Abstract
Objective
We hypothesized that aromatase inhibitor (AI)-induced interruption of estradiol negative feedback would modulate the reproductive hormone profile of obese women.
Design and Methods
Regularly cycling women aged 18–40 with a BMI of 18–25kg/m2 (normal weight, n=10) or >30kg/m2 (obese; n=12) were given AI daily for 7 days. Urinary hormone profiles were compared between groups. Fourteen eumenorrheic, normal weight women not receiving AI stimulation served as historical controls. Urinary metabolites for LH, FSH, estradiol (E1c), and progesterone (Pdg) were measured and normalized to a 28 day cycle. Serum estrone and estradiol were measured in the late follicular phase.
Results
Whole cycle LH, FSH and luteal Pdg excretion did not differ between obese (BMI= 37.1+7kg/m2) and normal weight women treated with AIs, although LH was greater in stimulated compared to unstimulated normal weight women. Whole cycle mean E1c was lower in AI stimulated obese and normal weight participants compared to non-stimulated normal weight controls, but obese women treated with AI excreted far less E1c (467.7±217.4ug/mgCr) than AI-treated normal weight women (911.4±361.8ug/mgCr; P=0.02). Follicular phase serum estrone and estradiol were also lower in AI-treated obese women vs. AI-treated normal weight women (61.7±22.8 and 18.3±3.7 pg/ml, versus 99.1±30.5 and 37.7±5.9 pg/ml, respectively; p=0.034 and 0.005).
Conclusions
Normal gonadotropin output and luteal function occur at the expense of reduced E1c excretion in AI-treated women, and this discrepancy is particularly evident in obese women.
Key Terms: Aromatase Inhibitors, Obesity, Estrogen Negative Feedback
Introduction
Female obesity is associated with menstrual cycle irregularities, ovulatory dysfunction, lower pregnancy rates, lower live birth rates, and higher miscarriage rates [1]. Ovulatory, obese women have a 4% decrease in fecundability with each unit increase in BMI [2]. Obesity’s effect on reproduction is thought to be, at least partially, secondary to effects on pituitary function. Increasing obesity, among ovulatory women, is associated with decreased LH pulse amplitude, lower follicular phase FSH, reduced whole menstrual cycle LH and progesterone excretion, and diminished estrogen excretion [3,4]. In anovulatory women with polycystic ovary syndrome (PCOS), increasing obesity is associated with lower LH pulse amplitude, despite an overall elevated LH in this condition [5]. With a growing obese population, it is becoming increasingly important to assess how obesity affects reproduction, how we can assist obese women in achieving their reproductive goals, and how we can mitigate the consequences of obesity on the subsequent pregnancy and offspring.
Aromatase inhibitors (AI), such as letrozole, are becoming increasingly popular for treatment of reproductive dysfunction. It is believed that AIs act by interrupting estrogen negative feedback and causing an increase in gonadotropins, thereby inducing or enhancing ovulation. Although AI-treated, normal weight women exhibit increased LH and increase LH pulse amplitude [6], no studies of AI treatment in spontaneously ovulating obese women have been performed to date. Since the deficit in LH pulse amplitude is seen in obesity [3,5] and because of the observed AI effect on gonadotropin output, we sought to assess the effect of interruption of estradiol negative feedback by AI in female obesity. We hypothesized that opening the negative feedback loop of estrogen in obese women would improve their hormone profile.
Material and Methods
We studied daily, first-morning voided urine hormone patterns in regularly cycling, ovulatory, obese women stimulated with letrozole in the early follicular phase and compared these findings to normal weight women who received similar AI and a group of normal weight women who did not receive any ovulation stimulation medication who served as historical controls [7, 8].
Participants
Twenty-two eumenorrheic women with no evidence of polycystic ovary syndrome were recruited and completed the study. Polycystic ovary syndrome was prospectively ruled out because all participants were required to have regular menstrual cycles between 25–35 days in length, we used the NIH definition of PCOS, which includes oligoamenorrhea as a central criterion [9]. Inclusion criteria were: age 18–40 at enrollment; BMI of 18–25 kg/m2 (normal weight) or greater than 30 kg/m2 (obese); regular menstrual cycles of 25–35 days; no evidence of chronic diseases known to affect reproductive hormones; normal TSH and prolactin; and no use of medications known to alter or interact with reproductive hormones. Women were excluded for excessive exercise, which was defined as greater than 4 hours per week. An additional 14 eumenorrheic, women who had not received any ovulation enhancing medication with a BMI of 18–25kg/m2 who underwent the same daily urine collection methods and whose data have been previously reported [3,7,8] were used as a comparison group. Participants were recruited by local campus-wide postings and email study advertisements. All participants provided written informed consent for their participation and the study was approved by the Institutional Review Boards of Albert Einstein College of Medicine and the University of Colorado School of Medicine’s Combined IRB (COMIRB).
Protocol
Each of the 12 obese and 10 normal weight women underwent AI with either 2.5 or 5mg of letrozole based upon body surface area [10]. Six obese women received 5 mg of letrozole, all other participants received the 2.5 mg dose. Letrozole was initiated on cycle day 2–5 and given for a total of 7 days. Daily, first-morning voided urine was collected over one entire menstrual cycle using previously described methodology [3,7]. Following the last day of letrozole administration, serum was obtained for estradiol, estrone, testosterone and progesterone. Daily urine samples were assayed for LH, FSH, estrone conjugates (E1c) and pregnanediol glucuronide (Pdg). Urinary LH and FSH were measured using a DELFIA immunofluorometric assay platform and E1c and Pdg were measured using an in house ELISA. All hormones were normalized to creatinine as described [3,7,8]. The interassay and intra-assay coefficients of variation were: for LH 7.7% and 4.6%, respectively; for FSH 7.3% and 4.1%, respectively, for E1c 9.3% and 3.1%, respectively; for Pdg 16.8% and 6.9%, for E2 10.6% and 3.7%, respectively; for E1 11.7% and 6.4%, respectively.
Statistical Analysis
Urinary LH, FSH, E1c, and Pdg concentrations were normalized to a 28 day cycle. Daily concentrations were evaluated using transverse sums and means across the entire menstrual cycle and in the follicular and luteal phases. Luteal activity and day of luteal transition were calculated using a previously validated algorithm that allowed us to determine whether ovulation had occurred and the likely day of ovulation [4]. Using this algorithm, luteal activity was first confirmed by identifying the Pdg nadir in the first 5 samples after menses and detecting a sustained, 3-fold rise from the nadir [3,5,7,8]. The day of luteal transition was calculated by a 60% drop in the E1c/Pdg ratio prior to the Pdg peak [4]. These criteria were then used to center the data for graphical representation (to the day of the luteal transition) and to allow for calculation of transverse sums across the follicular and luteal phases. Statistical analyses were performed using STATA and group means were compared as appropriate by ANOVA with Scheffe post hoc testing and Kruskal-Wallis tests for non-normally distributed data. A P value of 0.05 or less indicated statistical significance. Unless otherwise indicated, data are expressed as mean +/− SD. For serum hormones, group comparisons were performed using t testing or Mann Whitney when the data were not normally distributed.
The sample size was calculated based upon previous work indicating a large deficit in urinary Pdg excretion in obese women (3,10). We hypothesized that AI treatment would normalize Pdg excretion to 80% of that seen in non-obese women.
Results
Baseline characteristics of the study samples are summarized in Table 1. Transverse summed urinary hormones are presented in Table 2. All cycles were verified to be ovulatory by the Pdg algorithm criteria [4]. Whole cycle transverse summed FSH did not differ among the 3 groups (Table 2). Whole cycle transverse summed LH did not differ between the normal weight and obese AI treated women, but was significantly lower in the normal weight women who were not treated with any ovulation enhancing medication compared to normal weight, AI treated women (P=0.03; Table 2). Whole cycle Pdg was similar between both obese and normal weight AI treated women. However, the normal weight women who did not receive any ovulation enhancement medication had an overall higher whole cycle Pdg compared to normal weight, AI treated women (P<0.01). When luteal Pdg was examined separately, these group differences persisted (data not shown).
Table 1.
Baseline Characteristics of the Study Sample
| Weight | Rx | BMI (kg/m2) | Age (Y) | Cycle (Days) | T (nmol/L) |
|---|---|---|---|---|---|
| Obese | AI | 35.8 | 35 | 33 | 1.2 |
| Obese | AI | 36.5 | 24 | 28 | 1.4 |
| Obese | AI | 35.1 | 30 | 26 | 0.4 |
| Obese | AI | 32.3 | 30 | 50 | 0.7 |
| Obese | AI | 30.5 | 25 | 29 | 2.5 |
| Obese | AI | 36.5 | 30 | 29 | 1.5 |
| Obese | AI | 32.5 | 32 | 29 | 2.0 |
| Obese | AI | 34.9 | 28 | 31 | 1.3 |
| Obese | AI | 56.2 | 39 | 28 | 1.0 |
| Obese | AI | 45.0 | 33 | 31 | 1.6 |
| Obese | AI | 33.2 | 33 | 31 | |
| Obese | AI | 36.9 | 27 | 29 | |
| Normal | AI | 21.7 | 27 | 26 | 1.7 |
| Normal | AI | 23.6 | 28 | 27 | 2.9 |
| Normal | AI | 20.8 | 24 | 29 | 1.9 |
| Normal | AI | 23.3 | 31 | 23 | 0.8 |
| Normal | AI | 20.4 | 40 | 28 | 0.6 |
| Normal | AI | 19.7 | 31 | 28 | 2.0 |
| Normal | AI | 21.0 | 34 | 34 | |
| Normal | AI | 21.1 | 24 | 27 | |
| Normal | AI | 20.7 | 33 | 28 | |
| Normal | AI | 19.8 | 37 | 25 | |
| Normal | -- | 22.3 | 34 | 22 | |
| Normal | -- | 22.2 | 28 | 30 | |
| Normal | -- | 18.5–24.9 | 2826 | ||
| Normal | -- | 18.5–24.9- | 3826 | ||
| Normal | -- | 18.5–24.9- | 2937 | ||
| Normal | -- | 20.2 | 35 | 25 | |
| Normal | -- | 18.5–24.9-- | 2930 | ||
| Normal | -- | 18.5–24.9-- | 3921 | ||
| Normal | -- | 20.1 | 26 | 30 | |
| Normal | -- | 21.6 | 26 | 30 | |
| Normal | -- | 21.2 | 27 | 34 | |
| Normal | -- | 19.9 | 29 | 28 | |
| Normal | -- | 20.5 | 35 | 28 | |
| Normal | -- | 25.1 | 33 | 28 |
Precise BMI data was not available for some of the historical controls, and testosterone is not available. Missing values indicate absence of a serum sample.
TABLE 2.
Whole Cycle, Transverse-Summed Urine Hormone Metabolites
| Parameter | Obese-AI (O-AI) N=12 |
Normal weight-AI (N-AI) N=10 |
Normal weight Unstimulated (N-U) N=14 |
P values | |||
|---|---|---|---|---|---|---|---|
| overall | O-AI vs. N-AI | N-AI vs. N-U | O-AI vs. N-U | ||||
| BMI, kg/m2 | 37.1±7 | 21.2±1.3 | 21.5±1.6 | -- | <0.01 | NS | <0.01 |
| Age, years | 30.5±4.3 | 30.9±1 | 31.7±4.6 | 0.76 | -- | -- | -- |
| Pdg, ug/mg Cr | 54.1±33.0 | 88.7±64.6 | 157.4 ± 114.2 | <0.01 | 0.61 | 0.14 | 0.01 |
| E1c, ng/mg Cr | 467.7±217.4 | 911.4±361.8 | 1463.2 ± 422.4 | <0.01 | 0.02 | <0.01 | <0.01 |
| LH mIU/mg Cr | 242.3±144.3 | 335.2±165.7 | 164.0 ± 145.4 | 0.03 | 0.37 | 0.03 | 0.43 |
| FSH, mIU/mg Cr | 179.1±94.8 | 136.8±59.0 | 147.5 ± 52.6 | 0.35 | 0.23 | ||
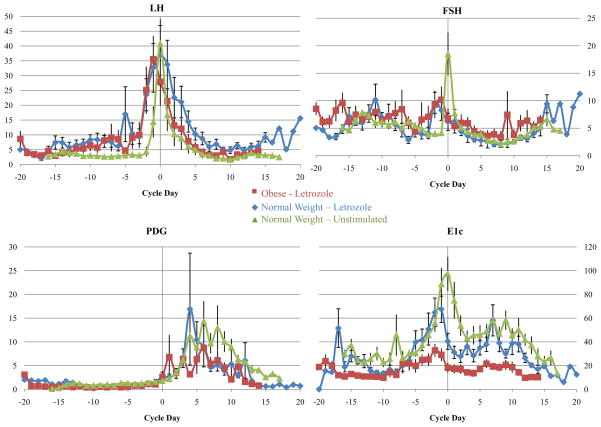
Whole cycle E1c excretion was dramatically reduced in AI-treated obese women compared to normal weight, AI-treated women (Table 2; Figure 1). Further, E1c excretion was found to be significantly decreased in both AI treated groups regardless of weight, compared to the historical, normal weight controls who did not receive any ovulation stimulation. The E1c pattern of obese women who received the higher dose of letrozole (5mg) did not differ from those who received the 2.5mg dose (P=0.4).
FIGURE 1.
Urinary hormone profiles in obese, AI treated women (n=12; red); normal weight, AI treated women (n=10; blue) and normal weight unstimulated historical controls (n=14; green).
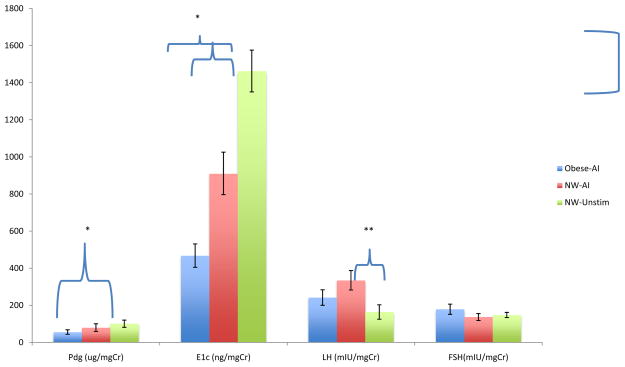
In order to clarify the unexpected decrement in urinary E1c in the obese women, we examined follicular phase serum estradiol and estrone from serum samples taken on the last day of letrozole administration, as part of a separate experiment. Both estradiol and estrone were significantly lower in obese AI-treated women compared to normal weight, AI treated women (Table 3). Obese women who received the higher dose of letrozole did not have significantly lower estradiol and estrone than those who received the lower dose. Testosterone and progesterone did not differ between these two groups (Table 3). Data are not available the normal weight women who did not receive ovulation enhancing medication. Figure 1 depicts daily mean urinary hormones for each group over the entire menstrual cycle, with day 0 as the day of luteal transition. Figure 2 is a pictorial display of the normalized transverse sum for each urinary metabolite, emphasizing the difference between the groups for E1c excretion.
Table 3.
Serum Follicular Phase Hormones in Aromatase Inhibitor-Treated Obese and Normal Weight Women
| Hormone | Normal Weight N=9 |
Obese N=10 |
P |
|---|---|---|---|
| Estradiol, pg/ml | 99.1±30.5 | 61.7±22.8 | 0.034 |
| Estrone, pg/ml | 37.7±5.9 | 18.3±3.7 | 0.005 |
| Testosterone, ng/dL | 42.3±6.9 | 48.5±5.5 | 0.8 |
| Progesterone, ng/ml | 0.32±0.06 | 0.21±0.03 | 0.07 |
FIGURE 2.
Transverse summed hormone concentrations +/− SEM throughout the menstrual cycle in obese, AI treated women (n=12; blue); normal weight, AI treated women (n=10; red) and normal weight unstimulated historical controls (n=14;green) demonstrating the large differences in E1c by group. Post-hoc differences between two groups are indicated by a bracket indicating the appropriate bars. *=P≤0.01; **=P<0.03
Discussion
Herein we demonstrated that interrupting estradiol negative feedback with aromatase inhibition results in similar hormone patterns in both obese and normal weight women. Following AI treatment, both obese and normal weight women demonstrated similar gonadotropin output and similar luteal progesterone excretion. The similarity of luteal function between obese and normal weight, AI-treated women suggests that AIs should be tested in treating the relative deficit in luteal progesterone production seen in obesity [3]. This creates a strong rational to conduct a study with untreated obese controls for comparison to definitely determine whether AI treatment improves luteal progesterone production.
Unexpectedly, this similar pattern of luteal progesterone excretion seen in both normal weight and obese AI treated women occurs in the face of drastically reduced E1c excretion in AI stimulated obese women compared to normal weight women (regardless of whether the normal weight women received an AI). E1c excretion was also reduced in both groups of AI-treated women regardless of BMI when compared to normal weight controls who did not receive any ovulation stimulation. These data suggest that the adiposity-related hypogonadotropic hypogonadal phenotype is at least partially receptive to modulation of estrogen negative feedback. We demonstrate dramatic increase in LH excretion after AI. This could be taken to mean that ovulatory, obese women appear to demonstrate sensitivity to interruption of estrogen negative feedback at the hypothalamic-pituitary level with AI treatment. Although we did not document a defect in luteal function in our obese sample of women before administering the AI, prior studies have consistently observed decrements in luteal Pdg when BMI is above 25 kg/m2[11,12]. E1c is also known to be lower in obese women compared to normal weight women, but the overall differences are small and require large sample sizes to establish [11,13]. In one study of 23 obese women, overall E1c excretion in very obese women (mean BMI=47.3 kg/m2) was 80% of that of normal weight women, but was not significantly lower [13]. In a much larger sample of 627 women who were studied over an entire menstrual cycle, whole cycle E1c in women with a BMI≥30 kg/m2 was 93% that of women with a BMI <25 kg/m2 [11].
On the other hand, whole cycle Pdg was lower in the obese women treated with an AI compared to normal weight, historical controls who had not received an AI. These findings may be due to the lack of concurrent collection of this control group and assay variation over time. It may also reflect a relative lowering of luteal progesterone secretion in response to AI treatment that occurs regardless of BMI. However, the normal weight women who received an AI did not have significantly lower whole cycle transverse summed Pdg exretion. Examination of isolated luteal phase data resulted in the same conclusion. As letrozole recently been shown to be superior to clomiphene for ovulation induction in women with polycystic ovary syndrome and led to a 8% greater rate of live births (Legro, American Society for Reproductive Medicine 2013 Annual Meeting, Boston MA October 14–17, Abstract O-167), it does not appear that this effect, should it be specific to AIs, compromises fertility and the ability to have a child.
AIs prevent the conversion of testosterone/adrostenedione into estradiol/estrone by competitively binding to the cytochrome P450 of aromatase [14]. The very large decrease in E1c among the obese, AI treated women has not been previously observed. Estradiol and estrone circulate in similar molar quantities in the serum of reproductive aged women. After menopause, the ratio of estradiol to estrone shifts to favor estrone, especially in obese women, as bioconversion of estrogen precursors occur primarily in adipose tissue. Obese, postmenopausal women who are given AI for breast cancer have higher circulating estradiol and estrone and greater likelihood of recurrence compared to women of normal weight [15,16]. This may indicate that inhibition of estrone production by AIs is less efficient in obese women only in adipose tissue, since postmenopausal women have only an adipose source of endogenous estrogens. Our study examined premenopausal women who have abundant ovarian estradiol and estrone production. The ovarian contribution to circulating estrogen in young women is far greater than the corresponding adipose tissue contribution. Taken together, our data imply that the ability of AIs to inhibit estrone production may be tissue or substrate specific. We are unaware of any other data that bear on the specificity of AI to preferentially inhibit utilization of one estrogen substrate over another, and, as such, this consideration will be important to confirm in future studies.
Estrogen negative feedback occurs in the pituitary and there is reason to believe that AI exhibit measurable and specific alteration in hormonal output at the level of the pituitary [6]. We previously reported increased LH pulse amplitude in response to AIs in women [6]. We hereby reported similar patterns of urinary LH and FSH in obese and normal weight AI treated women. This suggests that there was an increase in both LH and FSH in the obese women in response to AIs that brought them into the normal range for unstimulated, normal weight women. It is of interest, however, that the normal weight women had similar FSH transverse sums regardless of whether or not an AI was given. Although the unstimulated women were not concurrently collected controls [3,7,8] and therefore firm conclusions may not be drawn from these data, the lack of differences in FSH excretion suggests that increased absolute concentrations of circulating FSH may not be a primary mode of action for AIs. It is possible that AIs cause FSH output to differ qualitatively, possibly by altering the biological activity of secreted isoforms.
Strengths of this study include the detailed, daily sampling paradigm, confirmation of ovulation in all participants, and the use of two control groups (AI stimulated and unstimulated normal weight women). We also took the precaution of adjusting the AI dose to body surface area to take into account the large variation in body size between the two weight ranges. Relative weaknesses of our experimental design include the lack of concurrently collected normal weight women who had not received any ovulation enhancing medications and an obese control group receiving no ovulation enhancement medication for optimal comparisons. The relatively small sample sizes we used limit the applicability of these findings to larger populations of women; nonetheless the level of detail with which we were able to examine our participants will inform future, larger scale studies of overall effectiveness of AIs on fertility outcomes.
Taken together, these findings suggest that AIs may have a therapeutic role in the treatment of the obese non-PCOS patient, because they normalize gonadotropins and were associated with a Pdg profile that did not differ from that of normal weight, similarly treated women. This implies a benefit on corpus luteum function and is particularly relevant in the studied population as obesity is one of the very few conditions with documented decreased luteal progesterone and corpus luteum insufficiency [3, 10,17]. Clinical trials are necessary to evaluate if these findings can be translated into improved fecundity and pregnancy outcomes.
What is already known about this subject?
Obesity is associated with subfertility and reproductive hormone alterations.
Among ovulatory women, obesity is associated with decreased LH pulse amplitude, lower follicular phase FSH, reduced whole menstrual cycle LH and progesterone excretion, and diminished estrogen excretion.
Aromatase inhibitors (AI) are becoming increasingly popular for treatment of reproductive dysfunction but mechanism of action is unclear.
What does this study add?
Whole cycle excretion of FSH and progesterone did not differ between obese and normal weight women treated with AIs, and were similar to non AI-treated, normal weight women. LH was greater in stimulated compared to unstimulated normal weight women.
Normal gonadotropin output and luteal function occur at the expense of reduced E1c excretion in AI-treated women.
Reduced estrogen metabolite excretion is particularly evident in obese women.
Acknowledgments
Grants
NIH U54 HD058155 Center for the Study of Reproductive Biology
K24 HD041978 to NS, 1UL1 RR025780 (University of Colorado CTRC), 1UL1TR001073 (Albert Einstein College of Medicine CTSA)
The authors acknowledge the assistance of Bill Lasley, PhD and the Endocrine Core at the California National Primate Research Center, who supplied conjugate and antibody for the urinary steroid assays, and the Clinical and Translational Sciences Centers at the University of Colorado School of Medicine and the Albert Einstein College of Medicine.
Footnotes
DISCLOSURES
NS has investigator initiated grant support from Bayer Pharmaceuticals and stock options in Menogenix AJP has investigator initiated grant support from Bayer Pharmaceuticals
References
- 1.van der Steeg JW, et al. Obesity affects spontaneous pregnancy chances in subfertile, ovulatory women. Hum Reprod. 2008;23(2):324–8. doi: 10.1093/humrep/dem371. [DOI] [PubMed] [Google Scholar]
- 2.Rittenberg V, et al. Effect of body mass index on IVF treatment outcome: an updated systematic review and meta-analysis. Reprod Biomed Online. 2011;23(4):421–39. doi: 10.1016/j.rbmo.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 3.Jain A, et al. Pulsatile luteinizing hormone amplitude and progesterone metabolite excretion are reduced in obese women. J Clin Endocrinol Metab. 2007;92(7):2468–73. doi: 10.1210/jc.2006-2274. [DOI] [PubMed] [Google Scholar]
- 4.Santoro N, et al. Assessing menstrual cycles with urinary hormone assays. Am J Physiol Endocrinol Metab. 2003;284(3):E521–30. doi: 10.1152/ajpendo.00381.2002. [DOI] [PubMed] [Google Scholar]
- 5.Pagan YL, Srouji SS, Jimenez Y, Emerson A, Gill S, Hall JE. Inverse relationship between luteinizing hormone and body mass index in polycystic ovary syndrome: investigation of hypothalamic and pituitary contributions. J Clin Endocrinol Metab. 2006;91:3878–84. doi: 10.1210/jc.2005-2099. [DOI] [PubMed] [Google Scholar]
- 6.Kucherov A, Polotsky AJ, Menke M, Isaac B, McAvey B, Buyuk E, Bradford AP, Hickmon C, Babbs B, Berga S, Loucks T, Santoro N. Aromatase inhibition causes increased amplitude, but not frequency, of hypothalamic-pituitary output in normal women. Fertil Steril. 2011;95:2063–2066. doi: 10.1016/j.fertnstert.2011.01.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Santoro N, Brown JR, Adel T, Skurnick JH. Characterization of reproductive hormonal dynamics in the perimenopause. J Clin Endocrinol Metab. 1996;81:1495–501. doi: 10.1210/jcem.81.4.8636357. [DOI] [PubMed] [Google Scholar]
- 8.Pal L, Zhang K, Zeitlian G, Santoro N. Characterizing the reproductive hormone milieu in infertile women with diminished ovarian reserve. Fertil Steril. 2010;93:1074–1079. doi: 10.1016/j.fertnstert.2008.10.069. [DOI] [PubMed] [Google Scholar]
- 9.Zawadzki JK, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. In: Dunaif A, Givens JR, Haseltine FP, Merriam GR, editors. Polycystic ovary syndrome. Boston: Blackwell Scientific; 1992. pp. 377–384. [Google Scholar]
- 10.Sioufi A, Gauducheau N, Pineau V, Marfil F, Jaouen A, Cardot J, et al. Absolute bioavailability of letrozole in healthy postmenopausal women. Biopharm Drug Disp. 1997;18:779–89. doi: 10.1002/(sici)1099-081x(199712)18:9<779::aid-bdd64>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 11.Santoro N, Lasley B, McConnell D, Allsworth J, Crawford S, Gold EB, Finkelstein JS, Greendale GA, Kelsey J, Korenman S, Luborsky JL, Matthews K, Midgley R, Powell L, Sabatine J, Schocken M, Sowers MF, Weiss G. Body size and ethnicity are associated with menstrual cycle alterations in the early menopausal transition: the Study of Women’s Health Across the Nation (SWAN) J Clin Endocrinol Metab. 2004;89:2622–31. doi: 10.1210/jc.2003-031578. [DOI] [PubMed] [Google Scholar]
- 12.Grenman S, Ronnemaa T, Irjala K, Kalhola HL, Gronroos M. Sex steroid, gonadotropin, cortisol and prolactin levels in healthy, massively obese women: correlation with abdominal fat cell size and effect of weight reduction. J Clin Endocrinol Metab. 1986;63:1257–61. doi: 10.1210/jcem-63-6-1257. [DOI] [PubMed] [Google Scholar]
- 13.Rochester D, Jain A, Polotsky AJ, Polotsky H, Gibbs K, Isaac B, Zeitlian G, Hickmon C, Feng S, Santoro N. Partial recovery of luteal function after bariatric surgery in obese women. Fertil Steril. 2006;92:1410–5. doi: 10.1016/j.fertnstert.2008.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haynes BP, et al. The pharmacology of letrozole. J Steroid Biochem Mol Biol. 2003;87(1):35–45. doi: 10.1016/s0960-0760(03)00384-4. [DOI] [PubMed] [Google Scholar]
- 15.Folkerd EJ, Dixon JM, Renshaw L, A’Hern RP, Dowsett M. Suppression of plasma estrogen levels by letrozole and anastrozole is related to body mass index in patients with breast cancer. J Clin Oncol. 2012;30:2977–2980. doi: 10.1200/JCO.2012.42.0273. [DOI] [PubMed] [Google Scholar]
- 16.Pfeiler G, Konigsberg R, Fesl C, Mlineritsch B, Stoeger H, Singer CF, Postlberger S, Steger GG, Seifert M, Dubsky P, Taucher S, Samonigg H, Bjelic-Radisic V, Greil R, Marth C, Gnant M. Impact of body mass index on the efficacy of endocrine therapy in premenopausal patients with breast cancer: an analysis of the prospective ABCSG-12 trial. J Cin Oncol. 2011;29:2653–2659. doi: 10.1200/JCO.2010.33.2585. [DOI] [PubMed] [Google Scholar]
- 17.The Practice Committee of the American Society for Reproductive Medicine. . The clinical relevance of luteal phase deficiency: a committee opinion. Fertil Steril. 2012;98:1112–7. doi: 10.1016/j.fertnstert.2012.06.050. [DOI] [PubMed] [Google Scholar]