Abstract
Purpose
Both exercise and self-management are advocated in pulmonary rehabilitation for people with chronic obstructive pulmonary disease (COPD). The widely used 6-week, group-based Chronic Disease Self-Management Program (CDSMP) increases self-reported exercise, despite supervised exercise not being a program component. This has been little explored in COPD. Whether adding supervised exercise to the CDSMP would add benefit is unknown. We investigated the CDSMP in COPD, with and without a formal supervised exercise component, to address this question.
Patients and methods
Adult outpatients with COPD were randomized to the CDSMP with or without one hour of weekly supervised exercise over 6 weeks. The primary outcome measure was 6-minute walk test distance (6MWD). Secondary outcomes included self-reported exercise, exercise stage of change, exercise self-efficacy, breathlessness, quality of life, and self-management behaviors. Within- and between-group differences were analyzed on an intention-to-treat basis.
Results
Of 84 subjects recruited, 15 withdrew. 6MWD increased similarly in both groups: CDSMP-plus-exercise (intervention group) by 18.6±46.2 m; CDSMP-alone (control group) by 20.0±46.2 m. There was no significant difference for any secondary outcome.
Conclusion
The CDSMP produced à small statistically significant increase in 6MWD. The addition of a single supervised exercise session did not further increase exercise capacity. Our findings confirm the efficacy of a behaviorally based intervention in COPD, but this would seem to be less than expected from conventional exercise-based pulmonary rehabilitation, raising the question of how, if at all, the small gains observed in this study may be augmented.
Keywords: supervised exercise, physical capacity, 6-minute walk distance
Introduction
Developing self-management skills is now seen as a standard component of pulmonary rehabilitation (PR) programs.1–3 Indeed, PR is considered an integral component of managing chronic obstructive pulmonary disease (COPD), a progressive disabling respiratory and systemic condition.4 Guidelines also recommend that PR should include a detailed assessment, exercise, education, and psychosocial support.1,2
Exercise in PR has demonstrated improvements in physical capacity, health-related quality of life, dyspnea, and fatigue.5 Recommendations have stipulated at least three exercise sessions weekly, two of which are to be preferably supervised.6 However, the supporting evidence for this degree of supervised exercise is limited7 and was subsequently contradicted by others who provided only once-weekly exercise supervision.8–11 These later studies question the conventionally recommended degree of exercise supervision. The frequency of supervised exercise sessions has relevance to our study. As we will explain in the Materials and methods section, our intervention was once-weekly supervised exercise added to a self-management program, which we offered as a combined PR approach.
Condition-specific education in PR has traditionally been delivered in a didactic format and has not demonstrated additional benefit in terms of physical or exercise capacity.2,12–14 Consequently, exercise, not education, has become the cornerstone of PR. However, the benefits of the traditional format for PR, especially on physical capacity and exercise behavior, wane over time.15,16 This has led to an emphasis on behavioral strategies in PR as a potential means of maintaining the acute gains,3,16 but this is little tested.
Indeed, self-management interventions, underpinned by health psychology principles, have been seen as an alternative to the conventional PR approach. Such programs are seen particularly as strategies for improving health behaviors, such as regular exercise, and integrating them into daily life. Chronic disease self-management is defined as a process that facilitates an individual’s confidence and capability to engage in health-promoting behaviors in order to deal with the impact of their condition on all aspects of their health – namely, a sense of self, physical, emotional, social, and medical domains so as to maximize function and quality of life.17,18 Self-management education or training is recognized as needing to be interactive, to facilitate not only the acquisition of health behavior knowledge but its implementation, by fostering the self-management skills of collaborative goal-setting with associated action plans, problem solving, and decision-making.13 However, with the exception of increased uptake of a symptom-based action plan to manage COPD exacerbations (ie, self-management of symptoms),19 and some decrease in hospitalization rates,20 the evidence for the efficacy of holistic COPD-specific self-management approaches, while popular and continuing to increase in practice, has been limited.20–25
However, in more general chronic disease situations, statistically sustained significant improvements in health care utilization, health status, and health behaviors such as self-reported exercise have been reported for participants who attended the Stanford Chronic Disease Self-Management Program (CDSMP).26 The CDSMP, either provided at a medical center or community-based, is a generic, 6-week, group-based self-management education approach led by two trained leaders.26 Such benefits were subsequently supported in a review of self-management approaches27 and have been widely implemented, as indeed is the case in our institution. The CDSMP includes educational information delivered in a lecturette style and supplemented by a companion book. The topics cover generic health information, the basis of which is comparable to that relating to health behaviors in conventional PR (Table 1). Some condition-specific information is included in the companion book. The interactive format of the CDSMP deliberately fosters self-efficacy (“confidence”) to manage one’s health condition through mastery (practicing skills through setting action plans), vicarious experiences (role modelling and peer support), social persuasion (encouragement via guided feedback), and reinterpretation of symptoms (exploring different explanations of symptoms).28 Unlike PR, the CDSMP is not designed to include supervised exercise, yet has been reported to increase self-reported exercise26,29 and decrease dyspnea.29 Any added benefit of formally adding supervised exercise to the CDSMP has not been reported, especially in COPD.
Table 1.
Comparison of program content
| CDSMP program content | PR program content (Team member delivering the lecture) |
|---|---|
| Condition-specific information in the companion book | Heart and lungs: structure and function in relation to chronic heart and lung conditions (physiotherapist) |
| Symptom management: shortness of breath, breathing exercises | Monitoring and responding to symptoms: relaxation, breathing exercises, managing breathlessness |
| Muscle relaxation | |
| Endurance exercise (discussion) | Beginning an exercise program (physiotherapist) |
| Cognitive symptom management | |
| Symptom management: anger, fear, frustration, depression, fatigue, pain | Living with heart and lung conditions: emotional and social impact, communication (social worker) |
| Communication skills | |
| Advance directives for health care | |
| Working with and informing the health care team | |
| Medication usage: generic advice, specific information in companion book | Medications and delivery devices: condition-specific (pharmacist) |
| Healthy eating | Nutrition (dietician) |
| Specific suggestions in the companion book | Activity modification (occupational therapist) |
| How to set action plans and problem solve | Not formally addressed |
Notes: 1) The CDSMP course content is presented in comparison with PR and not as the content of the six individual CDSMP sessions. PR is shown as presented in the six sessions with the presenting health professional at our center. 2) Supervised exercise preceded the educational component of PR. The CDSMP has no supervised exercise component.
Abbreviations: CDSMP, Chronic Disease Self-Management Program; PR, pulmonary rehabilitation.
Thus, with an increasing worldwide focus on self-management of chronic conditions, the opinion of hospital management at our institution was that the rehabilitation needs of the target population might be most effectively met through the CDSMP rather than conventional PR. A small pilot of the CDSMP for people with COPD compared with our traditional PR30 found that the CDSMP alone improved physical capacity by 30 m, measured by the 6-minute walk distance (6MWD).31 However, with supervised exercise considered an essential component of PR and the benefits of such exercise for people with COPD established,5 we felt that it was incumbent on us to investigate more fully the likely benefits of the CDSMP approach on exercise capacity, specifically in COPD patients, as well as on more subjective endpoints in this context. We wished to establish whether supervised exercise in addition to the CDSMP would have added benefit compared with the CDSMP alone. This would be a step to informing us of whether the CDSMP with supervised exercise, or without, might offer an alternative to more traditional PR.
Materials and methods
Study design
This was a parallel group, randomized clinical trial aiming to investigate both the efficacy of the CDSMP itself in COPD and, more particularly, the addition of supervised exercise to the CDSMP on physical capacity measured by the 6MWD. The trial was registered with the Australian and New Zealand Clinical Trials Registry (ACTRN12610000781044).
In our pilot study already mentioned, which had included a similar population of people with COPD, we found the mean baseline 6MWD to be 365 m, with a standard deviation of 74 m.30 The acknowledged minimal clinical important difference (MCID) at that time was reported as 54 m.32 To achieve this difference in exercise capacity, for power of 0.8 and level of significance of 0.05, using a two-sided t-test for our primary outcome of 6MWD, we calculated that the current study would require 31 participants in each arm to allow demonstration of superiority for the active intervention by this amount. Allowing for the 25% dropout rate seen in the pilot, we estimated a total of 78 participants needed to be recruited, with 1:1 randomization to the exercise intervention arm of the CDSMP plus a single supervised exercise session versus the CDSMP alone.
Randomization used a random numbers table with allocation stored in opaque sealed envelopes until completion of baseline data measurement. Participants were assigned a depersonalized identification number, to provide blinding during data analyses. However, a double-blind clinical trial was not possible, as there was no appropriate dummy for the exercise component, and the whole population attended the CDSMP together.
Study subjects
Participants were recruited from patients referred by clinicians to PR at the Royal Hobart Hospital, a tertiary, university-affiliated, public hospital. Participants gave written informed consent. Ethical approval was granted by the Tasmanian Human Research Ethics Committee (H0008105).
Referring staff were aware that our rehabilitation service was intending to trial the addition of formal exercise to the CDSMP. Inclusion criteria included being over age 18 years, agreeable to attend supervised exercise as well as the CDSMP as randomized, a firm diagnosis of COPD, and there being at least 2 months since an acute exacerbation. Exclusion criteria were cognitive impairment, inability to provide informed consent or complete a self-administered questionnaire, previous CDSMP or PR attendance within the past 2 years, and standard contraindications to exercise.33
Detail of interventions
The intervention group underwent 6 weeks of a 1-hour, weekly, supervised group exercise session of aerobic and strengthening exercises for upper and lower limbs, individualized for each participant, in the same week as the 6-week CDSMP. We were able to offer only this 1-hour supervised exercise session per week due to operational constraints. However, this approach has been supported by others.8,11 Participants were offered a choice of attending the supervised exercise session either in the morning prior to the CDSMP or later in the week. The first exercise session took place in the week of the first CDSMP session. In collaboration with the physiotherapist–investigator, an individualized exercise regime was determined with each participant. A physiotherapy assistant trained in exercise supervision and not otherwise involved in the study supervised the actual exercise sessions, with the physiotherapist available for consultation if needed. Exercise intensity in the intervention group was determined by using the modified Borg Rating of Perceived Exertion (RPE) Scale (0–10; 10= maximum).34 A minimum of moderate intensity (RPE =3) exercise was aimed for, according to recommended exercise guidelines,35 and a maximum intensity of strong (RPE =5).
As it was not possible for us to offer separate CDSMP sessions for intervention and control participants, they attended the CDSMP together, each receiving the same encouragement and information about following a home exercise regime. To avoid contamination, the intervention group was requested not to discuss experience of supervised exercise with controls. Controls, receiving the CDSMP alone, were offered supervised exercise on completion of their study participation.
The participants attended the 6-week group-based CDSMP, with sessions of 2.5 hours duration offered once per week, facilitated by the respiratory nurse and the physiotherapist–investigator who had both undertaken leader training. The sessions were interactive with lecturettes, problem solving, brain storming, action planning, and reporting-back activities. There were up to 12 participants per cohort. A comparison of the CDSMP lecturette topics with those previously delivered in PR education is shown in Table 1.
Outcome measures and data analyses
Outcomes were assessed in the week prior to and the week following completion of the interventions. It was deemed more ethical to first determine whether or not a significant and clinically meaningful change resulted for the intervention in the short-term before extending the study to longer-term follow-up.
The primary outcome was physical capacity measured by the 6MWD31 and was assessed by an assistant not connected with the trial, affording some degree of objectivity at that point. Secondary outcomes, more directed to the likely efficacy outcomes of the CDSMP, are detailed in Table 2.31,35–41 We selected the Short-Form 36 Questionnaire, version 2 (SF-36) as the quality-of-life measure to account for the impact of comorbidities. It has been deemed as responsive as the COPD-specific Saint George’s Respiratory Questionnaire42 at detecting even small changes in people with COPD.43
Table 2.
Outcomes and measures
| Primary outcome and measure | |
|---|---|
| Physical capacity, measured by the 6MWD, a field walking test31 | |
| Secondary outcomes and measures | |
| Outcome | Measure |
| Self-reported exercise | CHAMPS Activities Questionnaire for Older Adults39* |
| Self-efficacy for exercise | Exercise: Self-Efficacy measure37* |
| Exercise participation criteria (achieving recommended level of daily exercise)minimum | Achieving weekly exercise35,36 “Regular Exercise is any planned physical activity (eg, brisk walking, aerobics, bicycling, swimming, line-dancing, tennis, doing formal exercises etc.) performed to increase or maintain health and physical fitness. Such exercise should be performed on all or at least 5 days of the week to accumulate 30 minutes or more per day. Exercise does not have to be painful to be effective but should be done at a moderate level that increases your breathing rate and makes you feel warmer Do you exercise regularly according to the definition above? Yes/No” |
| Stage of change for exercise | Exercise: Stages of Change – Short Form questionnaire36* |
| SOB | 10 cm VAS38 |
| Self-management behaviors | Flinders University PIH Scale40* |
| HRQoL | SF-36v2 Generic Health Survey41* |
Note:
Permission obtained for instrument use.
Abbreviations: 6MWD, 6-minute walk test distance; CHAMPS, Community Healthy Activities Model Program for Seniors; HRQoL, health-related quality of life; PIH, Partners in Health; SF-36v2, Short Form 36 version 2; SOB, shortness of breath; VAS, visual analog scale.
Data were analyzed on an intention-to-treat basis, using the statistical software package SPSS (SPSS, Chicago, IL, USA), version 15. The data analyst was blinded to participant allocation until all analyses were completed. Missing variables were replaced by carrying forward the last item measured, and for missing cases, the baseline data was carried forward to the post-data. The exception was for the CHAMPS (Community Healthy Activities Model Program for Seniors) self-report of physical activity, whereby all missing data were scored as zero.39
Results are reported as medians with ranges or means with standard deviations, depending on distribution of data points. Differences in outcomes were compared using Student’s t-tests for parametric data and Mann–Whitney U tests for nonparametric data. Differences in proportions were tested using chi-squared tests. Significance for the primary outcome was set at a P-value <0.05. Due to the large number of secondary outcomes, significance levels for these were calculated using a highly conservative Bonferroni correction (P<0.003).44
Results
Participants
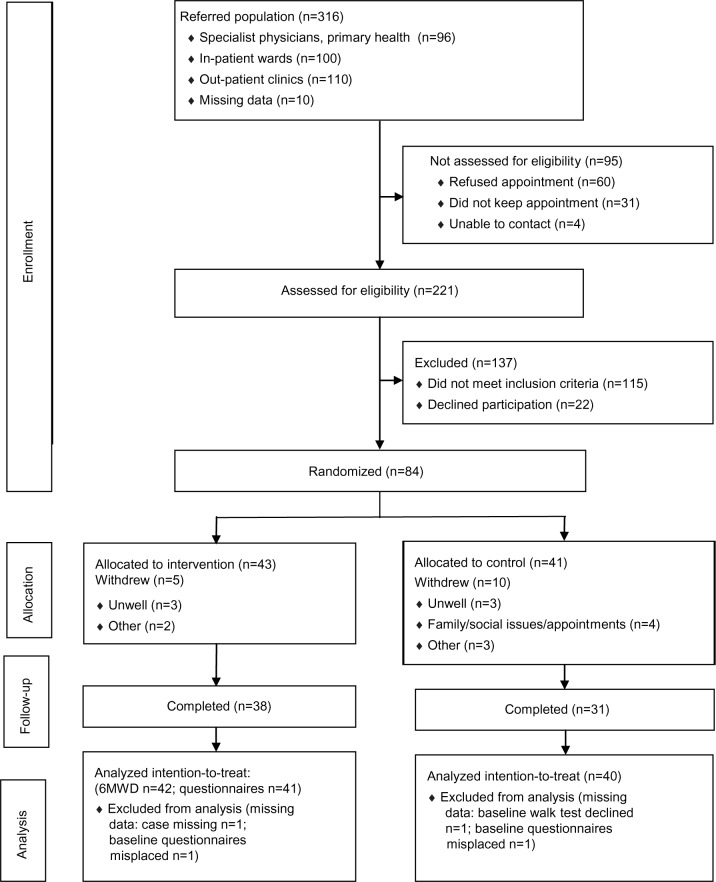
There were 316 potential participants referred for PR (Figure 1), with similar proportions from the private sector (30%), public inpatient service (32%), and public outpatient clinics (35%). The referral source was not recorded in 3%. Overall, attendance at screening was 70% (n=221). Of those not screened, the majority declined an appointment, while many others failed to keep their appointment (Figure 1). Those referred from the hospital wards were least likely, whereas those referred from the private sector were most likely, to attend a screening appointment (P<0.001). Of those deemed eligible at screening visit, 21% refused further involvement. Participants attended a median of five sessions of the CDSMP, and where appropriate, of the supervised exercise sessions.
Figure 1.
Flowchart showing participant’s progress through the study.
Abbreviation: 6MWD, 6-minute walk test distance.
There were 15 withdrawals (five intervention and ten controls) due to illness, family issues, or medical appointments. Data were missing for four participants: one could not cooperate, feeling “overwhelmed” by the process and subsequently withdrew permission for their data to be used; one could not complete the baseline walking test due to increasing breathlessness, and for two, baseline questionnaires were misplaced.
Participant characteristics are depicted in Table 3. The groups did not differ significantly in any baseline demographic measure. Outcome variables at baseline were similar, with no statistically significant differences between groups (Table 4). The 15 withdrawals had no baseline differences compared with completers, defined as those attending at least one CDSMP session and both data collections.
Table 3.
Participant characteristics
| Variable | Participants (n=84) |
I: CDSMP + exercise (n=43) |
C: CDSMP-only (n=41) |
P-value I versus C |
|---|---|---|---|---|
| Female | 39 (46%) | 20 (47%) | 19 (46%) | 0.99 |
| Age, years | 65.8±9.35 | 64.5±9.13 | 67.1±9.41 | 0.19 |
| Married | 50 (60%) | 25 (58%) | 25 (61%) | 0.97 |
| Education: to year 8 | 21 (26%) | 12 (29%) | 9 (23%) | 0.53 |
| to year 10 | 37 (45%) | 20 (48%) | 17 (43%) | |
| to year 12 | 24 (29%) | 10 (24%) | 14 (35%) | |
| Self-reported comorbidities: present | 22 (26%) | 9 (21%) | 13 (33%) | 0.35 |
| Referral source: primary health or private practice | 37 (44%) | 18 (42%) | 19 (46%) | 0.80 |
| Public hospital wards | 19 (23%) | 11 (26%) | 8 (20%) | |
| Public outpatient clinics | 28 (33%) | 14 (32%) | 14 (34%) | |
| Socioeconomic status: below State median | 43 (54%) | 21 (53%) | 22 (55%) | 1.00 |
| Body mass index, kg/m2 | 29.0±7.07 | 28.4±7.63 | 29.7±6.50 | 0.44 |
| COPD severity: | ||||
| Mild (60%–80%) | 20 (29%) | 8 (22%) | 12 (38%) | 0.23 |
| Moderate (40%–59%) | 16 (23%) | 11 (30%) | 5 (15.6%) | |
| Severe (<40%) | 33 (48%) | 18 (49%) | 15 (47%) |
Notes: Data are reported as either raw number (percent) within study group status and as means ± standard deviations. The P-values are from Student’s t-tests or chi-squared analyses. Level of significance was set at P<0.05. COPD severity classified according to COPD-X Plan Australian and New Zealand management guidelines for COPD.50
Abbreviations: C, control; CDSMP, Chronic Disease Self-Management Program; COPD, chronic obstructive pulmonary disease; I, intervention.
Table 4.
Intervention versus control group: baseline outcome variables
| Variable | Intervention (CDSMP + exercise) | Control (CDSMP-only) | P-value |
|---|---|---|---|
| Primary outcome | (n=42) | (n=40) | |
| 6MWD, m | 351.6±122.9 | 353.0±97.4 | 0.953 |
| Secondary outcomes | (n=41) | (n=40) | |
| All exercise: duration, hours per week | 5.250 (0.00–26.00) | 9.250 (0.00–52.25) | 0.011 |
| All exercise: frequency, times per week | 9.0 (0.0–49.0) | 13.5 (0.0–59.0) | 0.126 |
| Moderate exercise: duration, hours | 0.500 (0.00–19.50) | 1.500 (0.00–10.25) | 0.171 |
| Moderate exercise: frequency, times per week | 2.0 (0.0–20.0) | 2.0 (0.0–23.0) | 0.491 |
| Exercise self-efficacy, scale 0–5 | 2.7±1.1 | 2.8±1.0 | 0.763 |
| Self-management behaviors, scale 0–8 | 6.1±1.1 | 6.2±1.0 | 0.492 |
| Shortness of breath: severity, cm | 7.1±2.3 | 6.8±2.4 | 0.592 |
| Shortness of breath: frequency, cm | 6.9±2.6 | 6.4±2.6 | 0.330 |
| SF-36v2 Physical Function | 29.26±8.99 | 28.99±8.04 | 0.886 |
| SF-36v2 Role Physical | 31.95±10.37 | 32.12±9.42 | 0.938 |
| SF-36v2 Bodily Pain | 45.64±11.68 | 45.09±11.49 | 0.832 |
| SF-36v2 General Health | 30.30±9.88 | 32.41±8.96 | 0.317 |
| SF-36v2 Vitality | 41.74±9.19 | 40.77±9.69 | 0.648 |
| SF-36v2 Social Function | 42.75±13.19 | 39.94±11.37 | 0.309 |
| SF-36v2 Role Emotional | 36.25±16.10 | 37.22±15.28 | 0.782 |
| SF-36v2 Mental Health | 47.67±11.66 | 46.21±10.68 | 0.557 |
| SF-36v2 Physical Component Summary | 31.53±8.19 | 31.97±7.247 | 0.796 |
| SF-36v2 Mental Health Component Summary | 46.74±12.85 | 45.53±12.17 | 0.664 |
| Achieving exercise criteria: Yes | 11 (26.8%) | 6 (15.0%) | 0.301 |
| No | 30 (73.2%) | 34 (85.0%) | |
| Stage of change for exercise: Precontemplation | 4 (9.8%) | 6 (15.0%) | 0.789 |
| Contemplation | 14 (34.1%) | 17 (42.5%) | |
| Preparation | 9 (22.0%) | 7 (17.5%) | |
| Action | 6 (14.6%) | 5 (12.5%) | |
| Maintenance | 8 (19.5%) | 5 (12.5%) |
Notes: Data are reported as either raw number (percent) within study group, as mean ± standard deviation, or as median with range. The P-values are from Student’s t-tests, Mann–Whitney U tests or chi-squared analyses, with level of significance P<0.05 for 6MWT and P<0.003 for secondary outcomes, following a Bonferroni correction.
Abbreviations: 6MWD, 6-minute walk test distance; CDSMP, Chronic Disease Self-Management Program; SF-36v2, Short Form 36 version 2.
Primary outcome
There were statistically significant increases in 6MWD in both groups, of around 20 m on average (Table 5). However, there was no statistically significant difference between the groups. The number of participants in each group who reached the MCID of 54 m was similar, 22% in the CDSMP-plus-exercise group and 23% in the CDSMP-only group.
Table 5.
Change in outcomes: CDSMP + exercise versus CDSMP-only
| Variable | CDSMP + exercise (intervention)
|
CDSMP-only (control)
|
Change
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | Post | P-value | Baseline | Post | P-value | CDSMP + exercise | CDSMP-only | P-value | |
| 6MWD, m | 351.6±122.9 | 370.2±128.2 | 0.013 | 353.0±97.4 | 373.0±97.7 | 0.017 | 18.6±46.2 | 20.0±50.6 | 0.90 |
| Moderate exercise duration, hours (pw) | 0.50 (0.0–19.5) | 1.75 (0.0–30.8) | 0.002 | 1.50 (0.0–10.3) | 1.38 (0.0–15.8) | 0.350 | 1.00 (−5.8–14.0) | 0.000 (−9.8–10.3) | 0.230 |
| Moderate exercise frequency, times (pw) | 2.0 (0.0–20.0) | 3.0 (0.0–22.0) | 0.007 | 2.0 (0.0–23.0) | 3.0 (0.0–22.0) | 0.290 | 1.0 (−8.0–11.0) | 0.5 (−23.0–16.0) | 0.766 |
| Exercise self-efficacy, scale 0–5 | 2.7±1.1 | 2.9±1.1 | 0.354 | 2.8±1.0 | 3.0±1.0 | 0.290 | 0.2±1.1 | 0.2±1.1 | 0.892 |
| Self-management behaviors, scale 0–8 | 6.1±1.1 | 6.3±0.8 | 0.037 | 6.2±1.0 | 6.4±1.0 | 0.076 | 0.3±0.8 | 0.2±0.7 | 0.698 |
| Shortness of breath severity, cm | 7.1±2.3 | 6.3±2.5 | 0.032 | 6.8±2.4 | 6.8±2.3 | 0.989 | −0.8±2.4 | 0.0±2.3 | 0.118 |
| Stage of change | |||||||||
| Precontemplation | 4 (10%) | 3 (7%) | NS | 6 (15%) | 4 (10%) | NS | NS | NS | NS |
| Contemplation | 14 (34%) | 10 (24%) | 17 (43%) | 13 (33%) | |||||
| Preparation | 9 (22%) | 4 (10%) | 7 (18%) | 6 (15%) | |||||
| Action | 6 (15%) | 15 (36%) | 5 (12%) | 9 (22%) | |||||
| Maintenance | 8 (19%) | 9 (23%) | 5 (12%) | 8 (20.0%) | |||||
| Achieving weekly exercise criteria: Yes SF-36v2 domains below |
11 (27%) | 22 (54%) | 0.066 | 6 (15%) | 18 (45%) | 0.013 | 19 (47%) | 22 (55%) | NS |
| Physical function | 29.3±9.0 | 30.7±9.0 | 0.076 | 29.0±8.0 | 30.4±8.2 | 0.254 | 1.5±5.2 | 1.4±7.8 | 0.963 |
| Role physical | 32.0±10.4 | 34.3±10.3 | 0.033 | 32.1±9.4 | 35.1±9.8 | 0.038 | 2.4±6.9 | 2.9±8.0 | 0.752 |
| Bodily pain | 45.6±11.7 | 47.7±11.0 | 0.206 | 45.1±11.5 | 44.7±11.5 | 0.621 | 2.1±10.3 | −0.6±7.9 | 0.191 |
| General health | 30.3±9.9 | 31.2±8.6 | 0.467 | 32.4±9.0 | 31.8±10.1 | 0.580 | 0.9±8.2 | −0.6±6.8 | 0.361 |
| Vitality | 41.7±9.2 | 43.0±9.0 | 0.382 | 40.8±9.7 | 43.7±8.1 | 0.032 | 1.2±8.8 | 3.0±8.5 | 0.366 |
| Social function | 42.8±13.2 | 43.3±10.9 | 0.777 | 40.0±11.4 | 43.2±11.1 | 0.050 | 0.5±11.9 | 3.8±10.2 | 0.271 |
| Role emotional | 36.3±16.1 | 38.0±15.8 | 0.282 | 37.2±15.3 | 39.1±13.7 | 0.358 | 1.7±10.0 | 2.9±12.5 | 0.956 |
| Mental health | 47.7±11.7 | 47.9±10.0 | 0.849 | 46.2±10.7 | 47.7±10.1 | 0.428 | 0.2±6.4 | 1.3±10.0 | 0.567 |
| Physical component summary | 31.5±8.2 | 33.5±7.3 | 0.026 | 32.0±7.2 | 32.7±8.4 | 0.506 | 2.0±5.6 | 0.7±6.6 | 0.340 |
| Mental component summary | 46.7±12.9 | 47.2±11.3 | 0.707 | 45.5±12.2 | 47.9±10.4 | 0.205 | 0.4±7.1 | 2.9±11.7 | 0.362 |
Notes: Data are reported as either raw number (percentage) within study group status, or as mean ± standard deviation or median with range. The P-values are from Student’s t-tests, Mann–Whitney U-tests or chi-squared analyses, with level of significance P<0.05 for the primary outcome and P<0.003 for the secondary outcomes, following a Bonferroni adjustment. P-values in bold are those which were below or approximated a P-value of 0.05.
Abbreviations: 6MWD, 6-minute walk test distance; CDSMP, Chronic Disease Self-Management Program; NS, not significant as there were insufficient data to report significance; SF-36v2, Short Form 36 version 2; pw, per week.
The associations between the changes in 6MWT distance and the selected variables of age, sex, education, breathlessness, exercise duration, and frequency, SF-36 physical and mental component summaries, exercise self-efficacy, and body mass index were weak; all Pearson’s correlation coefficients were less than 0.3, and none reached statistical significance. Severity and frequency of breathlessness were selected rather than COPD grade, as the latter had missing data. The strongest correlations with the change in 6MWD were frequency of moderate exercise (r=−0.188, P=0.066), and exercise self-efficacy (r=0.140, P=0.132), although neither were statistically significant. When entered in a multivariable linear regression model, the frequency of moderate exercise (β=−0.257, P=0.048) and exercise self-efficacy (β=0.220, P=0.089) explained only 7.9% of the variance in the change in 6MWD.
Secondary outcomes
Both groups increased similarly in the frequency of moderate exercise, achieving 3 days per week, and showed small increases in physical function, “role physical”, and exercise self-efficacy (“confidence” to exercise), but none of these intragroup changes reached statistical significance. There were no statistically significant differences between the groups in these changes for any secondary outcome measure (Table 5). However, only the intervention group had a statistically significant increase, but only of 1 hour per week, in the duration of moderate intensity self-reported exercise (P=0.002).
Discussion
Summary of results
This is the first study to investigate the effect of the CDSMP itself on physical capacity in COPD, and whether limited supervised exercise produces additional benefit. This is important, because current formats of PR with an emphasis on lecture-style education and multiple weekly, health professional-supervised exercise sessions is costly, reaches only up to 50% of those to whom it is offered,3 and has waning effects over time.3 Wider-reaching, more cost-effective and sustainable alternatives are needed.
We found a statistically significant mean increase in 6MWD for both groups, but no extra benefit for the supervised exercise component, apart from some evidence for an increase in the mean duration of moderate self-reported exercise by 1 hour in the intervention group. However, this probably reflected just what participants had received in the intervention itself. There was also an increase in “role physical” of the SF-36 for both groups, although this lost statistical significance following the Bonferroni adjustment.
6MWD
Our study is the first on the CDSMP, when used for COPD self-management, to report in the literature a statistically significant increase in 6MWD for a self-management intervention alone (our control group). All participants in our study received a behaviorally-based educational and self-management skills training intervention (CDSMP), in contrast to other studies where controls have typically been assigned to usual medical care7,8,20,21 or usual activities only.9
However, the mean increase in 6MWD achieved by both groups was small and might be regarded as of borderline clinical significance. Although the direction of change is consistent with an updated systematic review5 and other randomized controlled studies,8,9 it does not approach the previously reported MCID of 54 m (95% confidence interval [CI] 37–71 m).32 On the other hand, our results do approach the more recently reported MCID of 25 m (95% CI 20–61 m),45 and others have also reported an MCID of 26±2 m in people with severe COPD following PR.46 Such small improvement may have particular relevance for severe COPD, and in our study, half of the participants were severely affected in this way. The 20 m change we observed is also within the lower limit of the CI observed by Holland et al.45
Other groups have also tried to reduce the PR frequency of weekly exercise component. These studies are relevant to our research, as we could offer only 1 weekly session of supervised exercise, in contrast to current recommendations.1,3 Thus, Singh et al9 compared twice-daily home-based walking recorded in a log and monitored once-weekly over 4 weeks with usual activities and found a significant increase in 6MWD for the intervention group but not the control group (54.2±26.7 m versus 6.7±10.3 m, P<0.001). Finnerty et al8 compared education, plus once-weekly supervised exercise, plus an unsupervised home exercise program of 5 days per week over 6 weeks with usual care and reported a median increase in 6MWD of 51 m (range 20–81 m) in the intervention group. These studies contrast with the limited earlier evidence7,47 on which the recommendation of at least twice-weekly supervised exercise sessions is based. Furthermore, no significant difference between once weekly or twice weekly exercisers for the incremental shuttle walking test was demonstrated in a recent randomized controlled trial.11 Others included a structured home exercise program to supervised exercise, also finding no additional benefit of two supervised sessions to the incremental shuttle walking test distance.10 These later studies of weekly versus twice-weekly supervised exercise point to uncertainty over the optimal frequency and mode of supervision required to increase physical capacity. They suggest that once-weekly exercise supervision with a structured home exercise program may be as good as more intensive regimes. To an extent, that is what we have tested, with negative results.
Self-reported exercise
In contrast with our study, others have reported a significant increase in self-reported exercise immediately following the CDSMP alone for people with chronic conditions, including COPD.48 One explanation could be that the measure of self-reported exercise we used is more comprehensive than the Stanford measure49 used previously. Thus, our study is the first CDSMP-related study to focus on COPD and the amount of moderate exercise which is required for optimal health benefits.35 Disappointingly, our results indicate that the CDSMP alone is of limited benefit for meeting the minimum recommendations of exercising in the community for 30 minutes on each of at least 5 days per week. Furthermore, the single supervised exercise session did not add anything in this regard either.
Study limitations and implications for future research
While a major strength of our study was its execution in “real world” clinical practice, utilizing existing resources, this also imposed some limitations. Firstly, due to ethical considerations, we were unable to include a second control group who did not receive any rehabilitation-type intervention. Secondly, it would have been informative to have a group in a twice-weekly or three-times-weekly supervised exercise schedule to determine whether this more exacting approach to exercise would add to the effects of the CDSMP. While this more intense exercise has its advocates,6 such an approach is highly resource-intensive; most other similar centers would have been unable to achieve this. Therefore, the effect of adding more than one supervised exercise session to the CDSMP is unknown, or indeed whether there are optimal numbers of weekly exercise sessions. This may be another area worthy of future research. However, even if more sessions are better, resource limitation will always be a major factor for generalizability within many health centers. Thirdly, participants were recruited from referrals to a hospital-based program that may differ from those who might self-refer to community-based CDSMPs. Nevertheless, our study reflects the usual practice for Australian PR, thus enhancing local generalizability. Fourthly, due to resource limitations, we were unable to offer separate sessions for CDSMP-exercise and CDSMP-only groups. While participants were requested not to discuss the exercise experience, vicarious “contamination” of the control group by the active intervention cannot be excluded. Fifthly, the leaders in this study were health professionals rather than peer leaders as is typical for other CDSMPs. Nevertheless, a recently published systematic review concluded that there were few differences between peer-led or health professional-led self-management programs,27 suggesting this was unlikely to be a source of bias. Sixthly, we did not stratify randomization according to COPD severity. Although this did not vary a great deal, it may have yielded information as to a differential effect of the intervention and would be a consideration for future research. Finally, the CDSMP does not include a structured home exercise program, since under the license agreement, we were precluded from doing so.
Conclusion
In conclusion, participants with COPD attending a CDSMP can expect a small increase in their physical capacity, but there seems little point in adding a single supervised exercise session. Either there needs to be a more intensive conventional exercise program as advocated in guidelines, or new ways need to be investigated for successfully fostering adequate amounts of home or community-based exercise which meet current recommendations for optimizing health benefits. Before completely abandoning the CDSMP plus limited supervised exercise approach, we are currently undertaking such a trial using an additional community-based mentoring component.
Acknowledgments
This work was supported in part by the Australian Physiotherapy Association Beryl Haynes Memorial Fund Grant, Australia; the Royal Hobart Hospital Research Foundation Grant, Hobart, TAS, Australia, and a University of Tasmania Scholarship, Hobart, TAS, Australia. The authors would like to thank the Physiotherapy Department, Royal Hobart Hospital, Hobart, TAS, Australia where this research took place. We thank Associate Professor Leigh Blizzard, Menzies Research Institute Tasmania, University of Tasmania for his invaluable assistance with statistical analysis. Finally, we are indebted to the people with COPD who willingly gave of their time and of themselves to participate in our research.
Footnotes
Disclosure
Helen L Cameron-Tucker received financial support for this research from the Australian Physiotherapy Association Beryl Haynes Memorial Fund Grant, Australia; the Royal Hobart Hospital Research Foundation Grant, Hobart, TAS, Australia, and a University of Tasmania Scholarship, Hobart, TAS, Australia. There are no other potential conflicts of interest pertaining to this work.
References
- 1.Bolton CE, Bevan-Smith EF, Blakey JD, et al. British Thoracic Society guideline on pulmonary rehabilitation in adults. Thorax. 2013;68(Suppl 2):ii1–ii30. doi: 10.1136/thoraxjnl-2013-203808. [DOI] [PubMed] [Google Scholar]
- 2.Ries AL, Bauldoff GS, Carlin BW, et al. Pulmonary rehabilitation: joint ACCP/AACVPR evidence-based clinical practice guidelines. Chest. 2007;131(Suppl 5):4S–42S. doi: 10.1378/chest.06-2418. [DOI] [PubMed] [Google Scholar]
- 3.Spruit MA, Singh SJ, Garvey C, et al. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188(8):e13–e64. doi: 10.1164/rccm.201309-1634ST. [DOI] [PubMed] [Google Scholar]
- 4.Global Strategy for the Diagnosis MaPoC, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2013. [Accessed June 21, 2013]. Available from: http://www.goldcopd.org/
- 5.Lacasse Y, Goldstein R, Lasserson TJ, Martin S. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2006;(4):CD003793. doi: 10.1002/14651858.CD003793.pub2. [DOI] [PubMed] [Google Scholar]
- 6.Nici L, Donner C, Wouters E, Zuwallack Z, et al. American Thoracic Society/European Respiratory Society statement on pulmonary rehabilitation. Am J Respir Crit Care Med. 2006;173(12):1390. doi: 10.1164/rccm.200508-1211ST. [DOI] [PubMed] [Google Scholar]
- 7.Ringbaek T, Broendum E, Hemmingsen L, et al. Rehabilitation of patients with chronic obstructive pulmonary disease. Exercise twice a week is not sufficient! Respir Med. 2000;94(2):150–154. doi: 10.1053/rmed.1999.0704. [DOI] [PubMed] [Google Scholar]
- 8.Finnerty J, Keeping I, Bullough I, Jones J. The effectiveness of outpatient pulmonary rehabilitation in chronic lung disease: a randomized controlled trial. Chest. 2001;119(6):1705–1710. doi: 10.1378/chest.119.6.1705. [DOI] [PubMed] [Google Scholar]
- 9.Singh V, Khandelwal DC, Khandelwal R, Abusaria S. Pulmonary rehabilitation in patients with chronic obstructive pulmonary disease. Indian J Chest Dis Allied Sci. 2003;45(1):13–17. [PubMed] [Google Scholar]
- 10.Liddell F, Webber J. Pulmonary rehabilitation for chronic obstructive pulmonary disease: a pilot study evaluating a once-weekly versus twice-weekly supervised programme. Physiotherapy. 2010;96(1):68–74. doi: 10.1016/j.physio.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 11.O’Neill B, McKevitt A, Rafferty S, et al. A Comparison of twice- versus once-weekly supervision during pulmonary rehabilitation in chronic obstructive pulmonary disease. Arch Phys Med Rehabil. 2007;88(2):167–172. doi: 10.1016/j.apmr.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 12.HyunSoo O, WhaSook S. Meta-analysis of the effects of respiratory rehabilitation programmes on exercise capacity in accordance with programme characteristics. J Clin Nurs. 2006;16:3–5. doi: 10.1111/j.1365-2702.2005.01387.x. [DOI] [PubMed] [Google Scholar]
- 13.Blackstock F, Webster K. Disease-specific health education for COPD: a systematic review of changes in health outcomes. Health Educ Res. 2007;22(5):703–717. doi: 10.1093/her/cyl150. [DOI] [PubMed] [Google Scholar]
- 14.Blackstock FC, Webster KE, McDonald CF, Hill CJ. Comparable improvements achieved in chronic obstructive pulmonary disease through pulmonary rehabilitation with and without a structured educational intervention: a randomized controlled trial. Respirology. 2013 Nov 21; doi: 10.1111/resp.12203. Epub. [DOI] [PubMed] [Google Scholar]
- 15.Griffiths Tl, Burr ML, Campbell IA, et al. Results at 1 year of outpatient multidisciplinary pulmonary rehabilitation: a randomised controlled trial. Lancet. 2000;355(9201):362–368. doi: 10.1016/s0140-6736(99)07042-7. Erratum in: Lancet 2000;355(9211):1280 Lonescu AA [corrected to Ionescu AA] [DOI] [PubMed] [Google Scholar]
- 16.Ries AL, Kaplan RM, Limberg TM, Prewitt LM. Effects of pulmonary rehabilitation on physiologic and psychosocial outcomes in patients with chronic obstructive pulmonary disease. Ann Intern Med. 1995;122(11):823–832. doi: 10.7326/0003-4819-122-11-199506010-00003. [DOI] [PubMed] [Google Scholar]
- 17.Clark NM, Becker MH, Lorig K, Rakowski W, Anderson L. Self-Management of chronic disease by older adults. J Aging Health. 1991;3(1):3–27. [Google Scholar]
- 18.Corbin S, Strauss J. Unending Work and Care: Managing Chronic Illness at Home. San Francisco, CA: Jossey-Bass; 1988. [Google Scholar]
- 19.Walters JA, Turnock AC, Walters EH, Wood-Baker R. Action plans with limited patient education only for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2010;(5):CD005074. doi: 10.1002/14651858.CD005074.pub3. [DOI] [PubMed] [Google Scholar]
- 20.Bourbeau J, Julien M, Maltais F, et al. Reduction of hospital utilization in patients with chronic obstructive pulmonary disease: a disease-specific self-management intervention. Arch Intern Med. 2003;163(5):585. doi: 10.1001/archinte.163.5.585. [DOI] [PubMed] [Google Scholar]
- 21.Monninkhof E, van der Valk P, van der Palen J, van Herwaarden C, Zielhuis G. Effects of a comprehensive self-management programme in patients with chronic obstructive pulmonary disease. Eur Respir J. 2003;22(5):815–820. doi: 10.1183/09031936.03.00047003. [DOI] [PubMed] [Google Scholar]
- 22.Cosgrove D, MacMahon J, Bourbeau J, Bradley J, O’Neill B. Facilitating education in pulmonary rehabilitation using the Living Well with COPD programme for pulmonary rehabilitation: a process evaluation. BMC Pulm Med. 2013;13(1):50. doi: 10.1186/1471-2466-13-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Apps LD, Mitchell KE, Harrison SL, et al. The development and pilot testing of the Self-management Programme of Activity, Coping and Education for Chronic Obstructive Pulmonary Disease (SPACE for COPD) Int J COPD. 2013;8:317–327. doi: 10.2147/COPD.S40414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taylor SJC, Sohanpal R, Bremner SA, et al. Self-management support for moderate-to-severe chronic obstructive pulmonary disease: a pilot randomised controlled trial. Br J Gen Pract. 2012:e687–e695. doi: 10.3399/bjgp12X656829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Effing T, Monninkhof Evelyn EM, van der Valk Paul PDLPM, et al. Self-management education for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2007;(4):CD002990. doi: 10.1002/14651858.CD002990.pub2. [DOI] [PubMed] [Google Scholar]
- 26.Lorig KR, Sobel DS, Stewart AL, et al. Evidence suggesting that a chronic disease self-management program can improve health status while reducing hospitalization: a randomized trial. Med Care. 1999;37(1):5–14. doi: 10.1097/00005650-199901000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Foster G, Taylor Stephanie JC, Eldridge S, Ramsay J, Griffiths Chris J. Self-management education programmes by lay leaders for people with chronic conditions. Cochrane Database Syst Rev. 2007;(4):CD005108. doi: 10.1002/14651858.CD005108.pub2. [DOI] [PubMed] [Google Scholar]
- 28.Bandura A. Social Foundations of Thought and Action: A Social Cognitive Theory. Englewood Cliffs, NJ: Prentice Hall; 1986. [Google Scholar]
- 29.Lorig K, Sobel DS, Ritter PL, Laurent D, Hobbs M. Effect of a self-management program on patients with chronic disease. Eff Clin Pract. 2001;4(6):256–262. [PubMed] [Google Scholar]
- 30.Cameron-Tucker HL, Wood-Baker R. COPD, Rehabilitation and the Stanford Chronic Disease Self- Management Programme (poster P-1-22) [Accessed February 3, 2008];Respirology. 2006 11(s5):A159. http://www.blackwell-synergy.com/doi/abs/10.1111/j.1440-1843.2006.00997.x. [Google Scholar]
- 31.Guyatt G, Thompson PJ, Berman BM, et al. How should we measure function in patients with chronic heart and lung disease? J Chron Dis. 1985;38:517–524. doi: 10.1016/0021-9681(85)90035-9. [DOI] [PubMed] [Google Scholar]
- 32.Redelmeier DA, Bayoumi AM, Goldstein RS, Guyatt GH. Interpreting small differences in functional status: the Six Minute Walk test in chronic lung disease patients. Am J Respir Crit Care Med. 1997;155(4):1278–1282. doi: 10.1164/ajrccm.155.4.9105067. [DOI] [PubMed] [Google Scholar]
- 33.Balady GJ. American College of Sports Medicine, ACSM’s Guidelines for Exercise Testing and Prescription. 6th ed. Philadelphia, London: Lipponcott Williams and Wilkins; 2000. [Google Scholar]
- 34.Borg GAV. Psychological bases of physical exertion. Medicine and Science in Sports and Exercise. 1982;14:377–381. [PubMed] [Google Scholar]
- 35.Australian Institute of Health and Welfare The Active Australia Survey: a guide and manual for implementation, analysis and reporting. 2003. [Accessed 20 April, 2004]. Available from: http://www.aihw.gov.au/publications/cvd/aas/aas.pdf.
- 36.Cancer Prevention Research Center [homepage on the Internet] Exercise: stages of change – short form. 1991. [Accessed September 20, 2004]. Available from: http://www.uri.edu/research/cprc/Measures/Exercise02.htm.
- 37.Cancer Prevention Research Center Exercise: self-efficacy measure. 1991. [Accessed September 20, 2004]. Available from: http://www.uri.edu/research/cprc/Measures/Exercise04.htm.
- 38.Gift AG. Validation of a vertical visual analogue scale as a measure of clinical dyspnea. Rehabil Nurs. 1989;14(6):323–325. doi: 10.1002/j.2048-7940.1989.tb01129.x. [DOI] [PubMed] [Google Scholar]
- 39.Stewart A, Mills KM, King AC, Haskell WL, Gillis D, Ritter PL. CHAMPS physical activity questionnaire for older adults. Med Sci Sports Exerc. 2001;33(7):1126–1141. doi: 10.1097/00005768-200107000-00010. [DOI] [PubMed] [Google Scholar]
- 40.Battersby M, Ask A, Reece M, Marwick M. The Partners in Health scale: the development and psychometric properties of a generic assessment scale for chronic condition self-management. Aust J Prim Health. 2003;9(2/3):41–49. [Google Scholar]
- 41.Ware JE, Kosinski M, Dewey JE. How to Score Version 2 of the SF-36 Health Survey. Lincoln, RI: Quality Metric Incorporated; 2000. [Google Scholar]
- 42.Jones PW. St George’s Respiratory Questionnaire: MCID. COPD. 2005;2(1):75–79. doi: 10.1081/copd-200050513. [DOI] [PubMed] [Google Scholar]
- 43.Jones PW, Kaplan RM. Methodological issues in evaluating measures of health as outcomes for COPD. Eur Respir J. 2003;21(Suppl 41):13S–18S. [PubMed] [Google Scholar]
- 44.Pallant J. SPSS Survival Manual. 2nd ed. Crows Nest: Allan and Unwin; 2005. [Google Scholar]
- 45.Holland A, Hill C, Rasekaba T, Lee A, Naughton M, McDonald C. Updating the minimal important difference for six-minute walk distance in patients with chronic obstructive pulmonary disease. Arch Phys Med Rehabil. 2010;91(2):221–225. doi: 10.1016/j.apmr.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 46.Puhan MA, Chandra D, Mosenifar Z, et al. The minimal important difference of exercise tests in severe COPD. Eur Respir J. 2011;37(4):784–790. doi: 10.1183/09031936.00063810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Puente-Maestu L, Sanz M, Sanz P, Cubillo J, Mayol J, Casaburi R. Comparison of effects of supervised versus self-monitored training programmes in patients with chronic obstructive pulmonary disease. Eur Respir J. 2000;15(3):517–525. doi: 10.1034/j.1399-3003.2000.15.15.x. [DOI] [PubMed] [Google Scholar]
- 48.Siu AM, Chan CC, Poon PK, Chui DY, Chan SC. Evaluation of the chronic disease self-management program in a Chinese population. Patient Educ Couns. 2007;65(1):42–50. doi: 10.1016/j.pec.2006.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lorig K, Stewart A, Ritter P, Gonzalez V, Laurent D, Lynch J. Outcome Measures for Health Education and Other Interventions. Thousand Oaks, CA: Sage Publications, Inc; 1996. [Google Scholar]
- 50.Abramson M, Crockett AJ, Dabscheck E, et al. Lung Foundation Australia and the Thoracic Society of Australia and New Zealand [Accessed August 19, 2013];The COPD-X Plan: Australian and New Zealand Guidelines for the management of Chronic Obstructive Pulmonary Disease. 2013 2(35) Available from: http://www.copdx.org.au/ [Google Scholar]