Abstract
Objective
To determine if lower extremity exercise-induced muscle injury (EMI) reduces vascular endothelial function of the upper extremity and if massage therapy (MT) improves peripheral vascular function after EMI.
Design
Randomized, blinded trial with evaluations at 90 minutes, 24 hours, 48 hours, and 72 hours.
Setting
Clinical research center at an academic medical center and laboratory
Participants
Thirty-six sedentary young adults were randomly assigned to one of three groups: 1) EMI + MT (n=15; mean age ± standard error (SE): 26.6±0.3), 2) EMI only (n=10; mean age ± SE: 23.6±0.4), and 3) MT only (n=11; mean age ± SE: 25.5 ± 0.4).
Intervention
Participants were assigned to either EMI only (a single bout of bilateral, eccentric leg-press exercise), MT only (30-minute lower extremity massage using Swedish technique), or EMI + MT.
Main outcome measures
Brachial artery flow-mediated dilation (FMD) was determined by ultrasound at each time point. Nitroglycerin-induced dilation was also assessed (NTG; 0.4 mg).
Results
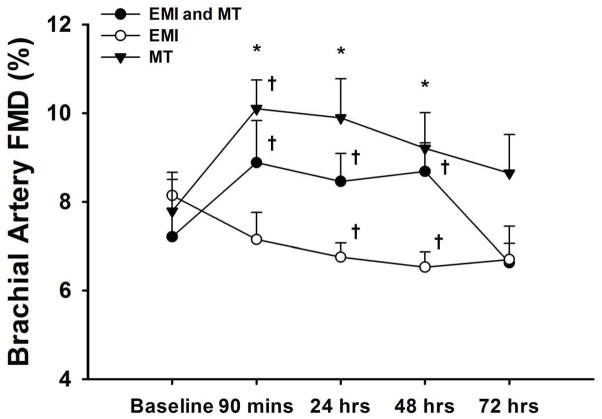
Brachial FMD increased from baseline in the EMI + MT group and the MT only group (7.38±0.18 to 9.02±0.28%, p<0.05 and 7.77±0.25 to 10.20±0.22%, p < 0.05, respectively) at 90 minutes remaining elevated until 72 hrs. In the EMI only group FMD was reduced from baseline at 24 and 48 hrs (7.78±0.14 to 6.75±0.11%, p<0.05 and 6.53±0.11, p<0.05, respectively) returning to baseline after 72 hrs. Dilations to NTG were similar over time.
Conclusions
Our results suggest that MT attenuates impairment of upper extremity endothelial function resulting from lower extremity EMI in sedentary young adults.
Keywords: Endothelial function, exertion, muscle injury, massage therapy
Exertion-induced muscle injury (EMI) is an important consideration, particularly following physical activities that entail high-force and/or repetitive eccentric muscle contractions1. This type of injury is associated with muscle pain, soreness, swelling, decreased range of motion (ROM) and reduced muscle strength, which can last from 5 to 7 days1. The pathogenesis of EMI is related to an inflammatory response triggered by damaged muscle fibers2,3, that can lead to systemic inflammation and altered endothelial function4, although these effects have not yet been fully elucidated. Currently, there is no universally accepted treatment for EMI; however, massage therapy (MT) is often recommended for reducing associated symptoms and has been shown to reduce post-injury inflammation5.
Massage therapy is a well-known comprehensive intervention that involves the utilization of a variety of manual techniques designed to manipulate the soft tissues and joints of the body6. Certain techniques are speculated to decrease pain, reduce intramuscular swelling and increase range of motion (ROM) following muscle injury7. It has also been suggested that some forms of MT influence physiological factors associated with muscle injury and recovery by increasing tissue blood flow6. Massage therapy has most frequently been studied within the context of indirect indices of muscle injury including: pain, muscle function (i.e. muscular strength, ROM), swelling, markers of muscle damage (i.e. creatine kinase and lactate dehydrogenase) and circulating neutrophils8.
Recent in vitro studies have revealed that MT attenuates production of inflammatory cytokines in muscle when administered following damage from acute eccentric exercise5. Inflammatory cytokines can initiate a systemic inflammatory response resulting in neutrophil activation and adhesion to vascular endothelial cells9,10. Such a response can ultimately culminate in impaired endothelial function4, an early prognostic indicator for the development of cardiovascular disease11,12. Previous research shows that flow-mediated dilation (FMD), a measure of endothelial function, is impaired in healthy but sedentary young adults after an acute bout of strenuous lower extremity resistance exercise involving both eccentric and concentric muscle contractions13. As such, MT may attenuate impaired endothelial function after acute eccentric exercise. Therefore, the purpose of this study was to 1) investigate the mechanisms of systemic endothelial dysfunction of the brachial artery which develops after EMI of the lower extremities and 2) determine if treatment of the lower extremities with MT reduces endothelial dysfunction. The hypothesis to be tested is that a MT treatment performed after exposure to EMI will protect against impaired endothelial function.
METHODS
Study Participants and Design
Thirty-six sedentary adults aged 18 to 40 years were studied. Subjects were randomly assigned to one of three groups: 1) MT treatment following exposure to EMI (EMI + MT), 2) a control intervention of EMI without MT treatment (EMI only), or 3) a control intervention of MT treatment without EMI (MT only). Eligibility was confirmed upon completion of a health history questionnaire and physical examination. Inclusion criteria were as follows: less than 150 minutes of moderate physical activity per week, no history of resistance or aerobic training within the past 6 months prior to enrollment, no history of cardiovascular disease or suspected collagen vascular disease such as systemic vasculitis, diabetes mellitus, thyroid dysfunction or orthopedic injuries, not pregnant, no history of cancer, no history of smoking (for at least 6 months prior to participation), no history of amenorrhea or irregular menses and no use of vasoactive medications. Written informed consent was obtained from all subjects prior to participation. The study protocol was approved by the Office for the Protection of Research Subjects and the Institutional Review Board of the University of Illinois at Chicago (UIC).
Initial Screening and Baseline Measurements
Subjects were evaluated in the UIC, Clinical Research Center (CRC) during a screening visit and at five time points before (baseline) and after (90 minutes, 24 hours, 48 hours and 72 hours) the intervention. All measurements were performed after a 12-hour overnight fast. During the initial screening visit, venous blood samples were drawn from an antecubital vein and plasma was separated by centrifugation for laboratory analysis of total cholesterol, high-density lipoproteins (HDLs), low-density lipoproteins (LDLs) and glucose. Total cholesterol, HDLs, and LDLs were measured using spectrophotometric assays. Glucose concentration was measured using the glucose oxidase procedure (Beckman Coulter Inc; Fullerton, CA). A food frequency questionnaire (Block Brief 2000 FFQ) was used to determine dietary intake and nutritional content14. Heart rate (HR), systolic and diastolic blood pressures (BP), and anthropometric measurements, including height, weight, and waist circumference (WC) were measured. Body composition was determined by bioelectrical impedance analysis (BIA; RJL Systems, Inc) as previously described15.
Brachial Artery Measurements of Flow-Mediated and Nitroglycerin-Induced Dilation
Approximately one week following the initial screening, subjects who met all inclusion criteria returned to the CRC for baseline assessment of endothelial function. Brachial artery flow-mediated dilation (FMD) was used as a measure of endothelial function using previously described techniques13,16. In premenopausal women, FMD may vary during the menstrual cycle17; therefore, female subjects underwent study procedures during the early follicular phase of menses. While subjects were in the supine position in a quiet, temperature controlled room, ultrasound imaging (Sonosite; Seattle, WA) of the brachial artery was performed in a longitudinal plane at a site one to three centimeters proximal to the antecubital fossa of the dominant arm18 using a high-frequency (7.5 MHz) linear array probe. Measurements were made at a depth of 30 mm to 50 mm according to each subject’s frame. The same anatomical distance and examination depth were used for each study visit. The dynamic range and pulse-repetition frequency for all examinations were set at 55 db to 60 db (B-mode) and 3.5 kHz, respectively. Baseline images were recorded after which a forearm blood pressure cuff was inflated to 50 mmHg above systolic BP for 5 minutes. Brachial artery diameter (mm) was determined during peak hyperemia after release of the cuff. To assess dilation, at least 30 seconds of images were captured during the first, second and third minutes after cuff release. Baseline brachial artery flow velocity and peak velocity after cuff release were recorded using central velocity measures described previously19. The velocity detecting range was from −30 cm/sec to 140 cm/sec within a sample volume of 2 mm. Subjects underwent repeated measures of brachial artery FMD and flow at four time points (90 minutes, 24 hours, 48 hours and 72 hours) after the intervention. Nitroglycerin-induced dilation was also assessed at these four time points. Ten minutes after recording the last brachial artery diameter measurement following hyperemia, 0.4 mg of sublingual nitroglycerin (NTG) was administered and endothelium-independent dilation was assessed for 5 minutes.
Offline analysis of FMD and NTG responses were made using edge-detection software (Medical Imaging; Iowa City, IA) as previously described13. Percent FMD and responses to NTG were calculated using the averaged minimum mean brachial artery diameter at baseline compared with the largest mean values obtained after release of the forearm occlusion or administration of NTG. Peak shear rate (SR) was calculated as flow velocity (cm/s) divided by vessel diameter20. Flow-mediated dilation was normalized for the peak SR using the following equation: .
Assessment of Perceived Muscle Soreness
Perceived muscle soreness was determined by subjective responses to palpation of relaxed quadriceps muscles. While subjects were in a seated position, the muscle belly and distal regions of the vastus medialis, vastus lateralis, and rectus femoris were palpated by a qualified exercise professional. Perceived soreness was then rated on a scale ranging from 1 (normal) to 10 (very, very sore), as previously describe21.
Protocol for Exertion-Induced Muscle Injury
After baseline assessment of endothelial function, subjects assigned to groups 1 (EMI + MT) and 2 (EMI only) underwent a single bout of bilateral eccentric exercise on an isotonic variable resistance leg press machine (Hoist Fitness Systems; San Diego, CA). Procedures for the eccentric exercise bout have been previously described22,23. Initially, subjects performed 1 to 2 sets of 8 to 12 repetitions using a weight of their choice in order to become familiarized with the leg press machine. Subjects were in a seated position and each repetition began with the hips and knees flexed (approximately 90°) performing single repetitions to exhaustion. The 10-point Borg rating of perceived exertion (RPE) scale was used as an index of intensity24 and weight was increased with each successive set as tolerated. The 1-RM was reached within 4 to 7 trials for all subjects. For eccentric exercise, subjects performed 6 to 8 sets of 10 repetitions at approximately 70 to 80% of the concentric 1-RM with 2 to 3 minutes of rest between each set. Heart rate, blood pressure, and the Borg RPE scale were used as indices of intensity. To perform eccentric muscle contractions, subjects were instructed to lower the weight by themselves for 5 seconds and received verbal encouragement to maintain the required lowering speed. If perceived fatigue inhibited subjects from lowering the weight at the required speed, a brief rest (approximately 10 to 15 seconds) was permitted. Heart rate and blood pressures were measured during and after each exercise trial. Peak systolic, diastolic and mean arterial pressures were recorded. All subjects completed the eccentric exercise bout within 30 minutes. Subjects assigned to EMI only rested for approximately 30 minutes after eccentric exercise as a time control.
Massage Therapy Treatment Protocol
Subjects assigned to group 1 (EMI + MT) underwent a MT treatment within 30 minutes after eccentric exercise while subjects assigned to group 3 (MT only) underwent treatment after 30 minutes of rest. The MT treatment protocol (30 minutes) targeted the bilateral lower-extremity muscle groups using Swedish techniques varying in depth from superficial to deep25. All treatments were performed by a single licensed and certified massage therapist. The hip extensors (gluteal and hamstring muscles) were treated while subjects lied in the prone position with the ankles bolstered. Superficial stroking and effleurage was applied to the gluteal and hamstring muscles (2 minutes, bilaterally) followed by superficial to deep effleurage (gliding strokes) and petrissage (palmar kneading and muscle stripping) on the gluteal muscle group (5 minutes, bilaterally). Superficial to deep effleurage (gliding strokes) and petrissage (palmar kneading, wringing and muscle stripping) were then performed on the biceps femoris, semitendinosus and semimembranosus, respectively (8 minutes, bilaterally). The hip extensor treatment was closed with application of superficial effleurage and stroking (2 minutes, bilaterally). The knee extensors (quadriceps muscles) were treated while subjects lied in the supine position with the knees bolstered. Superficial stroking and effleurage were applied to the quadriceps muscles (1 minute, bilaterally) followed by superficial to deep effleurage (gliding strokes) and petrissage (palmar kneading, wringing and muscle stripping) on the rectus femoris, vastus medialis and vastus lateralis, respectively (10 minutes, bilaterally). The knee extensor treatment was closed with application of superficial effleurage and stroking (2 minutes, bilaterally).
Statistical Analysis
Brachial artery FMD is expressed as the percent change in diameter from basal blood flow to peak hyperemia. Repeated measures analysis of variance (ANOVA) with between-group factors was used to assess differences in percent brachial artery FMD (before and after intervention) between the time points. Differences in brachial artery diameter, normalized FMD, peak flow velocity and NTG responses across time were also identified using repeated-measures ANOVA. Tukey’s post hoc test was used for multiple comparisons. Physical and physiological characteristics (i.e. age, gender, race/ethnicity, lipids, glucose, BP), anthropometric measurements, body fat percentage and nutritional data were compared using one-way between-groups ANOVA. For covariate testing, a random effect model with a group-by-time interaction in the model was used to compare FMD change over time between groups. The same random effect model with adding additional covariates (i.e. lipids, HR, BP, brachial artery diameter, peak SR) in the model was used for testing if the covariates impact the group effects on the FMD change over time. Pearson correlations were used to examine relationships between subjects’ vascular and physiological characteristics, anthropometric measurements, body fat percentage and nutritional data. All results are expressed as mean ± SE. The level of statistical significance for all analyses was set at P < 0.05. Data were analyzed using SPSS software (Version 17.0; SPSS Inc., Chicago, IL).
RESULTS
Participant Demographics and Baseline Characteristics
Subjects included 8 men and 28 women. Physical and physiological characteristics for subjects in groups 1 (EMI + MT), 2 (EMI only), and 3 (MT only) are presented in Table 1 and were similar among the groups. In addition, dietary patterns of macronutrients (fat, carbohydrate and protein) were similar among groups (Table 2).
TABLE 1.
Physical and physiological characteristics of the study population.
| EMI and MT | EMI Only | MT Only | P Value | |
|---|---|---|---|---|
| Number, n | 15 | 10 | 11 | N/A |
| Age, yr | 26.7 ± 0.3 | 23.6 ±0.4 | 25.5 ± 0.4 | 0.164 |
| Height, cm | 167.3 ± 0.4 | 166.9 ± 0.6 | 167.9 ± 0.7 | 0.614 |
| Weight, kg | 74.9 ± 0.9 | 76.3 ± 1.3 | 71.5 ± 1.5 | 0.860 |
| BMI, kg/m2 | 26.7 ± 0.3 | 27.4 ±0.5 | 25.3 ± 1.1 | 0.613 |
| Body Fat % | 28.8 ± 0.5 | 28.0 ± 1.0 | 25.8 ± 0.9 | 0.649 |
| Waist circumference, cm | 81.9 ± 0.8 | 83.2 ± 0.9 | 78.7 ± 1.1 | 0.649 |
| Total cholesterol, mmol/l | 4.2 ± 0.05 | 4.0 ± 0.05 | 3.9 ± 0.06 | 0.549 |
| LDLs, mmol/l | 2.2 ± 0.04 | 2.1 ± 0.07 | 2.0 ± 0.07 | 0.656 |
| HDL, mmol/l | 1.5 ± 0.03 | 1.5 ± 0.04 | 1.6 ± 0.03 | 0.796 |
| Triglycerides, mmol/l | 1.0 ± 0.05 | 0.7 ± 0.03 | 0.6 ± 0.02 | 0.168 |
| Glucose, mmol/l | 4.5 ± 0.02 | 4.8 ± 0.03 | 3.8 ± 0.02 | 0.220 |
| Heart Rate, bpm | 73.1 ± 0.7 | 74.6 ± 1.7 | 68.6 ± 1.0 | 0.448 |
| Systolic BP, mmHg | 117.1 ± 0.7 | 116.7 ± 1.2 | 118.5 ± 0.8 | 0.859 |
| Diastolic BP, mmHg | 71.9 ± 0.7 | 70.7 ± 1.0 | 72.2 ± 1.0 | 0.330 |
| Maximum perceived soreness | 5.3 ± 0.6* | 8.8 ± 0.2* | 1.2 ± 0.2* | <0.001 |
All values expressed as mean ± SE.
P < 0.05, statistically significant.
EMI, exertion-induced muscle injury; MT, massage therapy; LDL, low-density lipoprotein; HDL, high-density lipoprotein; BP, blood pressure. Maximum perceived soreness is a numeric rating based on a 1–10 scale.
TABLE 2.
Nutritional characteristics of the study population.
| EMI and MT | EMI Only | MT Only | P Value | |
|---|---|---|---|---|
| Energy, kcal/day | 1549.2 ± 316.1 | 2188.5 ± 430.8 | 1782.5 ± 343.6 | 0.497 |
| Total fat, g/day | 63.6 ± 13.8 | 96.3 ± 18.9 | 71.5 ± 15.0 | 0.383 |
| Total carbohydrate, g/day | 170.5 ± 35.7 | 225.3 ± 48.6 | 206.4 ± 38.8 | 0.628 |
| Total protein, g/day | 73.7 ± 16.3 | 87.0 ± 22.2 | 73.6 ± 17.7 | 0.870 |
| Vitamin C, mg/day | 93.0 ± 25.8 | 103.9 ± 35.1 | 101.7 ± 28.0 | 0.434 |
| Vitamin E, mg/day | 8.4 ± 1.4 | 9.2 ± 1.9 | 7.2 ± 1.5 | 0.941 |
All values expressed as mean ± SE.
P < 0.05, statistically significant.
EMI, exertion-induced muscle injury; MT, massage therapy.
Brachial Artery Reactivity to Flow-Mediated and Nitroglycerin-Induced Dilation
Vascular characteristics for subjects in groups 1 (EMI + MT), 2 (EMI only), and 3 (MT only) at baseline and following eccentric exercise across time are presented in Table 3. Subjects in each group exhibited significantly different responses to their interventions with perceived muscle soreness being the highest among subjects in group 2 (Table 1). Brachial artery diameters were similar among groups and were not altered after their interventions (Table 3). Ninety minutes after the intervention, FMD increased from baseline in the EMI + MT group as well as the MT only group (7.38 ± 0.18 to 9.02 ± 0.28%, p < 0.05 and 7.77 ± 0.25 to 10.20 ± 0.22%, p < 0.05, respectively), remaining elevated until 72 hours (Figure 1). In the EMI only group, FMD was reduced from baseline at 24 and 48 hours (7.78 ± 0.14 to 6.75 ± 0.11%, p < 0.05 and 6.53 ± 0.11, p < 0.05, respectively) returning to baseline after 72 hours (Figure 1). Significant differences in FMD were observed between the EMI only and MT only groups at 90 minutes, 24 hours and 48 hours (Figure 1). The magnitude of change in FMD among the groups was similar after normalization to peak SR (Table 3). No significant FMD-related differences were observed between male and female participants or across the various racial and ethnic groups. There were no differences in peak flow velocity among the groups or over time (Table 3). In addition, NTG-induced dilations were similar among the time points (Table 1) indicating that endothelium-independent dilation was unaltered. There was no correlation between vascular and physiological characteristics, anthropometric measurements, body fat percentage and nutritional data.
TABLE 3.
Vascular characteristics of the study population across time points.
| Characteristic | EMI and MT | EMI Only | MT Only |
|---|---|---|---|
| Baseline | |||
| Diameter, mm | 3.7 ± 0.1 | 3.8 ± 0.2 | 4.1 ± 0.3 |
| Peak FV, cm/s | 100.9 ± 4.2 | 101.5 ± 5.0 | 102.1 ± 3.4 |
| FMD peak SR, s−1 | 265.2 ± 24.7 | 260.5 ± 22.7 | 258.3 ± 24.2 |
| Normalized FMD | 0.028 ± 0.003 | 0.030 ± 0.002 | 0.030 ± 0.003 |
| NTG dilation, % | ND | ND | ND |
| 90 minutes | |||
| Diameter, mm | 3.6 ± 0.1 | 3.8 ± 0.2 | 3.8 ± 0.2 |
| Peak FV, cm/s | 109.8 ± 7.8 | 105.2 ± 7.2 | 111.2 ± 4.9 |
| FMD peak SR, s−1 | 285.7 ± 25.7 | 269.6 ± 27.5 | 269.6 ± 27.5 |
| Normalized FMD | 0.032 ± 0.003† | 0.026 ± 0.002* | 0.037 ± 0.003† |
| NTG dilation, % | 15.7 ± 0.02 | 15.0 ± 0.02 | 14.9 ± 0.02 |
| 24 hours | |||
| Diameter, mm | 3.8 ± 0.1 | 3.8 ± 0.2 | 3.9 ± 0.3 |
| Peak FV, cm/s | 109.4 ± 6.6 | 108.8 ± 7.9 | 107.8 ± 6.5 |
| FMD peak SR, s−1 | 276.5 ± 27.7 | 266.0 ± 25.4 | 271.6 ± 25.6 |
| Normalized FMD | 0.031 ± 0.003† | 0.025 ± 0.002*† | 0.034 ± 0.003 |
| NTG dilation, % | 14.1 ± 0.03 | 15.1 ± 0.03 | 16.5 ± 0.03 |
| 48 hours | |||
| Diameter, mm | 3.7 ± 0.1 | 3.8 ± 0.2 | 3.9 ± 0.3 |
| Peak FV, cm/s | 109.6 ± 8.0 | 107.5 ± 8.9 | 109.1 ± 8.6 |
| FMD peak SR, s−1 | 275.3 ± 25.8 | 264.3 ± 27.7 | 274.6 ± 21.9 |
| Normalized FMD | 0.032 ± 0.003† | 0.025 ± 0.002*† | 0.034 ± 0.003 |
| NTG dilation, % | 14.2 ± 0.02 | 18.0 ± 0.02 | 15.6 ± 0.02 |
| 72 hours | |||
| Diameter, mm | 3.7 ± 0.1 | 3.9 ± 0.3 | 3.9 ± 0.3 |
| Peak FV, cm/s | 108.1 ± 8.7 | 106.6 ± 8.4 | 110.6 ± 6.9 |
| FMD peak SR, s−1 | 277.0 ± 27.9 | 251.6 ± 22.9 | 266.6 ± 21.8 |
| Normalized FMD | 0.026 ± 0.002 | 0.032 ± 0.003 | 0.028 ± 0.002 |
| NTG dilation, % | 15.6 ± 0.02 | 17.4 ± 0.03 | 14.4 ± 0.02 |
All values expressed as mean ± SE.
Significant differences observed in EMI only group versus MT only group (P < 0.05).
Significant within group differences observed from baseline.
FV, flow velocity; FMD, flow-mediated dilation; SR, shear rate; NTG, nitroglycerin; ND, not determined.
Figure 1.
The effects of exertion-induced muscle injury (EMI) and/or massage therapy (MT) on brachial artery flow-mediated dilation (FMD) in sedentary adults at five time points during the study period. *Significant differences observed in EMI only group versus MT only group (p < 0.05). †Significant within group differences observed from baseline (p < 0.05).
DISCUSSION
The results of this study indicate that local MT has systemic effects on endothelial function. To our knowledge, this is the first study to determine that MT protects against reduced upper extremity endothelial function following lower extremity exertion. The primary finding of this study is that MT attenuates impairment of brachial artery FMD resulting from EMI in sedentary young adults. In addition, we present the novel and unexpected finding that lower extremity massage enhances brachial artery FMD even in the absence of EMI.
Pathophysiology of Exertion-Induced Muscle Injury
Muscle injury induced by eccentric contractions can lead to morphological changes in myofibrils and connective tissue2,26 that ultimately initiate a local inflammatory response and subsequent biochemical changes within the damaged muscle tissue27. The local inflammatory response to EMI is characterized by immediate leukocyte infiltration with neutrophils representing 50 to 60% of the total circulating pool28. Neutrophilic generation of ROS can contribute to systemic inflammatory and oxidative stress responses after EMI29 and thereby scavenge nitric oxide (NO). We have found that EMI impairs brachial artery FMD, which is an index of endothelial function associated with the L-arginine/NO-dependent vasodilator pathway30,31. Furthermore, the duration of this impairment was approximately 48 hours (Figure 1), which is consistent with the time course of exercise-induced inflammation3,27.
Potential Mechanisms of Endothelial Dysfunction after Muscle Injury
Under normal physiological conditions, NO is a chief vasodilator released by the endothelium and functions in modulating vascular smooth muscle tone. In addition, NO regulates inflammation by preventing leukocyte activation and adhesion32 and prevents atherosclerosis through its anti-proliferative effects on the vascular wall33. While, NO plays a critical role in vasodilation under conditions of increased blood flow, impaired endothelial function is associated with both decreased production and increased scavenging of NO by elevated ROS32. During inflammation, ROS generated by neutrophils can react with NO generated by endothelial cells creating a situation of oxidative stress34 reflected as a reduced FMD. Indeed we found that FMD is impaired after muscle injury (Figure 1) but future studies are required to further examine if this response is linked to the neutrophil-mediated inflammatory response or oxidative stress.
Massage Therapy as a Treatment Intervention for Exertion-Induced Muscle Injury
The performance of massage may alter local and systemic inflammatory responses after EMI through its effects on the circulation. Studies have demonstrated that MT enhances local circulation in both injured and uninjured muscle tissue through vasodilatory mechanisms induced by mechanical stimulation associated with pressure applied during various techniques5,6,25. If MT treatment is implemented after EMI, increased local blood flow may hasten the inflammatory response by reducing the time course of neutrophil infiltration and activation, thereby protecting against neutrophil-mediated tissue damage.
At the systemic level, reducing inflammation through MT may prevent reduced endothelial function. In our study, a single lower extremity MT treatment resulted in an elevation in brachial artery FMD that lasted for 48 hours after EMI (Figure 1). This treatment also increased FMD in the absence of EMI. Since shear stress has the capacity to alter function of the endothelium by increasing NO, vascular responses to the intervention could have been influenced by differences in shear35. However, shear rates were similar among groups (Table 3) and there was no association between FMD and peak SR.
Another possibility is that MT modulates the autonomic nervous system. Previous studies have shown that when moderate to deep pressure is employed a single MT treatment elicits a parasympathetic nervous system response characterized by decreased HR36 and reduced systolic BP37 Such changes in autonomic nervous system activity may have an impact on brachial artery FMD responses to MT. Our studies showed no significant changes in HR (EMI and MT: 73.1 ± 0.7; EMI only: 74.6 ± 1.7; and MT only: 68.6 ± 1.0, p > 0.05) or systolic BP (EMI and MT: 117.1 ± 0.7; EMI only: 116.7 ± 1.2; and MT only: 118.5 ± 0.8, p > 0.05) among the groups from baseline to 72 hours. Future studies are warranted to elucidate the mechanistic effects of MT on endothelial function in both the presence and the absence of EMI.
Study Limitations
There are some limitations of this study that need to be addressed. First, our results may be confounded by the absence of a true control group. Future studies implementing a no-intervention control group and larger sample sizes may be necessary to validate our findings. In addition, our study was not designed to determine gender or racial/ethnic-specific differences in massage and EMI but future studies will help to determine the influence of such variables on endothelial function. Second, although all subjects fit our criteria for being “sedentary”, differences in BMI may confound the effects of massage on FMD. To account for this, subjects in each group were carefully matched for BMI. Third, we were unable to test endothelium-independent dilation to nitroglycerin before EMI and/or MT because of the residual vasodilator effects of this compound on blood pressure. However, all subjects were healthy and absent of cardiovascular risk factors, lowering the risk of impaired endothelial function at baseline time points. Fourth, we cannot disregard the potential influence of alterations in autonomic function in response to massage. Although our studies showed no significant changes in HR or systolic BP among the groups, changes in autonomic nervous system activity could very well have an impact on endothelial responses to MT. Fifth, only peak SR was assessed. Other studies have shown that alterations of shear rate within a subject may limit and under-represent the normalized FMD compared with area under the curve (AUC) measurements taken until peak diameter38. Although AUC between cuff release and peak diameter was not measured, results shown in Table 3 suggest that the magnitude of change pre- and post-intervention using either normalized FMD or percent FMD were similar. Brachial diameter was not calculated continuously throughout the cuff release period. Consistent with other studies20, the diameter calculations were made during the first, second and third minutes after cuff release. Sixth, we did not include measures circulating markers of inflammation (i.e. TNF-α and IL-6) or oxidative stress; therefore, whether or not inflammation induced EMI was altered by massage is not known. Finally, our results may have been confounded by nutritional status due to potential differences in total calories as well as macronutrient and micronutrient consumption among subjects and between groups. While self-reported dietary intake is often underestimated, nutritional status was similar (Table 2).
Clinical Relevance
Massage therapy is a widely used and sought after treatment modality in numerous countries. Approximately 22 million American adults receive MT treatment annually and 32% of these adults do so for health reasons such as pain relief, injury recovery and rehabilitation39. In healthcare, 37% of hospitals currently offer complementary and alternative medicine therapies and 70% of them include MT among their offerings39. Although the use of MT is widespread, research to date does not provide evidence for the effects of massage on blood flow. We have demonstrated that a single MT treatment improves brachial artery endothelium-dependent FMD for up to 48 hours days. Since FMD correlates well with cardiovascular risk12,40, the results of our studies may indicate that the use of MT as a means of reducing EMI and post-exercise hypo-perfusion in at risk populations (i.e. heart disease) undergoing higher intensity exercise training regimens41,42. In addition, massage-induced improvements in endothelial function may help to protect against vascular responses to other physiological stressors such as acute hypertension, hypoxemia and wound healing.
Conclusions
In conclusion, the results of this study suggest that EMI impairs systemic endothelial function in sedentary but otherwise healthy young adults. Furthermore, massage therapy enhances endothelial function in both the presence and absence of muscle injury. The results of this study contribute to a better understanding of how massage therapy promotes faster recovery from EMI and may have broader implications for the clinical use of massage therapy, especially in the context of endothelial dysfunction.
Acknowledgments
This work was supported by a Massage Therapy Foundation research grant as well as National Heart, Lung, and Blood Institute grants K23HL85614, RO1HL095701, and HL095701-01A2S, and the University of Illinois at Chicago, Center for Clinical and Translational Science (CCTS), award UL1RR029879 from the National Center for Research Resources. We certify that no party having a direct interest in the results of the research supporting this article has or will confer a benefit on us or on any organization with which we are associated AND, if applicable, we certify that all financial and material support for this research (eg, NIH or NHS grants) and work are clearly identified in the title page of the manuscript.
Abbreviations
- BP
Blood pressure
- EMI
Exertion-induced muscle injury
- FMD
Flow mediated dilation
- HDL
High-density lipoprotein
- LDL
Low-density lipoprotein
- MT
Massage therapy
- NO
Nitric oxide
- NTG
Nitroglycerin
- ROM
Range of motion
- ROS
Reactive oxygen species
- SR
Shear rate
- WC
Waist circumference
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Howatson G, van Someren KA. The prevention and treatment of exercise-induced muscle damage. Sports medicine. 2008;38(6):483–503. doi: 10.2165/00007256-200838060-00004. [DOI] [PubMed] [Google Scholar]
- 2.Best TM, Hunter KD. Muscle injury and repair. Physical medicine and rehabilitation clinics of North America. 2000 May;11(2):251–266. [PubMed] [Google Scholar]
- 3.Ispirlidis I, Fatouros IG, Jamurtas AZ, et al. Time-course of changes in inflammatory and performance responses following a soccer game. Clinical journal of sport medicine : official journal of the Canadian Academy of Sport Medicine. 2008 Sep;18(5):423–431. doi: 10.1097/JSM.0b013e3181818e0b. [DOI] [PubMed] [Google Scholar]
- 4.Filippin LI, Cuevas MJ, Lima E, Marroni NP, Gonzalez-Gallego J, Xavier RM. Nitric oxide regulates the repair of injured skeletal muscle. Nitric oxide : biology and chemistry / official journal of the Nitric Oxide Society. 2011 Jan 1;24(1):43–49. doi: 10.1016/j.niox.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 5.Crane JD, Ogborn DI, Cupido C, et al. Massage therapy attenuates inflammatory signaling after exercise-induced muscle damage. Science translational medicine. 2012 Feb 1;4(119):119ra113. doi: 10.1126/scitranslmed.3002882. [DOI] [PubMed] [Google Scholar]
- 6.Goats GC. Massage--the scientific basis of an ancient art: Part 2. Physiological and therapeutic effects. British journal of sports medicine. 1994 Sep;28(3):153–156. doi: 10.1136/bjsm.28.3.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weerapong P, Hume PA, Kolt GS. The mechanisms of massage and effects on performance, muscle recovery and injury prevention. Sports medicine. 2005;35(3):235–256. doi: 10.2165/00007256-200535030-00004. [DOI] [PubMed] [Google Scholar]
- 8.Zainuddin Z, Newton M, Sacco P, Nosaka K. Effects of massage on delayed-onset muscle soreness, swelling, and recovery of muscle function. Journal of athletic training. 2005 Jul-Sep;40(3):174–180. [PMC free article] [PubMed] [Google Scholar]
- 9.Butterfield TA, Best TM, Merrick MA. The dual roles of neutrophils and macrophages in inflammation: a critical balance between tissue damage and repair. Journal of athletic training. 2006 Oct-Dec;41(4):457–465. [PMC free article] [PubMed] [Google Scholar]
- 10.Tidball JG. Inflammatory processes in muscle injury and repair. American journal of physiology. Regulatory, integrative and comparative physiology. 2005 Feb;288(2):R345–353. doi: 10.1152/ajpregu.00454.2004. [DOI] [PubMed] [Google Scholar]
- 11.Gokce N, Keaney JF, Jr, Hunter LM, Watkins MT, Menzoian JO, Vita JA. Risk stratification for postoperative cardiovascular events via noninvasive assessment of endothelial function: a prospective study. Circulation. 2002 Apr 2;105(13):1567–1572. doi: 10.1161/01.cir.0000012543.55874.47. [DOI] [PubMed] [Google Scholar]
- 12.Yoshida T, Kawano H, Miyamoto S, et al. Prognostic value of flow-mediated dilation of the brachial artery in patients with cardiovascular disease. Intern Med 2006. 2006;45(9):575–579. doi: 10.2169/internalmedicine.45.1534. [DOI] [PubMed] [Google Scholar]
- 13.Phillips SA, Das E, Wang J, Pritchard K, Gutterman DD. Resistance and aerobic exercise protects against acute endothelial impairment induced by a single exposure to hypertension during exertion. J Appl Physiol. 2011 Apr;110(4):1013–1020. doi: 10.1152/japplphysiol.00438.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coates RJ, Eley JW, Block G, et al. An evaluation of a food frequency questionnaire for assessing dietary intake of specific carotenoids and vitamin E among low-income black women. American journal of epidemiology. 1991 Sep 15;134(6):658–671. doi: 10.1093/oxfordjournals.aje.a116138. [DOI] [PubMed] [Google Scholar]
- 15.Fakhrawi DH, Beeson L, Libanati C, et al. Comparison of body composition by bioelectrical impedance and dual-energy x-ray absorptiometry in overweight/obese postmenopausal women. Journal of clinical densitometry : the official journal of the International Society for Clinical Densitometry. 2009 Apr-Jun;12(2):238–244. doi: 10.1016/j.jocd.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 16.Harris RA, Nishiyama SK, Wray DW, Richardson RS. Ultrasound assessment of flow-mediated dilation. Hypertension. 2010 May;55(5):1075–1085. doi: 10.1161/HYPERTENSIONAHA.110.150821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hashimoto M, Akishita M, Eto M, et al. Modulation of endothelium-dependent flow-mediated dilatation of the brachial artery by sex and menstrual cycle. Circulation. 1995 Dec 15;92(12):3431–3435. doi: 10.1161/01.cir.92.12.3431. [DOI] [PubMed] [Google Scholar]
- 18.Kizhakekuttu TJ, Gutterman DD, Phillips SA, et al. Measuring FMD in the brachial artery: how important is QRS gating? Journal of applied physiology. 2010 Oct;109(4):959–965. doi: 10.1152/japplphysiol.00532.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li S, Hoskins PR, Anderson T, McDicken WN. Measurement of mean velocity during pulsatile flow using time-averaged maximum frequency of Doppler ultrasound waveforms. Ultrasound Med Biol. 1993;19(2):105–113. doi: 10.1016/0301-5629(93)90002-6. [DOI] [PubMed] [Google Scholar]
- 20.Corretti MC, Anderson TJ, Benjamin EJ, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002 Jan 16;39(2):257–265. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 21.Clarkson PM, Byrnes WC, McCormick KM, Turcotte LP, White JS. Muscle soreness and serum creatine kinase activity following isometric, eccentric, and concentric exercise. International journal of sports medicine. 1986 Jun;7(3):152–155. doi: 10.1055/s-2008-1025753. [DOI] [PubMed] [Google Scholar]
- 22.Bloomer RJ, Falvo MJ, Schilling BK, Smith WA. Prior exercise and antioxidant supplementation: effect on oxidative stress and muscle injury. Journal of the International Society of Sports Nutrition. 2007;4:9. doi: 10.1186/1550-2783-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cooke MB, Rybalka E, Williams AD, Cribb PJ, Hayes A. Creatine supplementation enhances muscle force recovery after eccentrically-induced muscle damage in healthy individuals. Journal of the International Society of Sports Nutrition. 2009;6:13. doi: 10.1186/1550-2783-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scherr J, Wolfarth B, Christle JW, Pressler A, Wagenpfeil S, Halle M. Associations between Borg’s rating of perceived exertion and physiological measures of exercise intensity. European journal of applied physiology. 2013 Jan;113(1):147–155. doi: 10.1007/s00421-012-2421-x. [DOI] [PubMed] [Google Scholar]
- 25.Goats GC. Massage--the scientific basis of an ancient art: Part 1. The techniques. British journal of sports medicine. 1994 Sep;28(3):149–152. doi: 10.1136/bjsm.28.3.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stauber WT, Clarkson PM, Fritz VK, Evans WJ. Extracellular matrix disruption and pain after eccentric muscle action. Journal of applied physiology. 1990 Sep;69(3):868–874. doi: 10.1152/jappl.1990.69.3.868. [DOI] [PubMed] [Google Scholar]
- 27.Clarkson PM, Nosaka K, Braun B. Muscle function after exercise-induced muscle damage and rapid adaptation. Medicine and science in sports and exercise. 1992 May;24(5):512–520. [PubMed] [Google Scholar]
- 28.Peake JM. Exercise-induced alterations in neutrophil degranulation and respiratory burst activity: possible mechanisms of action. Exercise immunology review. 2002;8:49–100. [PubMed] [Google Scholar]
- 29.Powers SK, Jackson MJ. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiological reviews. 2008 Oct;88(4):1243–1276. doi: 10.1152/physrev.00031.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Celermajer DS, Sorensen KE, Bull C, Robinson J, Deanfield JE. Endothelium-dependent dilation in the sytemic arteries of asymptomatic subjects relates to coronary risk factors and their interaction. J Am Coll Cardiol. 1994;24:1468–1474. doi: 10.1016/0735-1097(94)90141-4. [DOI] [PubMed] [Google Scholar]
- 31.Joannides R, Haefeli WE, Linder L, et al. Nitric oxide is responsible for flow-dependent dilatation of human peripheral conduit arteries in vivo. Circ. 1995 Mar 1;91:1314–1319. doi: 10.1161/01.cir.91.5.1314. [DOI] [PubMed] [Google Scholar]
- 32.Vanhoutte PM, Shimokawa H, Tang EH, Feletou M. Endothelial dysfunction and vascular disease. Acta physiologica. 2009 Jun;196(2):193–222. doi: 10.1111/j.1748-1716.2009.01964.x. [DOI] [PubMed] [Google Scholar]
- 33.Landmesser U, Hornig B, Drexler H. Endothelial function: a critical determinant in atherosclerosis? Circulation. 2004 Jun 1;109(21 Suppl 1):II27–II33. doi: 10.1161/01.CIR.0000129501.88485.1f. [DOI] [PubMed] [Google Scholar]
- 34.Peake J, Suzuki K. Neutrophil activation, antioxidant supplements and exercise-induced oxidative stress. Exerc Immunol Rev. 2004;10:129–141. [PubMed] [Google Scholar]
- 35.Walther G, Nottin S, Karpoff L, Perez-Martin A, Dauzat M, Obert P. Flow-mediated dilation and exercise-induced hyperaemia in highly trained athletes: comparison of the upper and lower limb vasculature. Acta physiologica. 2008 Jun;193(2):139–150. doi: 10.1111/j.1748-1716.2008.01834.x. [DOI] [PubMed] [Google Scholar]
- 36.Field T, Diego M, Hernandez-Reif M. Moderate pressure is essential for massage therapy effects. The International journal of neuroscience. 2010 May;120(5):381–385. doi: 10.3109/00207450903579475. [DOI] [PubMed] [Google Scholar]
- 37.Kaye AD, Kaye AJ, Swinford J, et al. The effect of deep-tissue massage therapy on blood pressure and heart rate. Journal of alternative and complementary medicine. 2008 Mar;14(2):125–128. doi: 10.1089/acm.2007.0665. [DOI] [PubMed] [Google Scholar]
- 38.Pyke KE, Dwyer EM, Tschakovsky ME. Impact of controlling shear rate on flow-mediated dilation responses in the brachial artery of humans. J Appl Physiol. 2004 Aug;97(2):499–508. doi: 10.1152/japplphysiol.01245.2003. [DOI] [PubMed] [Google Scholar]
- 39.Association AMT. 2012 Feb 5; [Google Scholar]
- 40.Hashimoto M, Kozaki K, Eto M, et al. Association of coronary risk factors and endothelium-dependent flow-mediated dilatation of the brachial artery. Hypertens Res. 2000 May;23(3):233–238. doi: 10.1291/hypres.23.233. [DOI] [PubMed] [Google Scholar]
- 41.Mittleman MA, Maclure M, Tofler GH, Sherwood JB, Goldberg RJ, Muller JE. Triggering of acute myocardial infarction by heavy physical exertion. Protection against triggering by regular exertion. Determinants of Myocardial Infarction Onset Study Investigators. N Engl J Med. 1993 Dec 2;329(23):1677–1683. doi: 10.1056/NEJM199312023292301. [DOI] [PubMed] [Google Scholar]
- 42.Arena R, Myers J, Forman DE, Lavie CJ, Guazzi M. Should high-intensity-aerobic interval training become the clinical standard in heart failure? Heart failure reviews. 2013 Jan;18(1):95–105. doi: 10.1007/s10741-012-9333-z. [DOI] [PMC free article] [PubMed] [Google Scholar]