Abstract
We hypothesized that standardized systemic drug delivery would improve treatment safety and provide better leukemia control. We therefore developed an intravenous busulfan formulation and combined it with fludarabine instead of cyclophosphamide in preparation for allogeneic stem cell transplantation (alloSCT). We used a Bayesian method to compare the outcomes of 67 acute myeloid leukemia (AML)/myelodysplastic syndrome (MDS) patients who received intravenous busulfan–cyclophosphamide (BuCy2) with 148 subsequent AML/MDS patients who received busulfan–fludarabine (Bu-Flu). The groups had comparable pretreatment characteristics, except that the Bu-Flu patients were older, more often had unrelated donors and had a shorter follow-up. A greatly improved outcome in the Bu-Flu group is unlikely to be explained by improved supportive care during this time period. Overall, the data support replacing BuCy2 with or without antithymocyte globulin (ATG) with Bu-Flu with or without rabbit-ATG for AML or MDS. We further suggest that the high level of safety and fast recovery from conditioning therapy-related side effects after the Bu-Flu regimen makes it a suitable platform technology for testing additional adjuncts for improved posttransplant immune recovery and long-term disease control in patients who are at high risk of rapidly recurrent disease after alloSCT. The extremely low one-year treatment-related mortality as well as high overall and event-free survival of patients in the Bu-Flu group indicate that it is time to revisit the value of alloSCT compared with conventional maintenance chemotherapy for patients in first complete remission of AML/MDS.
Keywords: acute myeloid leukemia, allogeneic stem cell transplantation, cyclophosphamide, fludarabine, intravenous busulfan, myelodysplastic syndrome, reduced-toxicity conditioning therapy
Introduction
The ultimate long-term control of many hematological malignancies resides with allogeneic stem cell transplantation (alloSCT), commonly after myeloablative high-dose chemo- or chemoradiotherapy. Unfortunately, myeloablative conditioning therapy carries with it a risk of serious adverse effects, including treatment-related mortality, occurring in approximately 20–30% of patients already in the first 100 days posttransplant. The treatment-related mortality risk increases significantly with the use of alternative donors. In addition, the inclusion of total body irradiation (TBI) in the conditioning therapy is fraught with serious long-term complications, most notably stunted growth and retarded intellectual development in children, as well as endocrine abnormalities and secondary malignant tumors in both children and adults [1-4]. As an alternative to TBI-based conditioning treatment, oral busulfan–cyclophosphamide (BuCy) was introduced [5,6]. The follow-up of several randomized studies comparing oral BuCy with TBI–cyclophosphamide and TBI–etoposide revealed no significant differences in either short-term safety or long-term overall survival and disease-free survival (DFS) [7-9].
We hypothesized that standardized, uniform, systemic drug delivery would improve treatment outcome by both improved treatment safety and better tumor control, leading to improved overall survival and DFS.
To allow for controlled drug delivery, we designed an intravenous busulfan formulation that by definition allowed for controlled delivery and complete dose assurance [10]. When using intravenous busulfan with cyclophosphamide as conditioning therapy for chronic myeloid leukemia patients undergoing alloSCT, we observed first that the 100-day treatment-related mortality risk was reduced from an expected 20–30% to less than 5% [11]. Second, we confirmed a therapeutic interval for intravenous busulfan in this combination [12]. Third, there is probably a serious clinical interaction between busulfan and activated cyclophosphamide in the liver [13,14]. Both busulfan and cyclophosphamide have glutathione conjugation as their most important detoxification step. It is plausible that high-dose busulfan consumes most of the available hepatic glutathione, and when cyclophosphamide is administered in close time proximity it results in the serious liver toxicity, sinusoidal obstruction syndrome. To decrease the risk of glutathione depletion and sinusoidal obstruction syndrome from the conditioning regimen, we replaced cyclophosphamide as a conditioning partner to busulfan with the nucleoside analog fludarabine, which is metabolized via glutathione-independent pathways. Fludarabine is at least as immunosuppressive as cyclophosphamide [15], and it is likely to potentiate bifunctional DNA-alkylating agent cytotoxicity if sequenced optimally [16]. The long half-life of fludarabine also recommends it to once-daily dosing, something that is possible to extend to busulfan with its intravenous formulation. This is both convenient and cost-saving. Here we describe our experience with the novel once daily intravenous busulfan-fludarabine (Bu-Flu) regimen in relation to our earlier data with the BuCy2 regimen in patients undergoing alloSCT for acute myeloid leukemia (AML) or myelodysplastic syndrome (MDS).
Patients and methods
The once-daily intravenous Bu-Flu conditioning programme used busulfan at 130 mg/m2 given over 3 h once a day for 4 days, each dose preceded by intravenous fludarabine at 40 mg/m2 over 1 h. Patients with a one antigen-mismatched related donor or an unrelated donor received rabbit-antithymocyte globulin (ATG) on days −3 to −1; the total dose was 4 mg/kg. There were 148 Bu-Flu patients with a median age of 46 years (19–66 years). Seventy-eight (53%) had a related donor, whereas 70 (47%) had a matched unrelated donor. The BuCy2 comparison group was younger (median of 39 years, 13–64, P < 0.01), and had a higher fraction of related donors (53 of 67, 79%, P < 0.0003). This cohort received the every 6 h BuCy2 regimen as described by Tutschka et al. [6], modified by Andersson et al. [17]. The BuCy2 patients who had a one antigen-mismatched related donor or a matched unrelated donor received equine ATG (at a dose of 20 mg/kg on days −3 to −1 for a total dose of 60 mg/kg). The eligibility criteria included AML, all clinical stages and of intermediate or high-risk cytogenetic classes, and MDS with high-risk criteria or failing induction chemotherapy, or both, and normal organ function testing within acceptable limits [18,19]. Other characteristics, such as the fraction of patients in remission at the time of stem cell transplant (SCT), and cytogenetic risk groups were similar between these busulfan-based treatment groups.
As the busulfan-based programmes were executed consecutively we could not determine whether the observed improvement in outcome was related partly or completely to improved supportive care during the time period 1997–2005. We therefore introduced a group of 78 AML/MDS patients who were conditioned with the melphalan–fludarabine (MF) regimen [20]. The MF regimen was unchanged between 1997 and 2004, spanning the changeover timepoint from intravenous BuCy2 to intravenous Bu-Flu, and was used to provide an estimate of the ‘time–period effect’. We divided this MF group into two groups spanning the time 1997 to 18 April 2001 (MF-A, 50 patients) and a group treated after 18 April 2001 to the end of 2004 (MF-B, 28 patients), generally covering the time periods for using BuCy2 and Bu-Flu, respectively. The ages and disease characteristics of these two MF cohorts were similar, except that the latter MF-B group had a higher fraction of patients in complete remission at the time of SCT (MF-A, six out of 50, 12% versus MF-B, 10 out of 28, 36% in complete remission at SCT, P < 0.02).
Results and discussion
The Bu-Flu patients had a 3-year DFS of 75% if transplanted in (any) complete response compared with approximately 45% for the BuCy2 group. The 100-day and one-year treatment-related mortality rates were 2% and 6%, respectively, for the Bu-Flu group, compared with approximately 7% and 21%, respectively, for the BuCy2 group. The Bu-Flu results appear better than those obtained with BuCy2. There are, however, inherent problems in this comparison because the regimens were not assigned in a randomized manner but were administered sequentially during consecutive studies over an 8-year period [19]. Therefore, evolving supportive care routines may favor the more recently used programme. Furthermore, different patient populations were included in the trials; the Bu-Flu patients were older (median of 46 versus 39 years, P < 0.01) and had a higher fraction of alternative (matched unrelated donor) donors (39% versus 15%, P < 0.0003).
Overall, our comparison included 215 AML/MDS patients who received alloSCT after intravenous busulfan-based conditioning therapy. From August 1996 until April 2001, 67 individuals received BuCy2, and from April 2001 until August 2005, a total of 148 patients received Bu-Flu. As mentioned, these programmes were executed consecutively. Consequently, the possibility that improved supportive care accounted for the improved outcome could not be directly excluded. The group of 78 AML/MDS patients who were conditioned with the MF regimen [20], spanning the changeover timepoint from intravenous BuCy2 to intravenous Bu-Flu, was introduced to provide an estimate of the ‘time–period effect’, i.e. the total effect of changes in supportive care, changes in referral pattern, etc. that may affect outcome over time in addition to already well-recognized prognostic factors such as cytogenetic risk pattern and remission status at the time of alloSCT. The outcome of the 50 patients in the MF-A group was thus compared with the 28 patients in the MF-B group who were treated after the 18 April 2001 timepoint. The characteristics of the MF subgroups were comparable, except that the MF-B group had a higher fraction in complete remission at the time of alloSCT (P < 0.02). We assumed that any change in referral pattern or significant improvements in supportive care that would be detected in the MF groups were similarly applicable to the BuCy2 and Bu-Flu populations treated during the corresponding time periods.
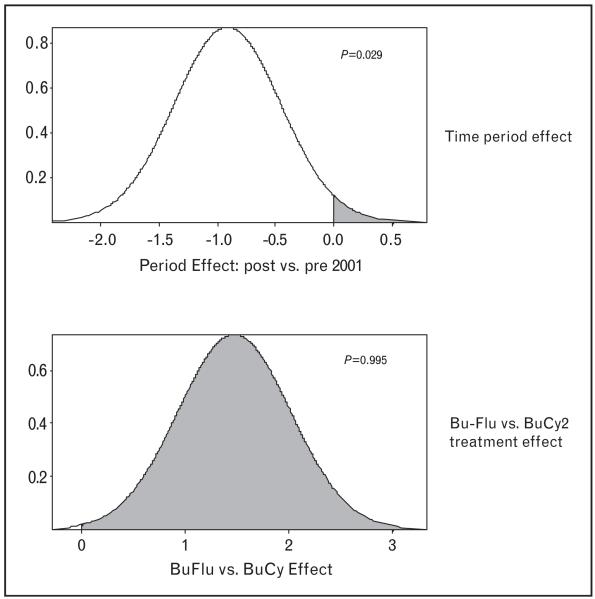
A comparative analysis of the 215 intravenous busulfan patients revealed that the only significant imbalances were that the Bu-Flu patients were on average 7 years older than the BuCy2 patients, and that they had a lower fraction of matched related donors (53% versus 79%). Whereas one patient in the Bu-Flu group failed to engraft, there was no engraftment failure in the BuCy2 group (0/67 versus 1/148, P = n.s.). The Bu-Flu cohort had a shorter median follow-up, 30 months versus 70 months for the BuCy2 group. The Kaplan–Meier estimates for overall survival in these two groups indicate that the Bu-Flu patients survived significantly longer, but had a shorter follow-up time. A Bayesian log normal regression model for overall survival fit indicated that the longer overall survival was significantly associated with having a matched related donor, younger age, the absence of circulating myeloblasts and a higher platelet count at the time of alloSCT. An absence of poor prognosis cytogenetics and being in complete remission at the start of conditioning therapy were associated with longer overall survival, but did not reach statistical significance. After accounting for covariate effects, the Bu-Flu regimen was associated with significantly lower treatment-related mortality at 100 days and at one year after alloSCT, and with significantly longer overall survival when compared with BuCy2. This was, however, confounded by the fact that we were dealing with sequential, non-randomized studies. We separately addressed this time–period effect by introducing the comparator group of 78 patients transplanted after the MF regimen between 1997 and 2004. The 28 MF-B patients who were treated after 18 April 2001 (when BuCy2 was replaced by Bu-Flu), had significantly higher probabilities of being in complete remission and of having no circulating blasts. Therefore, they had a more favorable prognosis than the 50 MF-A patients treated before 18 April 2001. Fifty-four of the 78 MF patients (69%) died, yielding a median overall survival of 8 months (95% confidence interval 5.4-22). A beneficial outcome after alloSCT using the MF regimen was significantly less likely in the MF-B group, treated after 18 April 2001. This indicated a negative time–period effect (Fig. 1). We fit a Bayesian lognormal regression model for overall survival fit to the combined total dataset of 293 patients. This fit accounted for the time–period effect, and the fludarabine versus cyclophosphamide effect within intravenous busulfan patients, as well as two treatment-covariate interactions. In particular, the ‘Bu-Flu versus BuCy2’ effect is the combined effect of the confounded variables (intravenous Bu-Flu and enrolled after 18 April 2001) versus (intravenous BuCy2 and enrolled before 18 April 2001). This fitted model indicated that, in terms of the effects on overall survival, all covariate effects were significant. After accounting for covariate effects, the time period effect showed a significant benefit to being treated before 18 April 2001 (P = 0.971). After accounting for the effects of all covariates and also for the time period, there was a large, significant benefit to being treated with intravenous Bu-Flu compared with being treated with BuCy2 (P = 0.995; Fig. 1). Finally, we could not discern any statistically significant difference in anti-tumor efficacy when comparing relapse-free survival after Bu-Flu and BuCy2. The encouragingly low treatment-related mortality and high overall survival beyond 3 years after alloSCT in a large fraction of the Bu-Flu patients suggest that it may be time to perform a systematic comparison of alloSCT and conventional maintenance chemotherapy in first complete remissions. A recent matched-pair analysis of patients who received Bu-Flu followed by alloSCT for AML/MDS in first complete emission at M.D. Anderson versus matched pairs who received conventional maintenance therapy demonstrated a more than threefold higher 3-year event-free survival in the Bu-Flu group (de Lima and Kantarjian, unpublished, 2009). This is contrary to the standard treatment recommendations of reserving alloSCT for patients who progress beyond the first complete remission. This standard recommendation is based on the historical use of alloSCT after TBI-based conditioning therapy with a resulting high, 40–50%, treatment-related mortality at one year after transplant, which effectively neutralized most of the allogeneic graft-versus-leukemia effect [21,22].
Figure 1. Regression model for the time-period effect (upper panel) and for the busulfan-fludarabine (Bu-Flu) versus busulfan-cyclophosphamide 2 (BuCy2) effect.
The probability of benefit is represented by the shaded region with the time–period effect being negative; the overall benefit of Bu-Flu is strong in this patient population.
All of the above discussed data were derived with a fixed-dosing algorithm for intravenous busulfan. In reference to pharmacokinetics, we demonstrated that within a dosing interval of 32–150 mg/m2 the elimination of busulfan is linear over time and that systemic exposure therefore increases linearly with graded dose increments. Furthermore, if busulfan dosing is based on pharmacokinetic parameters, incremental increases in systemic exposure corresponded to increasing toxicity levels; in this aspect, and in collaboration with Dr Russell and his group, we demonstrated what appears to be an upper recommendable limit in systemic exposure, represented by the area under the concentration versus time curve (AUC) of approximately 6000 μmol/l ·min daily in the 4-day intravenous Bu-Flu regimen, or a total course AUC of approximately 24 000 μmol/l ·min [23]. Additional data should be obtained and a stringent analysis should be performed to confirm precisely the borders of a therapeutic interval for intravenous busulfan in the 4-day Bu-Flu regimen.
We conclude that the once-daily 4-day intravenous Bu-Flu with or without ATG regimen constitutes a safe reduced-toxicity platform technology that allows systematic testing of a series of hypotheses, including whether leukemia control can be improved by either (1) adding mobilization-stimulating agents such as plerixafor before the conditioning chemotherapy, or (2) replacing fludarabine with later generation nucleoside analog(s) (e.g. clofarabine). Both of these additions will potentially improve cytoreduction and therefore also leukemia control. The value of (3) pharmacokinetic-guided busulfan delivery might likewise enhance anti-tumor effects and safety but limit graft-versus-host disease. The safety experienced with this novel pretransplant conditioning ‘platform’ allows the addition of posttransplant adjuncts such as cyclophosphamide or pentostatin to enhance graft-versus-host disease control and accelerate immune recovery. It will also allow the safe addition of posttransplant ‘maintenance therapy’ with e.g. hypomethylating agents to prevent recurrent leukemia in patients who are deemed at high risk of rapidly recurrent disease.
Acknowledgments
Sponsorship: This work was supported by National Institutes of Health grants 2PO1 CA55164 and 2P30CA16672-26 and the Stephen L. and Lavinia P. Boyd Fund for Leukemia Research.
Footnotes
Conflicts of interest: B.S.A. is consulting for Otsuka Pharmaceuticals America for clinical trial development.
The other authors report no conflicts of interest.
References
- 1.Curtis RE, Rowlings PA, Deeg JH, et al. Solid cancers after bone marrow transplantation. N Engl J Med. 1997;336:897–904. doi: 10.1056/NEJM199703273361301. [DOI] [PubMed] [Google Scholar]
- 2.Socie G, Curtis RE, Deeg JH, et al. New malignant diseases after allogeneic marrow transplantation for childhood acute leukemia. J Clin Oncol. 2000;18:348–357. doi: 10.1200/JCO.2000.18.2.348. [DOI] [PubMed] [Google Scholar]
- 3.Baker SK, DeFor TE, Burns LJ, et al. New malignancies after blood and marrow stem-cell transplantation in children and adults: incidence and risk factors. J Clin Oncol. 2003;21:1352–1358. doi: 10.1200/JCO.2003.05.108. [DOI] [PubMed] [Google Scholar]
- 4.Brown JR, Yeckes H, Friedberg JW, et al. Increasing incidence of late second malignancies after conditioning with cyclophosphamide and total-body irradiation and autologous bone marrow transplantation for non-Hodgkin’s lymphoma. J Clin Oncol. 2005;23:2208–2214. doi: 10.1200/JCO.2005.05.158. [DOI] [PubMed] [Google Scholar]
- 5.Santos GW, Tutschka PJ, Brookmeyer R, et al. Marrow transplantation for acute nonlymphocytic leukemia after treatment with busulfan and cyclophosphamide. N Engl J Med. 1983;309:1347–1353. doi: 10.1056/NEJM198312013092202. [DOI] [PubMed] [Google Scholar]
- 6.Tutschka PJ, Copelan EA, Klein JP. Bone marrow transplantation for acute leukemia following a new busulfan and cyclophosphamide regimen. Blood. 1987;70:1382–1388. [PubMed] [Google Scholar]
- 7.Blume KG, Kopecky KJ, Henslee-Downey JP, et al. A prospective randomized comparison of total body irradiation-etoposide versus busulfan-cyclophosphamide as preparatory regimens for bone marrow transplantation in patients with leukemia who were not in first remission: a Southwest Oncology Group study. Blood. 1993;81:2187–2193. [PubMed] [Google Scholar]
- 8.Hartman AR, Williams S, Dillon JJ. Survival, disease-free survival and adverse effects of conditioning for allogeneic bone marrow transplantation with busulfan/cyclophosphamide vs total body irradiation: a meta-analysis. Bone Marrow Transplant. 1998;22:439–443. doi: 10.1038/sj.bmt.1701334. [DOI] [PubMed] [Google Scholar]
- 9.Socie G, Clift RA, Blaise D, et al. Busulfan plus cyclophosphamide compared with total-body irradiation plus cyclophosphamide before marrow transplantation for myeloid leukemia: long-term follow-up of 4 randomized studies. Blood. 2001;98:3569–3574. doi: 10.1182/blood.v98.13.3569. [DOI] [PubMed] [Google Scholar]
- 10.Bhagwatwar HP, Phadungpojna S, Chow DS, Andersson BS. Formulation and stability of busulfan for intravenous administration in high-dose chemotherapy. Cancer Chemother Pharmacol. 1996;37:401–408. doi: 10.1007/s002800050404. [DOI] [PubMed] [Google Scholar]
- 11.Thall PF, Champlin RE, Andersson BS. Comparisson of 100-day mortality rates associated with i.v. busulfan and cyclophosphamide vs other preparative regimens in allogeneic bone marrow transplantation for chronic myelogenous leukemia: Bayesian sensitivity analyses of confounded treatment and center effects. Bone Marrow Transplant. 2004;33:1191–1199. doi: 10.1038/sj.bmt.1704461. [DOI] [PubMed] [Google Scholar]
- 12.Andersson BS, Thall PF, Madden T, et al. Busulfan systemic exposure relative to regimen-related toxicity and acute graft-versus-host disease: defining a therapeutic window for i.v. BuCy2 in chronic myelogenous leukemia. Biol Blood Marrow Transplant. 2002;8:477–485. doi: 10.1053/bbmt.2002.v8.pm12374452. [DOI] [PubMed] [Google Scholar]
- 13.Hassan M, Ljungman P, Ringden O, et al. The effect of busulphan on the pharmacokinetics of cyclophosphamide and its 4-hydroxy metabolite: time interval influence on therapeutic efficacy and therapy-related toxicity. Bone Marrow Transplant. 2000;25:915–924. doi: 10.1038/sj.bmt.1702377. [DOI] [PubMed] [Google Scholar]
- 14.McDonald GB, Slattery JT, Bouvier ME, et al. Cyclophosphamide metabolism, liver toxicity, and mortality following hematopoietic stem cell transplantation. Blood. 2003;101:2043–2048. doi: 10.1182/blood-2002-06-1860. [DOI] [PubMed] [Google Scholar]
- 15.Terenzi A, Aristei C, Aversa F, et al. Efficacy of fludarabine as an immunosupressor for bone marrow transplantation conditioning: preliminary results. Transplant Proc. 1996;28:3101. [PubMed] [Google Scholar]
- 16.Ghandi V, Plunkett W. Cellular and clinical pharmacology of fludarabine. Clin Pharmacokinet. 2002;41:93–103. doi: 10.2165/00003088-200241020-00002. [DOI] [PubMed] [Google Scholar]
- 17.Andersson BS, Kashyap A, Gian V, et al. Conditioning therapy with intravenous busulfan and cyclophosphamide (IV BuCy2) for hematologic malignancies prior to allogeneic stem cell transplantation: a phase II study. Biol Blood Marrow Transplant. 2002;8:145–154. doi: 10.1053/bbmt.2002.v8.pm11939604. [DOI] [PubMed] [Google Scholar]
- 18.De Lima M, Couriel D, Thall PF, et al. Once-daily intravenous busulfan and fludarabine: clinical and pharmacokinetic results of a myeloablative, reduced-toxicity conditioning regimen for allogeneic stem cell transplantation in AML and MDS. Blood. 2004;104:857–864. doi: 10.1182/blood-2004-02-0414. [DOI] [PubMed] [Google Scholar]
- 19.Andersson BS, de Lima M, Thall PF, et al. Once daily IV busulfan and fludarabine compares favorably with IV busulfan and cyclophosphamide (IV BuCy2) as pretransplant conditioning therapy in AML/MDS. Biol Blood Marrow Transplant. 2008;14:672–684. doi: 10.1016/j.bbmt.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giralt S, Thall PF, Khouri I, et al. Melphalan and purine analog-containing preparative regimens: reduced-intensity conditioning for patients with hematologic malignancies undergoing allogeneic progenitor cell transplantation. Blood. 2001;97:631–637. doi: 10.1182/blood.v97.3.631. [DOI] [PubMed] [Google Scholar]
- 21.Burnett AK, Wheatley K, Goldstone AH, et al. The value of allogeneic bone marrow transplant in patients with acute myeloid leukaemia at differing risk of relapse: results of the UKMRC AML 10 trial. BrJHaematol. 2002;118:385–400. doi: 10.1046/j.1365-2141.2002.03724.x. [DOI] [PubMed] [Google Scholar]
- 22.Burnett AK. Evaluating the contribution of allogeneic and autologous transplantation to the management of acute myeloid leukemia in adults. Cancer Chemother Pharmacol. 2001;48(Suppl. 1):S53–S58. doi: 10.1007/s002800100306. [DOI] [PubMed] [Google Scholar]
- 23.Geddes M, Kangarloo BS, Naveed F, et al. High busulfan exposure is associated with worse outcomes in daily i.v. busulfan and fludarabine allogeneic transplant regimen. Biol Blood Marrow Transplant. 2008;14:220–228. doi: 10.1016/j.bbmt.2007.10.028. [DOI] [PubMed] [Google Scholar]