in the different studies. For example, we may not have obtained a statistically significant effect of folic acid omission on axenic Drosophila performance because the dietary yeast used in this study may have had higher concentrations of folic acid than the yeast used in previous studies. In broad terms, these findings for Drosophila are congruent with evidence that various animals, including other insects and mammals, bear bacteria that are capable of synthesizing B vitamins and contribute to the vitamin nutrition of their host (Akman et al., 2002; Goodman et al., 2009; Hill, 1997; LeBlanc et al., 2013; Roscoe, 1931; Wu et al., 2006).
Riboflavin deficiency in axenic Drosophila is predicted to have far-reaching nutritional and metabolic consequences, contributing to explanations for multiple phenotypic traits of these flies. Riboflavin is metabolized to flavin adenine dinucleotide (FAD) and flavin mononucleotide (FMN) via riboflavin kinase and FAD synthetase, respectively, both of which are coded by the Drosophila genome. FAD and FMN are the defining required cofactors for all flavoproteins, with crucial roles in energy metabolism (e.g. in the respiratory electron transport chain, decarboxylation of pyruvate, and fatty acyl CoA dehydrogenase in fatty acid oxidation) and redox reactions, including reduction of oxidized glutathione (GSSG) to the reduced form (GSH), and are also crucial for animal-mediated synthesis of the active forms of other B vitamins, specifically the transformation of folate (vitamin B9) to 5-methyl-tetrahydrofolate, and conversion of pyridoxal (vitamin B6) to pyridoxal phosphate (McCormick, 2012; Powers, 2005). Riboflavin deficiency and the consequent reduced availability of other cofactors may contribute, for example, to the depressed metabolic rate (Ridley et al., 2012) and elevated lipid content (Newell and Douglas, 2013) (this study) of axenic Drosophila.
A reduced protein content of axenic flies was found with most diets for females and with diets of high glucose content in males. The protein content of conventional flies may be protected by digestion of protein-rich microbial cells or by the specific release of certain amino acids from members of the microbiota; both processes are known in other animal–microbial symbioses (Douglas, 2010). Additionally or alternatively, the bacteria may modulate host regulation of nutrient allocation such that the protein content is maintained, even on unbalanced diets. The importance of the microbiota-dependent protection of protein content underlines the central role of protein homeostasis to the nutritional health of animals. In particular, the defense of the protein content has been implicated in apparently maladaptive responses of animals to unbalanced diets, such that animals raised on high energy/low protein diets tend to overfeed to obtain sufficient protein, resulting in the accumulation of excess energy storage and obesity. However, this process, known as protein leverage of obesity (Simpson and Raubenheimer, 2005), cannot readily account for the high energy storage of axenic Drosophila because the elevated lipid content is associated with reduced feeding (Fig. 4). It is more likely that the microbiota-dependent protein nutrition in Drosophila is linked to microbiota-dependent insulin signaling in Drosophila (Shin et al., 2011; Storelli et al., 2011). Specifically, insulin signaling is generally stimulated by protein nutrition (Prentki et al., 2013), raising the possibility that the promotion of Drosophila protein nutrition by the microbiota may contribute to the microbial stimulation of insulin signaling. Also, depressed insulin signaling in axenic flies is predicted to increase gluconeogenesis from the breakdown products of protein-derived amino acids, resulting in protein wasting and reduced protein content of the flies.
The axenic flies displayed hyperlipidemia and hyperglycemia, as is found in conventional Drosophila only on diets of exceptionally high carbohydrate content (Musselman et al., 2011; Na et al., 2013). This effect was particularly pronounced in axenic flies of low body mass, which occurred on diets of low yeast content. Larval growth and adult size are likely limited by dietary protein on low yeast diets (yeast is the sole source of protein in the diets used in this study), and this effect would be particularly acute on diets with high glucose content, which tends to depress insect feeding rates (Edgecomb et al., 1994; Lee et al., 2008) (this study). The bacterial sparing of dietary protein would protect the mass of conventional Drosophila on diets of low yeast/high glucose content. The disproportionately high lipid and carbohydrate content of axenic flies may also be attributed to the absence of metabolic activity from the gut bacteria. The dominant gut bacteria (Acetobacteraceae and Lactobacillales) in Drosophila are capable of utilizing glucose, raising the possibility that they reduce the glucose concentration in the food ingested by conventional flies, and hence energy intake per unit food consumed, relative to axenic flies on the same diet. In addition (as considered above), the supply of riboflavin by the bacteria would buffer conventional flies on low yeast diets against impaired energy metabolism, arising from reduced activity of FAD/FMN-dependent enzymes in lipolysis, energy metabolism and redox reactions.
Intriguingly, this effect is the reverse of observations on germ-free mice, which are significantly leaner than conventional mice (Bäckhed et al., 2004). The difference between conventional and germ-free mice can be attributed to microbiota in the mammalian colon that ferment complex polysaccharides to short chain fatty acids, thereby enhancing the caloric content of the food ingested by these hosts by up to 10–20% (Smith et al., 2007). If the microbiota in Drosophila, similarly, degraded complex polysaccharides [which dominate the carbohydrate content of yeast (Suomalainen and Pfaffli, 1961)] to simple sugars or organic acids that are utilized by the fly, we would predict reduced energy storage in axenic flies. Our evidence that microbial metabolism does not enhance the availability of dietary carbon to Drosophila points to an important difference between the host–microbiota relationship in mammals and Drosophila.
Although eliminating the microbiota has reverse effects on lipid/carbohydrate storage in Drosophila and mouse, the axenic Drosophila is reminiscent of mouse models with microbiota perturbed by mutation or sub-therapeutic antibiotic treatment (Cho et al., 2012; Vijay-Kumar et al., 2010). This raises the possibility that microbial perturbation elicits a global metabolic response that includes reduced energy storage and is conserved between Drosophila and mammals. This effect would be masked in mammalian hosts by microbial fermentation, which enhances the caloric value of food for conventional animals.
We have, additionally, found that the microbial effects on Drosophila are not uniform between males and females, indicating that aspects of the interaction between the microbiota and the host metabolic program are sex specific. To our knowledge, the microbiota has not been considered previously in the many demonstrations of between-sex differences in Drosophila metabolism and metabolism gene expression patterns (Ayroles et al., 2009; Bauer et al., 2006; Bharathi et al., 2003; Greenberg et al., 2011; Jumbo-Lucioni et al., 2010; Lushchak et al., 2014; Scheitz et al., 2013), although there is evidence for sex-specific impacts of the microbiota on metabolic traits of the mouse (Markle et al., 2013). The processes contributing to interactions between the microbiota and host metabolism are likely multiple and interactive. The host signaling pathways regulating metabolism of males and females may respond differently to microbial products and their absence; and the metabolic traits of the microbiota may be influenced by the many metabolic and other physiological differences between the sexes, especially the nutritional demand in females for egg production.
In conclusion, this study has identified a set of host–microbe interactions that shapes the metabolic phenotype of Drosophila: specifically, microbial provisioning of B vitamins and protein, and microbial modulation of lipid/carbohydrate allocation. Our analysis, based on comparisons between conventional and axenic hosts, provides the essential framework for future investigations of metabolite exchange between the partners and microbial engagement with the signaling networks that regulate metabolic flux and nutrient allocation.
MATERIALS AND METHODS
The insects and diets
A culture of Wolbachia-free Drosophila melanogaster strain Canton S was maintained in routine culture at 25°C under a 12 h:12 h light:dark cycle on yeast–glucose medium [Y–G, comprising 100 g l−1 glucose (Sigma, St Louis, MO, USA), 100 g l−1 inactive yeast (MP Biomedicals, Santa Ana, CA, USA) and 12 g l−1 agar (MP Biomedicals) and preservatives (0.04% phosphoric acid, 0.42% propionic acid; Sigma)]. The 16 test diets comprised yeast (Y) and glucose (G) at each combination of 25, 50, 100 or 200 g l−1, giving Y:G ratios ranging from 1:8 to 8:1. For some experiments, casein protein (Sigma) at 78.8 g l−1 final concentration was added to diet containing 25 g yeast and 200 g l−1 glucose, to give protein content equivalent to that of diets with 200 g l−1 yeast (yeast comprises 45% protein). The vitamin supplement to diets comprised thiamine (1.4 mg l−1), riboflavin (0.7 mg l−1), nicotinic acid (8.4 mg l−1), pantothenate (10.8 mg l−1), pyridoxine (1.7 mg l−1), biotin (0.1 mg l−1) and folic acid (9 mg l−1), as elsewhere (Sang, 1956; Blatch et al., 2010). Filter-sterilized supplements were added aseptically to autoclaved diet cooled to 55°C.
Experiments were initiated with eggs deposited overnight by mated females, and axenic treatment was obtained by dechorionating eggs in 0.6% sodium hypochlorite flies as described previously (Ridley et al., 2012). For all experiments, 20 eggs were added to 7.5 ml diet in sterile 50 ml Falcon tubes using aseptic technique in a laminar flow cabinet.
Drosophila performance and metabolic indices
Axenic and conventional flies were raised in five replicate vials (one replicate of every diet formulation on five different days). Vials were monitored daily and larval development time to pupation and eclosion was recorded. For most experiments, the number of individuals surviving to adulthood was scored, but survival to pupation was adopted as the most direct index of larval performance on vitamin-supplemented diets. Experiments were terminated 30 days after egg transfer.
Each sample of five male flies or five female flies at 4/5 days post-eclosion was weighed on a microbalance (Mettler, MX5; Columbus, OH, USA) to an accuracy of 1 μg. The sample was then hand-homogenized in 125 μl ice-cold TE buffer (pH 7.4) comprising 10 mmol l−1 Tris, 1 mmol l−1 EDTA and 0.1% Triton-X-100, followed by centrifugation at 7000 g at 4°C for 1 min. A portion (20 μl) of each supernatant was immediately stored at −80°C for analysis of total protein, while the remaining supernatant was heat treated at 72°C for 20 min to inactivate enzymatic activity before analysis of glucose, trehalose glycogen and triglyceride content, and then stored at −80°C prior to analysis. Metabolic assays were conducted in 96-well plates using commercial kits/reagents following manufacturers' instructions, as described previously (Ridley et al., 2012): the DC Protein Assay kit (Bio-Rad, 500-0116; Hercules, CA, USA), Triglyceride Assay kit (Sigma, TG-5-RB), and Glucose (GO) Assay kit (Sigma, GAGO20) for glucose, and for trehalose and glycogen after treatment with trehalase (1 U ml−1, Sigma, T8778) or amyloglucosidase (2 U ml−1, Sigma, A7420), respectively, at 37°C for 1 h. All colorimetric readings were obtained using a microplate spectrophotometer (Bio-Rad, xMark) with standards, and the nutritional indices are expressed as μg mg−1 body mass.
Feeding assays
All feeding assays were initiated 6 h after onset of the light period. For short-term feeding trials, 110 flies of each treatment (diet, sex and conventional/axenic) were anesthetized by CO2 and sorted into 11 vials of 10 flies, and then starved for 2 h. Ten groups of flies were transferred to diet labeled with a blue dye (0.5% Xylene Cyanol and 0.1% Bromophenol Blue) and one group was transferred to dye-free diet as a control. After 30 min, the flies were frozen at −80°C until analyzed. Samples of frozen flies were thawed for 2 min, rinsed gently in water, and the number of flies that had fed, as indicated by blue dye in the abdomen, was scored by examination under a dissecting microscope (7×). Each sample of fed flies was then homogenized in 100 μl TE buffer (10 mmol l−1 Tris, 1 mmol l−1 EDTA and 0.1% Triton-X-100, pH 7.4) with 1.4 mm ceramic beads (MP Biomedicals) in FastPrep-24 Instrument (MP Biomedicals) for 1 min, diluted with an additional 500 μl TE buffer and centrifuged at 7000 g for 3 min. The absorbance of the supernatant were measured at 614 nm using a microplate spectrophotometer (Bio-Rad, xMark). Absorbance values were transformed to μg food ingested per fly, by reference to a standard curve generated with dilution series of the dye (0–200 ng dye ml−1).
To quantify food consumption over long periods, the capillary feeder (CAFÉ) system of Ja et al. (Ja et al., 2007) was used. Liquid food (100 g yeast extract and 100 g glucose l−1 sterile water) was delivered to 18 replicate groups of five, 5 day old adult flies of each sex in 5 μl microcapillary tubes (Drummond Microcaps, Drummond Scientific, Broomall, PA, USA). Food consumption over 48 h was quantified as the change in height of the liquid column in the microcapillary tube, after subtraction of the height difference of control vials containing no flies.
Data analysis
All data were analyzed in R (version 2.15.1). Data were fitted to the three-way interaction of dietary glucose, dietary yeast and microbiota treatment, with experimental replicate as a random effect. Dietary yeast and glucose content were log2 transformed for analysis of development, as this transformation yielded a better model fit. Development data were analyzed by fitting a mixed-effects log-rank (frailty) model from the coxme library. In all analyses, triglyceride content was square-root transformed and the 30 min feeding rate was log transformed to achieve normal distributions. The mass, nutrient content and food uptake over 30 min of the flies were first analyzed with linear mixed-effects models using the lme function from the nlme library. Analyses of energy stores yielded congruent results, and so the glucose, trehalose, glycogen and triglyceride content were reanalysed together, using a multivariate linear mixed model. PCA was performed using prcomp.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Stephanie Westmiller and Sara Hermann for technical assistance, and Dr Nicolas Buchon, Dr John Chaston, Dr Brian Lazzaro and Dr Peter Newell for discussions and valuable comments on the manuscript.
FOOTNOTES
Competing interests
The authors declare no competing financial interests.
Funding
This work was supported by National Institutes of Health grant 1R01GM095372 and graduate student fellowship (to A.C.-N.W.) of The Sarkaria Institute for Insect Physiology and Toxicology. Deposited in PMC for release after 12 months.
Supplementary material
Supplementary material available online at http://jeb.biologists.org/lookup/suppl/doi:10.1242/jeb.101725/-/DC1
References
- Akman L., Yamashita A., Watanabe H., Oshima K., Shiba T., Hattori M., Aksoy S. (2002). Genome sequence of the endocellular obligate symbiont of tsetse flies, Wigglesworthia glossinidia. Nat. Genet. 32, 402-407 [DOI] [PubMed] [Google Scholar]
- Ayroles J. F., Carbone M. A., Stone E. A., Jordan K. W., Lyman R. F., Magwire M. M., Rollmann S. M., Duncan L. H., Lawrence F., Anholt R. R., et al. (2009). Systems genetics of complex traits in Drosophila melanogaster. Nat. Genet. 41, 299-307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäckhed F., Ding H., Wang T., Hooper L. V., Koh G. Y., Nagy A., Semenkovich C. F., Gordon J. I. (2004). The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. USA 101, 15718-15723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakula M. (1969). The persistence of a microbial flora during postembryogenesis of Drosophila melanogaster. J. Invertebr. Pathol. 14, 365-374 [DOI] [PubMed] [Google Scholar]
- Bauer M., Katzenberger J. D., Hamm A. C., Bonaus M., Zinke I., Jaekel J., Pankratz M. J. (2006). Purine and folate metabolism as a potential target of sex-specific nutrient allocation in Drosophila and its implication for lifespan-reproduction tradeoff. Physiol. Genomics 25, 393-404 [DOI] [PubMed] [Google Scholar]
- Benson A. K., Kelly S. A., Legge R., Ma F., Low S. J., Kim J., Zhang M., Oh P. L., Nehrenberg D., Hua K., et al. (2010). Individuality in gut microbiota composition is a complex polygenic trait shaped by multiple environmental and host genetic factors. Proc. Natl. Acad. Sci. USA 107, 18933-18938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharathi N. S., Prasad N. G., Shakarad M., Joshi A. (2003). Variation in adult life history and stress resistance across five species of Drosophila. J. Genet. 82, 191-205 [DOI] [PubMed] [Google Scholar]
- Blatch S. A., Meyer K. W., Harrison J. F. (2010). Effects of dietary folic acid level and symbiotic folate production on Drosophila melanogaster. Fly (Austin) 4, 312-319 [DOI] [PubMed] [Google Scholar]
- Broderick N. A., Lemaitre B. (2012). Gut-associated microbes of Drosophila melanogaster. Gut Microbes 3, 307-321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummel T., Ching A., Seroude L., Simon A. F., Benzer S. (2004). Drosophila lifespan enhancement by exogenous bacteria. Proc. Natl. Acad. Sci. USA 101, 12974-12979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caricilli A. M., Saad M. J. (2013). The role of gut microbiota on insulin resistance. Nutrients 5, 829-851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler J. A., Lang J. M., Bhatnagar S., Eisen J. A., Kopp A. (2011). Bacterial communities of diverse Drosophila species: ecological context of a host-microbe model system. PLoS Genet. 7, e1002272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho I., Yamanishi S., Cox L., Methé B. A., Zavadil J., Li K., Gao Z., Mahana D., Raju K., Teitler I., et al. (2012). Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature 488, 621-626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas A. E. (2010). The Symbiotic Habit. Princeton, NJ: Princeton University Press; [Google Scholar]
- Edgecomb R. S., Harth C. E., Schneiderman A. M. (1994). Regulation of feeding behavior in adult Drosophila melanogaster varies with feeding regime and nutritional state. J. Exp. Biol. 197, 215-235 [DOI] [PubMed] [Google Scholar]
- Erkosar B., Storelli G., Defaye A., Leulier F. (2013). Host-intestinal microbiota mutualism: ‘learning on the fly’. Cell Host Microbe 13, 8-14 [DOI] [PubMed] [Google Scholar]
- Faith J. J., McNulty N. P., Rey F. E., Gordon J. I. (2011). Predicting a human gut microbiota's response to diet in gnotobiotic mice. Science 333, 101-104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint H. J., Scott K. P., Louis P., Duncan S. H. (2012). The role of the gut microbiota in nutrition and health. Nature reviews. Gastroenterol. Hepatol. 9, 577-589 [DOI] [PubMed] [Google Scholar]
- Goodman A. L., McNulty N. P., Zhao Y., Leip D., Mitra R. D., Lozupone C. A., Knight R., Gordon J. I. (2009). Identifying genetic determinants needed to establish a human gut symbiont in its habitat. Cell Host Microbe 6, 279-289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon H. A., Pesti L. (1971). The gnotobiotic animal as a tool in the study of host microbial relationships. Bacteriol. Rev. 35, 390-429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg A. J., Hackett S. R., Harshman L. G., Clark A. G. (2011). Environmental and genetic perturbations reveal different networks of metabolic regulation. Mol. Syst. Biol. 7, 563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill M. J. (1997). Intestinal flora and endogenous vitamin synthesis. Eur. J. Cancer Prev. 6 Suppl. 1, S43-S45 [DOI] [PubMed] [Google Scholar]
- Human Microbiome Project Consortium (2012). Structure, function and diversity of the healthy human microbiome. Nature 486, 207-214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jumbo-Lucioni P., Ayroles J. F., Chambers M. M., Jordan K. W., Leips J., Mackay T. F., De Luca M. (2010). Systems genetics analysis of body weight and energy metabolism traits in Drosophila melanogaster. BMC Genomics 11, 297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasov W. H., Douglas A. E. (2013). Comparative digestive physiology. Comprehensive Physiology 3, 741-783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kau A. L., Ahern P. P., Griffin N. W., Goodman A. L., Gordon J. I. (2011). Human nutrition, the gut microbiome and the immune system. Nature 474, 327-336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc J. G., Milani C., de Giori G. S., Sesma F., van Sinderen D., Ventura M. (2013). Bacteria as vitamin suppliers to their host: a gut microbiota perspective. Curr. Opin. Biotechnol. 24, 160-168 [DOI] [PubMed] [Google Scholar]
- Lee K. P., Simpson S. J., Clissold F. J., Brooks R., Ballard J. W. O., Taylor P. W., Soran N., Raubenheimer D. (2008). Lifespan and reproduction in Drosophila: new insights from nutritional geometry. Proc. Natl Acad. USA 105, 2498-2503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lushchak O. V., Gospodaryov D. V., Rovenko B. M., Yurkevych I. S., Perkhulyn N. V., Lushchak V. I. (2014). Specific dietary carbohydrates differentially influence the life span and fecundity of Drosophila melanogaster. J. Gerontol. A 69, 3-12 [DOI] [PubMed] [Google Scholar]
- Markle J. G., Frank D. N., Mortin-Toth S., Robertson C. E., Feazel L. M., Rolle-Kampczyk U., von Bergen M., McCoy K. D., Macpherson A. J., Danska J. S. (2013). Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science 339, 1084-1088 [DOI] [PubMed] [Google Scholar]
- McCormick D. B. (2012). Riboflavin. In Present Knowledge in Nutrition, 10th edn (ed. Erdman J. W., Macdonald I. A., Zeisel S. H.), pp.280-292 Oxford, UK: Wiley-Blackwell; [Google Scholar]
- Musselman L. P., Fink J. L., Narzinski K., Ramachandran P. V., Hathiramani S. S., Cagan R. L., Baranski T. J. (2011). A high-sugar diet produces obesity and insulin resistance in wild-type Drosophila. Dis. Model. Mech. 4, 842-849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na J., Musselman L. P., Pendse J., Baranski T. J., Bodmer R., Ocorr K., Cagan R. (2013). A Drosophila model of high sugar diet-induced cardiomyopathy. PLoS Genet. 9, e1003175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell P.D., Douglas A.E. (2013). Among-species interactions determine the impact of gut microbiota on nutrient allocation in Drosophila melanogaster. Appl. Environ. Microbiol. [Epub ahead of print] doi: 10.1128/AEM.02742-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson J. K., Holmes E., Kinross J., Burcelin R., Gibson G., Jia W., Pettersson S. (2012). Host-gut microbiota metabolic interactions. Science 336, 1262-1267 [DOI] [PubMed] [Google Scholar]
- Parks B. W., Nam E., Org E., Kostem E., Norheim F., Hui S. T., Pan C., Civelek M., Rau C. D., Bennett B. J., et al. (2013). Genetic control of obesity and gut microbiota composition in response to high-fat, high-sucrose diet in mice. Cell Metab. 17, 141-152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper M.D., Blanc E., Leitao-Goncalves R., Yang M., He X., Linford N.J., Hoddinott M.P., Hopfen C., Soultoukis G.A., Niemeyer C., et al. (2013). A holidic medium for Drosophila melanogaster. Nat. Methods. 11, 100-105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers H. J. (2005). Interaction among folate, riboflavin, genotype, and cancer, with reference to colorectal and cervical cancer. J. Nutr. 135 Suppl., 2960S-2966S [DOI] [PubMed] [Google Scholar]
- Prentki M., Matschinsky F. M., Madiraju S. R. (2013). Metabolic signaling in fuel-induced insulin secretion. Cell Metab. 18, 162-185 [DOI] [PubMed] [Google Scholar]
- Ren C., Webster P., Finkel S. E., Tower J. (2007). Increased internal and external bacterial load during Drosophila aging without life-span trade-off. Cell Metab. 6, 144-152 [DOI] [PubMed] [Google Scholar]
- Ridley E. V., Wong A. C., Westmiller S., Douglas A. E. (2012). Impact of the resident microbiota on the nutritional phenotype of Drosophila melanogaster. PLoS ONE 7, e36765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roscoe M. H. (1931). The effects of coprophagy in rats deprived of the vitamin B complex. Biochem. J. 25, 2056-2067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang J. H. (1956). The quantitative nutritional requirements of Drosophila melanogaster. J. Exp. Biol. 33, 45-72 [Google Scholar]
- Sang J. H. (1962). Relationships between protein supplies and B-vitamin requirements, in axenically cultured Drosophila. J. Nutr. 77, 355-368 [DOI] [PubMed] [Google Scholar]
- Scheitz C. J. F., Guo Y., Early A. M., Harshman L. G., Clark A. G. (2013). Heritability and inter-population differences in lipid profiles of Drosophila melanogaster. PLoS ONE 8, e72726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin S. C., Kim S. H., You H., Kim B., Kim A. C., Lee K. A., Yoon J. H., Ryu J. H., Lee W. J. (2011). Drosophila microbiome modulates host developmental and metabolic homeostasis via insulin signaling. Science 334, 670-674 [DOI] [PubMed] [Google Scholar]
- Simpson S. J., Raubenheimer D. (2005). Obesity: the protein leverage hypothesis. Obes. Rev. 6, 133-142 [DOI] [PubMed] [Google Scholar]
- Smith K., McCoy K. D., Macpherson A. J. (2007). Use of axenic animals in studying the adaptation of mammals to their commensal intestinal microbiota. Semin. Immunol. 19, 59-69 [DOI] [PubMed] [Google Scholar]
- Smith M. I., Yatsunenko T., Manary M. J., Trehan I., Mkakosya R., Cheng J., Kau A. L., Rich S. S., Concannon P., Mychaleckyj J. C., et al. (2013). Gut microbiomes of Malawian twin pairs discordant for kwashiorkor. Science 339, 548-554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stecher B., Maier L., Hardt W. D. (2013). ‘Blooming’ in the gut: how dysbiosis might contribute to pathogen evolution. Nat. Rev. Microbiol. 11, 277-284 [DOI] [PubMed] [Google Scholar]
- Storelli G., Defaye A., Erkosar B., Hols P., Royet J., Leulier F. (2011). Lactobacillus plantarum promotes Drosophila systemic growth by modulating hormonal signals through TOR-dependent nutrient sensing. Cell Metab. 14, 403-414 [DOI] [PubMed] [Google Scholar]
- Suomalainen H., Pfaffli S. (1961). Changes in the carbohydrate reserves of baker's yeast during growth and on standing. J. Inst. Brew. 67, 249-254 [Google Scholar]
- Turnbaugh P.J., Ridaura V.K., Faith J.J., Rey F.E., Knight R., Gordon J.I. (2009). The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci. Transl. Med. 1, 6ra14*** [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijay-Kumar M., Aitken J. D., Carvalho F. A., Cullender T. C., Mwangi S., Srinivasan S., Sitaraman S. V., Knight R., Ley R. E., Gewirtz A. T. (2010). Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science 328, 228-231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong C. N., Ng P., Douglas A. E. (2011). Low-diversity bacterial community in the gut of the fruitfly Drosophila melanogaster. Environ. Microbiol. 13, 1889-1900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong A. C., Chaston J. M., Douglas A. E. (2013). The inconstant gut microbiota of Drosophila species revealed by 16S rRNA gene analysis. ISME J. 7, 1922-1932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D., Daugherty S. C., Van Aken S. E., Pai G. H., Watkins K. L., Khouri H., Tallon L. J., Zaborsky J. M., Dunbar H. E., Tran P. L., et al. (2006). Metabolic complementarity and genomics of the dual bacterial symbiosis of sharpshooters. PLoS Biol. 4, e188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi P., Li L. (2012). The germfree murine animal: an important animal model for research on the relationship between gut microbiota and the host. Vet. Microbiol. 157, 1-7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.