Summary
Long distance cell-cell communication is essential for organ development and function. Whereas neurons communicate at long distances by transferring signals at sites of direct contact (i.e. at synapses), it has been presumed that the only way other cell types signal is by dispersing signals through extracellular fluid - indirectly. Recent evidence from Drosophila suggests that non-neuronal cells also exchange signaling proteins at sites of direct contact, even when long distances separate the cells. Here, we review contact-mediated signaling in neurons and discuss how this signaling mechanism is shared by other cell types.
Keywords: cytoneme, synapse, morphogen, neuron
Long distance cell-cell communication
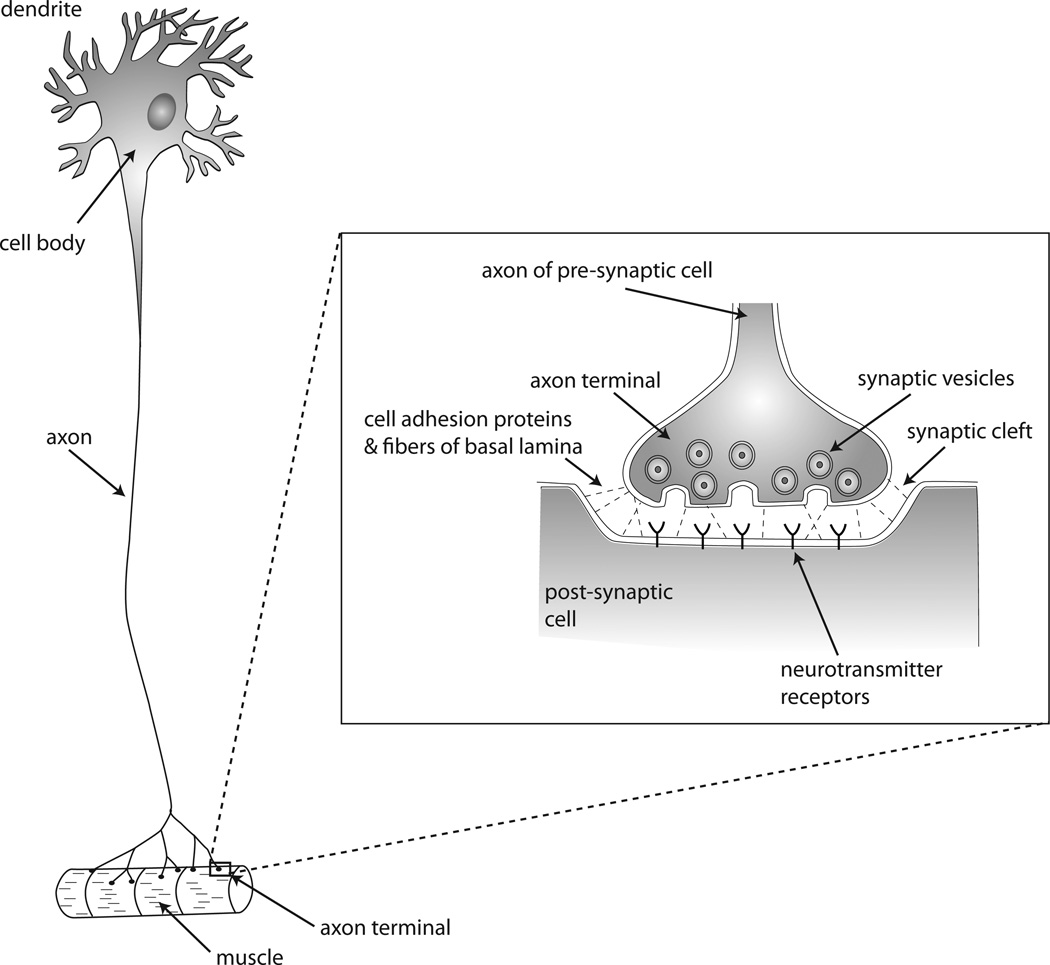
Cells employ many types of signals to coordinate growth and function. Some signals are released systemically and transmit information in the absence of direct cell-cell contact. For example, the hormone insulin distributes throughout the bloodstream, and cells respond similarly regardless if hormone is secreted by the pancreas or is injected intravenously. In contrast, other signals are exposed at the cell surface and convey information while tethered to the plasma membrane. These signals require cell-cell contact. The Notch signaling pathway is an example. Both the Notch receptor and its ligands are transmembrane proteins, and activation of its signal transduction pathway depends on the direct binding of their respective extracellular domains. Yet one of the enigmas of Notch signaling is that its effects are not limited to neighboring cells but can extend to cells that are more distant [reviewed in 1]. Thus, whereas long distance signaling is a general property of systemic signals, not all contact-dependent signaling is short-range. In addition, not all contact-dependent signaling is mediated by tethered signals. Neurons extend axons and dendrites that reach long distances over intervening cells and that focus neurotransmitter signaling to specialized contact sites known as synapses. Synaptic signaling requires release of neurotransmitters from the pre-synaptic cell, travel across the synaptic cleft, and binding to surface receptors (or channels) on the post-synaptic cell [Fig. 1; reviewed in 2].
Figure 1.
Diagram of a neuron that synapses with a muscle cell. The axon that extends from the neuron has specialized terminals that contact the target muscle cell via cell adhesion proteins and basal lamina connections. The synaptic cleft separates the pre- and post-synaptic cells at a defined, set distance, juxtaposing the axon terminal that contains many synaptic vesicles filled with neurotransmitters with the target cell membrane that concentrates neurotransmitter receptors.
Recent evidence indicates that long distance, contact-mediated signaling is not unique to neurons, but may be common to many cell types. The existence of cellular projections that extend from non-neuronal cells and that correlate with long distance signaling have been described in many contexts [3–16]. Recent work directly implicates a particular type of cellular projection called a cytoneme in long distance signaling [17]. Cytonemes are filopodia that are specialized for signaling [3], and new work shows that Drosophila cytonemes synapse with target cells to directly link communicating cells. Moreover, cytoneme synapses are essential for signal uptake and downstream signaling, suggesting a general mechanism for long distance signaling whereby signals exchange between communicating cells specifically and exclusively at specialized sites of contact. In this review, we compare signaling by neurons at axonal and dendritic synapses with signaling by non-neuronal cells at cytoneme synapses, and discuss the conceptual and structural similarities shared by these two types of cell-cell communication.
Information transfer by neurons
Neurons communicate with target cells by extending cellular processes (dendrites and axons) that make functional synapses, which either capture signals released from synaptic partners or deliver signals to them [reviewed in 2]. The distances that separate the cell body of the neuron and its target cells may be long, but by transporting signals through cell extensions, neurons direct information transfer specifically to the sites of direct cell-cell contact. This mechanism pre-selects signaling partners, ensuring specificity, and allowing both signal amplitude and duration to be controlled with exquisite precision.
Neuronal synapses are complex structures in which many cell adhesion proteins, extracellular matrix components, receptors, ion channels and other proteins involved in signal release and uptake are localized and organized. Although there are many types of neurotransmitters (e.g., small molecules such as acetylcholine and glutamate, ions, neurotrophins, and signaling protein such as Wnt [18, 19], transforming growth factor (TGF-β) [20–22], Hedgehog (Hh) [23], Epidermal Growth Factor (EGF) [24], or Fibroblast Growth Factor (FGF) [25]), all neurotransmitters signal by moving from pre- to post-synaptic cells. They are not simply released into extracellular fluid, but are placed specifically in the synaptic cleft, which is a privileged environment. Synaptic partners regulate the properties of the synaptic cleft so that neurotransmitter movement is constrained and half-life is controlled. Therefore, although neurotransmitters move across the synaptic cleft by diffusion, their dispersion is fundamentally different from that of hormones, whose dissemination is broad and essentially random. At synapses, exchange between a pre-synaptic cell and a post-synaptic cell is direct and between defined, pre-selected partners. Specifically, the way information is transferred during this exchange is the key attribute of neuronal signaling that is relevant to this discussion, and not the particulars of neuronal patterning, circuitry or signal processing, the stability of synapses or the directionality of signaling, the pathfinding process that establishes synaptic contacts or the cell biological mechanisms that generates them, or the process of electrical transmission [reviewed in 2].
Because neurons focus neurotransmitter release to synapses rather than disperse it either by a general extracellular route or systemically, they do not leave to chance which cells, or even which receptors in a cell, will respond to neurotransmitter. The mechanism that axons employ to transmit the information over long distances (i.e., the propagation of electrical impulses) may be remarkable, and electrical transmission may be a defining feature of neurons, but the key point is that information transfer between distant neurons is juxtacrine at synaptic contacts. This feature conveys information efficiently so that regardless of the distances between the cell bodies of contacting cells, signaling is controlled temporally and spatially [reviewed in 2].
Long distance signaling in non-neuronal tissues
The non-cell autonomous activity of signaling proteins that “act at a distance” was inferred from the properties of developmental organizers/signaling centers first described over 100 years ago [26–29]. For many years, the “inducer” molecules responsible for organizer activity were assumed to be small organic molecules that move freely in and out of cells, and that once released from producing cells, diffuse across a developmental field [30]. The discovery that inducers are signaling proteins (morphogens) did not change the general perception that these signals act non-autonomously by diffusing in extracellular fluid after release from producing cells. The fact that signaling proteins have signal peptide sequences has been equated with unregulated release, and the non-autonomous functionality of signaling proteins and their presence at considerable distances from sites of production have been interpreted to support an extracellular route of dispersion. However, the evidence suggesting an extracellular presence remains indirect, and is based either on kinetics of dispersion and mathematical modeling, which can only establish correlations, or on patterns of distribution that did not distinguish between protein tethered to cells from protein free of cells in the extracellular space [31–37]. Recent experimental evidence now shows that morphogens can transfer at points of direct contact and can be transported long distances along cytonemes.
The development and function of many (and perhaps all) tissues are regulated by signaling proteins such as TGF-β, Wnt, Hh, EGF and FGF. The current understanding is that discrete signaling centers in each tissue produce and secrete morphogens to act on cells in the local target field. The question therefore arises how a signaling center communicates exclusively with its target field in the context of closely juxtaposed tissues, each of which have separate and independent signaling centers that employ the same signals. An example in the Drosophila wing imaginal disc is the TGF-β family member Dpp, which signals to cells across the epithelial layer where Dpp is expressed, but not to cells in a separate layer but closer than most of the target cells [38]. The mechanism specifically directing morphogens to their intended targets appears to be similar to the mechanism neurons employ by focusing signals through contact. We cite three examples of non-neuronal long distance, contact-dependent signaling below.
Signaling between the wing disc and the air sac primordium in Drosophila
The Drosophila air sac primordium (ASP) is a branch of the tracheal system that grows across the basal surface of the wing disc epithelium (Fig. 2). The ASP of the late third instar larva is a tube consisting of approximately 120 cells arranged as a single-layered epithelium surrounding a lumen. At this stage, FGF expressed by a small group of wing disc cells induces the ASP to bud from an existing tracheal branch, and subsequent ASP growth and morphogenesis are dependent on Dpp and FGF produced by the disc [17, 39]. The distances between wing disc cells that express FGF or Dpp and the responding cells in the ASP vary as the ASP and disc grow, but separation can be as great as 40 µm. Yet, although signaling can extend over many cells (the diameter of a wing disc cell is ≤2.2 µm in a late third instar disc), signaling from the disc to the ASP is contact-dependent [17].
Figure 2.
Cytonemes link signal-producing cells in the wing disc to the ASP. (A) The wing imaginal disc is bound to a branch of the tracheal system (green); the Dpp (blue) it produces along the A/P compartment border and the FGF (blredue) it produces in a group of cells near the wing blade primordium control the development of the ASP. (B, C) Expression of CD8:GFP in the ASP marks cytonemes that extend out from the tip toward FGF-expressing wing disc cells (red, marked by bnl>LacZ in (C)) and from the medial region (arrow) toward Dpp-expressing disc cells. (D) GRASP fluorescence marks contacts that ASP tip cytonemes (labeled with mCherry:CAAX) make with FGF-expressing disc cells; the CD4:GFP11 was expressed in the ASP and the CD4:GFP1-10 was expressed in the FGF domain. (E) Anti-pERK antibody (red) marks cells at the tip of the ASP that extend cytonemes toward FGF-expressing cells, receive FGF from these cells, and activate FGF signal transduction. Anti-Discs large antibody (green) marks membranes of ASP cells.
The ASP extends several hundred cytonemes toward the wing disc, many of which contact disc cells that express Dpp or FGF. These contacts can be marked by GRASP (GFP Reconstitution Across Synaptic Partners) fluorescence [40], a technique that places complementary fragments of GFP on the external surface of apposing cells and generates fluorescence if the distance between the cells is spanned and if the contact time is sufficient for GFP to fold. GRASP is an effective way to map synaptic connections in neuronal networks [40]; it also identifies cytoneme contacts at which the Dpp and FGF signaling proteins transfer between signaling cells [17]. Neuronal synapses juxtapose pre- and post-synaptic membranes at approximately 20 nm and are long-lived. GRASP fluorescence at the tips of ASP cytonemes indicates that these cytonemes juxtapose ASP and disc cells at similar distances and are sufficiently stable for GFP to fold.
Some of the proteins that assemble neuronal synapses are also present and required at cytoneme contacts, and both types of contact enable cell-cell communication. Diaphanous, (Dia; an actin-binding formin [41]), Shibire (Shi; a dynamin [42]), Neuroglian, (Nrg; an L1-CAM [43]), and Capricious (Caps; an LRR transmembrane protein [44]) are essential for neuronal synapses [45–49] and for cytoneme-mediated signaling [17]. Nrg, Caps and activated Dia concentrate at cytoneme tips, and whereas all four proteins are required by ASP cytonemes, their roles are not the same [17]. The number of ASP cytonemes declines in cells that lack functional Dia, Shi or Nrg, indicating that ASP cells require them to make or stabilize cytonemes. In contrast, ASP cytonemes are not reduced in the absence of Caps in the ASP, but contact-dependent GRASP fluorescence declines. Furthermore, both the uptake of signaling protein and signaling are reduced, suggesting that signaling in these ASPs is defective despite the normal state of the disc cells that produce the proteins. Collectively, these findings indicate that information transfer between the disc and ASP depends on cytoneme contacts, and signaling in these ASPs is defective despite the normal state of the disc cells that produce the signaling proteins. Although electron microscopy images of cytoneme contacts have not been obtained, the structural and functional relations to neurons and neuronal synapses – shared components, similar distance of membrane juxtaposition, contact-associated information exchange – suggest that cytoneme contacts could be considered a type of signaling synapse.
Cytonemes pattern bristles in Drosophila
Cytonemes may also be responsible for delivering signals associated with patterning the mechanosensory bristles on the dorsal thorax of the fly (Fig. 3). These bristles are spaced at an approximate density of 1/4.5 cells by a process called “lateral inhibition” [50]. Each bristle forms from a single precursor cell in the epithelial sheet that develops into the thoracic epidermis. Although many of the epithelial cells are competent to adopt the bristle fate, Notch-Delta signaling prevents most from doing so: Notch-Delta signaling inhibits over 20 cells surrounding each bristle precursor cell from adopting the bristle fate. In theory, a diffusing signal secreted by a bristle-precursor cell could control the fate of the neighboring cells, but as noted above, Notch and Delta are transmembrane proteins, and the soluble form of Delta lacks biological activity [51, 52]. Several observations indicate that Notch-Delta signaling is likely to be mediated by direct contact via cytonemes [10, 53].
Figure 3.
Dynamic cytonemes appear to mediate lateral inhibition and Notch-Delta signaling. Cells that give rise to mechanosensory organs of the Drosophila thorax are depicted in purple; they extend dynamic cytonemes in a “sunburst” pattern, appearing to contact cells in the epithelial sheet as far as 4–5 cell diameters away and to mediate Notch signaling.
Bristle precursors extend cytonemes [53, 54] whose presence is dependent on Delta [10, 55] and whose length and lifetime tightly correlate with both the pattern and density of bristles [10]. In addition, Delta is present in the cytonemes of the bristle precursors [55], and although the presumptive contacts between these cytonemes and their putative target cells have not been observed or characterized, the Notch extracellular domain has 36 tandem EGF domains and extends approximately 25 nm [56], a distance that could span the gap at a neuronal or cytoneme synapse.
Oriented cell protrusions in the Drosophila leg disc epithelium
A role for long distance, contact mediated signaling was recently found in the Drosophila leg imaginal disc, where patterning requires signaling that is both selective and spatially biased (Fig. 4). Portions of the Drosophila adult leg cuticle are populated by ordered rows of leg sensory organs (LSOs), each of which has a single sensory bristle that is surrounded by a socket cell. There is also a short, pointed structure at the base of each bristle called a bract. Like a bristle, a bract is an outgrowth of a single cell, which is recruited to the LSO from the group of epithelial cells surrounding each socket cell. EGF signaling by a socket cell induces a bract cell fate in a nearby cell. Although as many as eight neighbors are competent to make a bract, only one does – the one to its proximal side. This choice of cell fate is dependent on polarized cellular protrusions that extend from the socket cell to envelop partially its most proximal neighbor. Although the shape of these protrusions is different from filopodia, like cytonemes, they are actin-rich and require the formin Dia. They also appear to be responsible for directional delivery of a signaling protein and are associated with asymmetric, spatially biased EGF signaling [9].
Figure 4.
Oriented cell protrusions direct EGF signaling in the leg disc epithelium. (A) Socket cells (purple) in the leg disc epithelium produce oriented protrusions that activate EGF signal transduction proximally and induce the bract fate in only one cell (brown) among the surrounding cells (light brown) that are competent to respond to EGF and develop into a bract. (B) A short, thick, pigmented and pointed structure called a bract is on the proximal side of Drosophila leg bristles.
Lessons from Drosophila
Taken together, it is clear that cell contact-dependent morphogen signaling is different from the diffuse nature of hormonal signaling. In hormonal signaling, different cell types can respond in distinct ways if their signal transduction components and chromatin states are cell type-specific [reviewed in 57], but the hormone’s spatial distribution is systemic and effectively uniform. In contrast, the specificity of the morphogen signaling proteins appears to be achieved by physically pairing particular cells. Therefore, in contexts in which many cells in the locale of the signal source are equipped to respond, only some are activated. In the ASP and LSO, oriented cell processes promote oriented signaling by linking specific cells for the directed exchange of signaling proteins. For bristle patterning of the fly thorax, bristle density is dependent on signaling by membranetethered proteins and on cytonemes that juxtapose membranes of cells that may not be nearest neighbors. These mechanisms, which focus signaling to sites of direct contacts, has direct parallels with neuronal signaling, in which neurons also direct and constrain signaling to post-synaptic cells.
Concluding remarks
In the human, over half of the 100 billion neurons in the central nervous system release the neurotransmitter glutamate into synaptic gaps, and almost all neurons express receptors that are sensitive to glutamate [58]. Signaling specificity requires targeting and spatial localization that widespread diffusion of extracellular signals cannot provide. Neurons in a section sliced from a brain can generate chemical and electrophysiological responses to neurotransmitter released from a pipette, but this does not imply that neurotransmitter receptors at synapses normally respond to extra-synaptic inputs. Similarly, morphogens that diffuse from polystyrene beads can activate responses in distant cells, but this also does not imply that unregulated release into extracellular space is the normal route of dissemination. The same issue pertains to cultured cells, which may release proteins into culture medium that may normally move between cells in the restricted environment of a synapse in vivo [19]. Furthermore, in the context of genetically engineered production of signaling proteins, over-expression may overwhelm a cell’s available capacity for regulated release [59]. Both neurons and non-neuronal cells make asymmetric extensions that synapse with distant cells to mediate information transfer, and both processes require the specificity and control that direct contact provides.
The established need for cell-cell contact for signaling by both neurons and non-neuronal cells suggests a relationship between the cellular processes that are responsible for this signaling. Axons are large, complex structures that contain microtubule cores and can be very long. The pathfinding process that mates them to their targets involves a complex growth cone with dynamic growth cone filopodia. Although cytonemes differ in many respects – length, diameter, microtubule vs actin core, growth cone specializations, stability, and electrophysiology [2, 4, 16, 17] – the kinship between signaling at axonal and cytoneme synapses is arguably significant. As noted above, neuronal synapses stably juxtapose pre- and postsynaptic cells at ≤20 nm, and the GRASP fluorescence at cytoneme contacts indicates that cytoneme and neuronal synapses juxtapose apposing membranes at similar distances [17]. Moreover, genetic and molecular studies suggest that neurons and cytonemes use some of the same proteins to make functional contacts [17]. These observations establish that the synapses at the tips of axons and the synapses of cytonemes are created in similar ways, possess common components, and juxtapose the plasma membranes of signaling cells at comparable distances. Importantly, they are both sites of cell-cell communication. The key functional feature of a neuronal synapse is that information transfer is restricted to a pre-specified site, and the cytoneme synapse appears to play a similar role for non-neuronal cells. Both types of synapses endow the communicating cells with the ability to target signaling to specific sites.
In neurobiology, questions about development (pathfinding and synapse generation), function (signal capture, propagation and processing) and network circuitry are considered independently. It is not known how these attributes evolved, but the existence of contact-dependent signaling in other cell types and the kinship with cytoneme synapses suggests that the complex functionalities of neurons may have evolved from a simpler cytoneme-like structure. We do not yet understand how cytonemes find their targets or convey signals to enable quantitative and (perhaps) temporal control of signaling, but it is now established that cytonemes make specific contacts that mediate signal exchange. Therefore, despite our current state of unawareness, the critical take home message is that both non-neuronal cells and neurons use the same mode of long distance signaling – contact-dependent exchange.
Highlights.
Cytonemes synapse with cells they target for signal exchange.
Signal exchange is cytoneme, axonal and dendritic synapses is analogous.
Contact-dependent signal exchange is not unique to neurons.
Acknowledgments
The authors thank Chris Doe, Larry Zipursky, Markus Noll and Valerie Wallace for comments on the manuscript. Research received funding from NIH: R01GM030637 (to T.B.K.) and K99HL114867 (to S.R.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Axelrod JD. Delivering the lateral inhibition punchline: it's all about the timing. Sci Signal. 2010;3:pe38. doi: 10.1126/scisignal.3145pe38. [DOI] [PubMed] [Google Scholar]
- 2.Kandel ER, et al. Principles of Neural Science. McGraw-Hill Medical; 2013. [Google Scholar]
- 3.Ramirez-Weber FA, Kornberg TB. Cytonemes: cellular processes that project to the principal signaling center in Drosophila imaginal discs. Cell. 1999;97:599–607. doi: 10.1016/s0092-8674(00)80771-0. [DOI] [PubMed] [Google Scholar]
- 4.Hsiung F, et al. Dependence of Drosophila wing imaginal disc cytonemes on Decapentaplegic. Nature. 2005;437:560–563. doi: 10.1038/nature03951. [DOI] [PubMed] [Google Scholar]
- 5.Roy S, et al. Specificity of Drosophila cytonemes for distinct signaling pathways. Science. 2011;332:354–358. doi: 10.1126/science.1198949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Callejo A, et al. Dispatched mediates Hedgehog basolateral release to form the long-range morphogenetic gradient in the Drosophila wing disk epithelium. Proc Natl Acad Sci U S A. 2011;108:12591–12598. doi: 10.1073/pnas.1106881108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rojas-Rios P, et al. Cytoneme-mediated delivery of hedgehog regulates the expression of bone morphogenetic proteins to maintain germline stem cells in Drosophila. PLoS Biol. 2012;10:e1001298. doi: 10.1371/journal.pbio.1001298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McClay DR. The role of thin filopodia in motility and morphogenesis. Exp Cell Res. 1999;253:296–301. doi: 10.1006/excr.1999.4723. [DOI] [PubMed] [Google Scholar]
- 9.Peng Y, et al. Planar polarized protrusions break the symmetry of EGFR signaling during Drosophila bract cell fate induction. Dev Cell. 2012;23:507–518. doi: 10.1016/j.devcel.2012.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen M, et al. Dynamic filopodia transmit intermittent Delta-Notch signaling to drive pattern refinement during lateral inhibition. Dev Cell. 2010;19:78–89. doi: 10.1016/j.devcel.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 11.Fifadara NH, et al. Interaction between activated chemokine receptor 1 and FcepsilonRI at membrane rafts promotes communication and F-actin-rich cytoneme extensions between mast cells. International immunology. 2010;22:113–128. doi: 10.1093/intimm/dxp118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galkina SI, et al. Nitric oxide-induced membrane tubulovesicular extensions (cytonemes) of human neutrophils catch and hold Salmonella enterica serovar Typhimurium at a distance from the cell surface. FEMS Immunol Med Microbiol. 2009;56:162–171. doi: 10.1111/j.1574-695X.2009.00560.x. [DOI] [PubMed] [Google Scholar]
- 13.Gupta N, DeFranco AL. Visualizing lipid raft dynamics and early signaling events during antigen receptor-mediated B-lymphocyte activation. Mol Biol Cell. 2003;14:432–444. doi: 10.1091/mbc.02-05-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koizumi K, et al. RhoD activated by fibroblast growth factor induces cytoneme-like cellular protrusions through mDia3C. Mol Biol Cell. 2012;23:4647–4661. doi: 10.1091/mbc.E12-04-0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lidke DS, et al. Reaching out for signals: filopodia sense EGF and respond by directed retrograde transport of activated receptors. J Cell Biol. 2005;170:619–626. doi: 10.1083/jcb.200503140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bischoff M, et al. Cytonemes are required for the establishment of a normal Hedgehog morphogen gradient in Drosophila epithelia. Nature cell biology. 2013;15:1269–1281. doi: 10.1038/ncb2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roy S, et al. Cytoneme-mediated contact-dependent transport of the Drosophila Decapentaplegic signaling protein. Science. 2014 doi: 10.1126/science.1244624. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hall AC, et al. Axonal remodeling and synaptic differentiation in the cerebellum is regulated by WNT-7a signaling. Cell. 2000;100:525–535. doi: 10.1016/s0092-8674(00)80689-3. [DOI] [PubMed] [Google Scholar]
- 19.Korkut C, et al. Trans-synaptic transmission of vesicular Wnt signals through Evi/Wntless. Cell. 2009;139:393–404. doi: 10.1016/j.cell.2009.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henriquez JP, et al. The Wnt and BMP families of signaling morphogens at the vertebrate neuromuscular junction. Int J Mol Sci. 2012;12:8924–8946. doi: 10.3390/ijms12128924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Williamson WR, Hiesinger PR. Synaptic patterning by morphogen signaling. Sci Signal. 2008;1:pe20. doi: 10.1126/stke.118pe20. [DOI] [PubMed] [Google Scholar]
- 22.Salinas PC. Signaling at the vertebrate synapse: new roles for embryonic morphogens? J Neurobiol. 2005;64:435–445. doi: 10.1002/neu.20159. [DOI] [PubMed] [Google Scholar]
- 23.Huang Z, Kunes S. Hedgehog, transmitted along retinal axons, triggers neurogenesis in the developing visual centers of the Drosophila brain. Cell. 1996;86:411–422. doi: 10.1016/s0092-8674(00)80114-2. [DOI] [PubMed] [Google Scholar]
- 24.Yogev S, et al. Polarized secretion of Drosophila EGFR ligand from photoreceptor neurons is controlled by ER localization of the ligand-processing machinery. PLoS Biol. 2010;8 doi: 10.1371/journal.pbio.1000505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Umemori H, et al. FGF22 and its close relatives are presynaptic organizing molecules in the mammalian brain. Cell. 2004;118:257–270. doi: 10.1016/j.cell.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 26.Morgan TH. An attempt to analyze the phenomena of polarity in Tubularia. J. Exp. Zool. 1904;1:587–591. [Google Scholar]
- 27.Browne NE. The production of new hydranths in Hydra by the insertion of small grafts. J Exp. Zool. 1909;7:1–23. [Google Scholar]
- 28.Lenhoff HM. Ethel Browne, Has Spemann, and the Discovery of the Organizer Phenomenon. Biol. Bull. 1991;181:72–80. doi: 10.2307/1542490. [DOI] [PubMed] [Google Scholar]
- 29.Spemann H, Mangold H. Über Weckung organisatorischer Fähigkeiten durch Verplanzung in organisatorische Umgebung. Roux's Arch. 1924;109:557–577. doi: 10.1007/BF02079696. [DOI] [PubMed] [Google Scholar]
- 30.Crick F. Diffusion in embryogenesis. Nature. 1970;225:420–422. doi: 10.1038/225420a0. [DOI] [PubMed] [Google Scholar]
- 31.Entchev EV, et al. Gradient formation of the TGF-beta homolog Dpp. Cell. 2000;103:981–991. doi: 10.1016/s0092-8674(00)00200-2. [DOI] [PubMed] [Google Scholar]
- 32.Kicheva A, et al. Kinetics of morphogen gradient formation. Science. 2007;315:521–525. doi: 10.1126/science.1135774. [DOI] [PubMed] [Google Scholar]
- 33.Schwank G, et al. Formation of the long range dpp morphogen gradient. PLoS Biol. 2011;9:e1001111. doi: 10.1371/journal.pbio.1001111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Teleman AA, Cohen SM. Dpp gradient formation in the Drosophila wing imaginal disc. Cell. 2000;103:971–980. doi: 10.1016/s0092-8674(00)00199-9. [DOI] [PubMed] [Google Scholar]
- 35.Toyoda R, et al. FGF8 acts as a classic diffusible morphogen to pattern the neocortex. Development. 2010;137:3439–3448. doi: 10.1242/dev.055392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu SR, et al. Fgf8 morphogen gradient forms by a source-sink mechanism with freely diffusing molecules. Nature. 2009;461:533–536. doi: 10.1038/nature08391. [DOI] [PubMed] [Google Scholar]
- 37.Zhou S, et al. Free extracellular diffusion creates the dpp morphogen gradient of the Drosophila wing disc. Curr Biol. 2012;22:668–675. doi: 10.1016/j.cub.2012.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kornberg TB, Guha A. Understanding morphogen gradients: a problem of dispersion and containment. Current opinion in genetics & development. 2007;17:264–271. doi: 10.1016/j.gde.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sato M, Kornberg TB. FGF is an essential mitogen and chemoattractant for the air sacs of the drosophila tracheal system. Dev Cell. 2002;3:195–207. doi: 10.1016/s1534-5807(02)00202-2. [DOI] [PubMed] [Google Scholar]
- 40.Feinberg EH, et al. GFP Reconstitution Across Synaptic Partners (GRASP) defines cell contacts and synapses in living nervous systems. Neuron. 2008;57:353–363. doi: 10.1016/j.neuron.2007.11.030. [DOI] [PubMed] [Google Scholar]
- 41.Castrillon DH, Wasserman SA. Diaphanous is required for cytokinesis in Drosophila and shares domains of similarity with the products of the limb deformity gene. Development. 1994;120:3367–3377. doi: 10.1242/dev.120.12.3367. [DOI] [PubMed] [Google Scholar]
- 42.van der Bliek AM, Meyerowitz EM. Dynamin-like protein encoded by the Drosophila shibire gene associated with vesicular traffic. Nature. 1991;351:411–414. doi: 10.1038/351411a0. [DOI] [PubMed] [Google Scholar]
- 43.Bieber AJ, et al. Drosophila neuroglian: a member of the immunoglobulin superfamily with extensive homology to the vertebrate neural adhesion molecule L1. Cell. 1989;59:447–460. doi: 10.1016/0092-8674(89)90029-9. [DOI] [PubMed] [Google Scholar]
- 44.Shishido E, et al. Drosophila synapse formation: regulation by transmembrane protein with Leu-rich repeats, CAPRICIOUS. Science (New York, N.Y.) 1998;280:2118–2121. doi: 10.1126/science.280.5372.2118. [DOI] [PubMed] [Google Scholar]
- 45.Kohsaka H, Nose A. Target recognition at the tips of postsynaptic filopodia: accumulation and function of Capricious. Development (Cambridge, England) 2009;136:1127–1135. doi: 10.1242/dev.027920. [DOI] [PubMed] [Google Scholar]
- 46.Matusek T, et al. Formin proteins of the DAAM subfamily play a role during axon growth. J Neurosci. 2008;28:13310–13319. doi: 10.1523/JNEUROSCI.2727-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Milan M, et al. The LRR proteins capricious and Tartan mediate cell interactions during DV boundary formation in the Drosophila wing. Cell. 2001;106:785–794. doi: 10.1016/s0092-8674(01)00489-5. [DOI] [PubMed] [Google Scholar]
- 48.Murphey RK, et al. Targeted expression of shibire ts and semaphorin 1a reveals critical periods for synapse formation in the giant fiber of Drosophila. Development (Cambridge, England) 2003;130:3671–3682. doi: 10.1242/dev.00598. [DOI] [PubMed] [Google Scholar]
- 49.Hall SG, Bieber AJ. Mutations in the Drosophila neuroglian cell adhesion molecule affect motor neuron pathfinding and peripheral nervous system patterning. J Neurobiol. 1997;32:325–340. [PubMed] [Google Scholar]
- 50.Simpson P. Lateral inhibition and the development of the sensory bristles of the adult peripheral nervous system of Drosophila. Development. 1990;109:509–519. doi: 10.1242/dev.109.3.509. [DOI] [PubMed] [Google Scholar]
- 51.Mishra-Gorur K, et al. Down-regulation of Delta by proteolytic processing. J Cell Biol. 2002;159:313–324. doi: 10.1083/jcb.200203117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Parks AL, et al. Ligand endocytosis drives receptor dissociation and activation in the Notch pathway. Development. 2000;127:1373–1385. doi: 10.1242/dev.127.7.1373. [DOI] [PubMed] [Google Scholar]
- 53.Renaud O, Simpson P. scabrous modifies epithelial cell adhesion and extends the range of lateral signalling during development of the spaced bristle pattern in Drosophila. Dev Biol. 2001;240:361–376. doi: 10.1006/dbio.2001.0482. [DOI] [PubMed] [Google Scholar]
- 54.Lai EC, Rubin GM. neuralized functions cell-autonomously to regulate a subset of notch-dependent processes during adult Drosophila development. Dev Biol. 2001;231:217–233. doi: 10.1006/dbio.2000.0124. [DOI] [PubMed] [Google Scholar]
- 55.De Joussineau C, et al. Delta-promoted filopodia mediate long-range lateral inhibition in Drosophila. Nature. 2003;426:555–559. doi: 10.1038/nature02157. [DOI] [PubMed] [Google Scholar]
- 56.Kelly DF, et al. Molecular structure and dimeric organization of the Notch extracellular domain as revealed by electron microscopy. PloS one. 2010;5:e10532. doi: 10.1371/journal.pone.0010532. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 57.Burd CJ, Archer TK. Chromatin architecture defines the glucocorticoid response. Molecular and cellular endocrinology. 2013;380:25–31. doi: 10.1016/j.mce.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van der Zeyden M, et al. Microdialysis of GABA and glutamate: analysis, interpretation and comparison with microsensors. Pharmacology, biochemistry, and behavior. 2008;90:135–147. doi: 10.1016/j.pbb.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 59.Gallet A, et al. Cholesterol modification is necessary for controlled planar long-range activity of Hedgehog in Drosophila epithelia. Development. 2006;133:407–418. doi: 10.1242/dev.02212. [DOI] [PubMed] [Google Scholar]