Abstract
Objective
Obesity in childhood is associated with an inflammatory state in adipose tissue and liver, which elevates risk for diabetes and liver disease. No prior study has examined associations between pathologies occurring in adipose tissue and liver to identify elements of tissue damage associated with type 2 diabetes risk. This study sought to determine if inflammation and fibrosis in abdominal subcutaneous adipose tissue (SAT) in obese/overweight children (BMI-z 2.3±0.76) was related to the extent of observed liver disease or type 2 diabetes risk.
Design & Methods
Biopsy samples of abdominal (SAT) and liver were simultaneously collected from 33 Italian children (mean BMI 28.1±5.1 kg/m2 and mean age 11.6±2.2 years) with confirmed NAFLD. Histology and immunohistochemistry were conducted on biopsies to assess inflammation and fibrosis in adipose tissue and fibrosis and inflammation in liver.
Results
Presence vs. absence of crown like structures (CLS) in SAT was significantly related to liver fibrosis scores (1.7±0.7 vs. 1.2±0.7, p=0.04) independent of BMI. SAT fibrosis was significantly correlated with a lower disposition index (r=−0.48, p=0.006). No other adipose measures were associated with liver disease parameters.
Conclusion
Markers of subcutaneous WAT inflammation are associated with greater extent of liver fibrosis independent of obesity and SAT fibrosis may contribute to diabetes risk through reduced insulin secretion.
Keywords: Fibrosis, Inflammation, Pediatric, NAFLD, Adipose
Introduction
Obesity is marked by excess storage of lipids and a chronic pro-inflammatory state in adipocytes (1). The obesity-associated low-grade inflammation is sustained by the increase of macrophages in adipose tissue (2) and is strongly associated with increased ectopic fat accumulation in the liver (3), which can lead to non-alcoholic fatty liver disease (NAFLD), a process that begins early in life (4). Pediatric obesity prevalence continues to rise internationally (5) and is a strong risk factor for NAFLD, which is now the primary cause of liver disease in children (6) and is increasing in prevalence in the US (7). Adipose and liver tissues demonstrate similar yet independent relationships between excess fat deposition and the ensuing cell-signalling responses and resultant inflammation, however there is very little research that examines how inflammation in both adipose tissue (8) and liver (9) may be linked in children. This is surprising given that the prevalence of NAFLD amongst obese children can be as high as 70% in the United States (10) and even at an early age some children may progress to NASH, marked by the presence of liver fibrosis and inflammation (4).
Increases in adipose tissue have been linked to metabolic dysregulation (11) and increased ectopic lipid deposition in the liver promotes pro-inflammatory mediators (12) that are thought to activate specific liver-resident macrophages known as Kupffer cells, which may mediate the progression from NAFLD to NASH (13) by inducing hepatic injury and fibrosis (14). Similarly, amongst adipocytes, a pro-inflammatory state perturbs normal extracellular matrix (ECM) remodelling and favors fibrous collagen deposition (15) that can result in insulin resistance (16) and a diminished capacity for fat mass expansion (17). This inability of adipose fat mass to expand under fibrotic conditions may force ectopic deposition of fat into other organs such as the liver (18), which suggests a potential link between inflammation in adipose and hepatic tissues, yet the specific role of markers of subcutaneous WAT inflammation in metabolic or liver disease is unclear. In support of this potential link, the Scherer group has recently proposed that abnormal adipose tissue, marked by the presence of fibrosis, may be involved in several pathological changes to tissue locally and at a systems level (19) including impaired glucose homeostasis and ectopic fat deposition.
In order to investigate potential links between obesity-induced adipose inflammation and fibrosis and liver disease in children, we conducted immunohistochemical analyses on biopsied tissue from both subcutaneous adipose (SAT) depots and liver. The aim of this analysis was to determine and characterize the extent of inflammation and fibrosis in the adipose tissue of overweight and obese children and examine their relationship with liver disease and type 2 diabetes risk.
Methods
Patients
A total of 40 consecutive patients with biopsy-proven NAFLD seen at Bambino Gesù Children’s Hospital were prospectively included in the study from January 2011 to December 2012, and represented a subset of a population previously reported on (9). The study was approved by the Ethics Committee of the Bambino Gesù Children’s Hospital and Research Institute in Rome, Italy and all participants signed approved informed consent or assent documents. Inclusion criteria and exclusion criteria were the same as previously reported (9). Autoimmune liver disease, metabolic liver disease, Wilson’s disease, celiac disease, and alpha-1-antitrypsin deficiency were ruled out using standard clinical, laboratory, and histological criteria. Adipose samples from 7 of the 40 children biopsied were not viable and these participants were excluded from formal analysis. Complete data was obtained in the remaining 33 participants.
Anthropometric measures
Weight and height were measured using standard procedures. Body mass index (BMI) (kg/m2) and its standard deviation score (Z score) were calculated using US reference data (20, 21). Waist circumference (WC) was measured at the highest point of the iliac crest.
Laboratory assessment
Alanine and aspartate transferases (ALT and AST, respectively), gamma-glutamyl-transpeptidase, total triglycerides, and total low density (LDL) and high-density lipoprotein (HDL) cholesterol were evaluated using standard laboratory methods. Plasma insulin was measured using a radioimmunoassay (Myria Technogenetics, Milan, Italy). All participants underwent a standard oral glucose tolerance test (OGTT) performed with 1.75 grams of glucose per kilogram of body weight (up to 75g), and glucose and insulin were measured at 0, 30, 60, 90 and 120 minutes. The degree of insulin sensitivity/resistance was determined via the homeostatic model assessment (HOMA) (22) and by the OGTT-derived insulin sensitivity index (ISI) (23). Both the HOMA and the OGTT-derived ISI have a significant correlation with the gold standard euglycemic hyperinsulinemic glucose clamp technique (23). A HOMA value >2 or ISI value <6 were considered an indication of insulin resistance.
Pro-inflammatory markers and adipocytokines
Serum C-reactive protein (CRP) was determined via a high sensitivity latex agglutination method on HITACHI 911 Analyser (Sentinel Ch., Milan). The kit had a minimum detection of less than 0.05 mg/L, and a measurable concentration range up to 160 mg/L. The intra-assay and inter-assay variation coefficients were, respectively, 0.8–1.3 and 1.0–1.5%. Serum tumor necrosis factor (TNF) α and interleukin (IL)-6 were measured by sandwich ELISA (R&D System Europe Ltd, Abingdon, UK). For TNF α, the kit had a sensitivity of 0.12 pg/mL in a 200 µL sample size and a range of 0.5 to 32 pg/mL. The intra and inter assay coefficients of variation were 5.9% and 12.6%, respectively. For IL-6, the kit had a sensitivity of 0.25 pg/mL in a 50 µL sample size and a range of 3.9 to 250 ng/mL. The intra and inter assay coefficients of variation were 3.4% and 5.8%, respectively. Serum adiponectin was measured by ELISA kit according to the manufacturer's protocol (Ray Biotech, Norcross, GA, USA)
Liver histology
The clinical indication for biopsy was either to assess the presence of NASH and degree of fibrosis and/or to rule out potential other liver diseases. Liver biopsy was performed in all children after an overnight fast, using an automatic core biopsy 18 gauge needle (Biopince, Amedic, Sweden) under general anesthesia and ultrasound guidance. A Sonoline Omnia ultrasound machine (Siemens, Munich, Germany) equipped with a 5-MHz probe (5.0 C 50, Siemens) and a biopsy adaptor were employed. The length of liver specimen was recorded and only samples with a length ≥15 mm and including at least 5–6 complete portal tracts were considered adequate for the purpose of the study. It is worth noting that we have previously shown that a high prevalence of necro-inflammatory fibrosis can be found in children with ultrasonographic evidence of steatosis yet normal ALT levels at the time of biopsy, with 81% of patients with normal ALT presenting with fibrosis (24). Therefore, liver biopsies were conducted even in some patients with normal levels of ALT. Additionally, the use of an approach that allowed for the obtainment of a fat biopsy at the time of liver biopsy through implementing an additional pass of the biopsy needle minimized any additional risk to the patient. Biopsies were routinely processed (ie, formalin-fixed and paraffin-embedded) and sections of liver tissue were stained with hematoxylin-eosin, Van Gieson, Periodic acid-Schiff diastase and Prussian blue stain. Biopsies were evaluated by a single hepatopathologist who was blinded to clinical and laboratory data. Steatosis, inflammation, hepatocyte ballooning and fibrosis were scored using the NAFLD Clinical Research Network (CRN) criteria (25): Briefly, steatosis was graded on a 4-point scale: grade 0 = steatosis involving <5% of hepatocytes; grade 1 = steatosis involving up to 33% of hepatocytes; grade 2 = steatosis involving 33–66% of hepatocytes; and grade 3 = steatosis involving >66% of hepatocytes. Lobular inflammation was graded on a 4-point scale: grade 0 = no foci; grade 1 = <2 foci per 200× field; grade 2 = 2–4 foci per 200× field; and grade 3 = >4 foci per 200× field. Hepatocyte ballooning was graded from 0 to 2: 0 = none; 1= few balloon cells; and 2 =many/prominent balloon cells. The stage of fibrosis was quantified using a 5-point scale: stage 0 = no fibrosis; stage 1 = perisinusoidal or periportal (1a = mild, zone 3, perisinusoidal; 1b = moderate, zone 3, perisinusoidal; 1c = portal/periportal); stage 2 =perisinusoidal and portal/periportal; stage 3 = bringing; and stage 4 = cirrhosis.
Features of steatosis, lobular inflammation, and hepatocyte ballooning were combined to obtain the NAFLD activity score (NAS). As recently recommended by the NASH Clinical Research Network (25), a microscopic diagnosis based on overall injury pattern (steatosis, hepatocyte ballooning, inflammation) as well as the presence of additional lesions (e.g. zonality of lesions, portal inflammation and fibrosis) was assigned to each case. Accordingly, biopsies were subdivided into: not-NASH and definite NASH subcategories (26).
Adipose Histology
Following the liver biopsy, a second pass with the same needle through the same insertion point was used to obtain an abdominal subcutaneous adipose tissue (SAT) biopsy in all children. Biopsies were routinely processed (ie, formalin-fixed and paraffin-embedded) and sections of adipose tissue were stained with hematoxylin-eosin and CD68 antibody (Leica Biosystems, Newcastle, UK) or picrosirius red. The CD68 antibody incubation time was 15 min and antigen retrieval was at PH 8 for 20 minutes on the Leica bond. Biopsies were evaluated by a single technician who was blinded to clinical and laboratory data. For the CD68 stained sections, 4 consecutive 5-micron sections were obtained for each subject. Two independent fields at 20x magnification were captured for each section, for a total of 8 images per subject. Adipose cell size (micron2), adipose cell count, isolated macrophages and crown like structure (CLS) counts were obtained for each field captured using Fiji quantitative microscopy software (27). For each field, isolated macrophage per cm2 and CLS per cm2 were calculated by dividing by the area of the field measured. For each subject, the mean values for adipose cell size, macrophages per cm2 and CLS cm2 were obtained. For the collagen analysis, 3 non-serial 5 micron sections were obtained for each subject and stained with picrosirius red and prepared according to Bedossa et al. (28). Two independent fields at 20x magnification were captured for each section. Fiji software (27) was used to obtain total collagen area and a collagen to adipose ratio.
Data analysis
Values are expressed mean ± standard deviation. Variables were assessed for normality and non-normal data was log (ln) transformed for analyses. Wilcoxon test was used to compare between-group differences. Non-parametric Spearman’s correlations were used to examine correlations and partial correlations were conducted controlling for age, gender and BMI as covariates. Statistical significance was set at p<0.05. All analyses were performed on SPSS v18 (SPSS Inc, Chicago IL, USA).
Results
Anthropometric associations with SAT and liver tissue measures
Complete measures were obtained in 33 of the 40 participating children. Anthropometric characteristics are reported in Table 1together with a panel of adipocytokines and additional subject descriptors. Mean BMI was 28.1±5.1 kg/m2 with a mean BMI Z-score of 2.3±0.76 (lean, n=7; overweight, n=16, obese, n=10). Twenty of the 33 children were male and the mean age was 11.6±2.2 years.
Table 1.
Subject Characteristics
| Population Characteristics | All (n=33) | Lean (n=7) |
Overweight (n=16) |
Obese (n=10) |
|---|---|---|---|---|
| Age (yrs) | 11.6 (2.2) | 10.8 (2.2) | 11.6 (2.1) | 12.4 (2.5) |
| Gender (M:F) | 20/13 | 4/3 | 11/5 | 5/5 |
| BMI (kg/m2) | 28.1 (5.1) | 21.8 (2.2) | 27 (1.1) | 34.2 (3.8) |
| BMI z-score | 2.3 (.76) | 1.8 (.42) | 2.6 (.99) | 2.4 (.28) |
| WC (cm) | 89.3 (8.5) | 83.3 (11.8) | 89.9 (7.8) | 92.6 (4.8) |
| Biochemical Measures | ||||
| Fasting Plasma Glucose (mg/dL) | 80.2 (13) | 80.7 (7.6) | 80 (16.8) | 80.3 (9.7) |
| Fasting Plasma Insulin (µU/L) | 16.7 (11.2) | 13.3 (9.6) | 14.54 (7) | 22.8 (15.4) |
| Matsuda Index | 3.9 (3.1) | 3.8 (2.1) | 3.9 (3.2) | 4 (3.8) |
| HOMA-IR | 3.3 (2.3) | 2.7 (1.9) | 2.8 (1.4) | 4.6 (3.3) |
| Insulinogenic Index (IGI) | 2.9 (6.4) | 2.1 (1.4) | 4.2 (9) | 4.6 (3.2) |
| Disposition Index (IGI*ISI) | 9 (16.5) | 6.8 (5.6) | 12.9 (23.4) | 4.6 (3.6) |
| AST (IU/L) | 29.2 (8.8) | 30.3 (6.3) | 29.9 (8.6) | 27.1 (10.9) |
| ALT (IU/L) | 34.4 (15.6) | 45.2 (20.9) | 33.1 (14.2) | 28.6 (10.3) |
| GGT (ng/dL) | 20.6 (9.2) | 19.9 (7.6) | 19.9 (11.2) | 22.4 (7.4) |
| Adiponectin (ng/dL) | 21.9 (2.3) | 21.9 (2.7) | 22 (1.9) | 21.7 (2.8) |
| CRP (ng/dL) | 1.6 (.4) | 1.9 (.4) | 1.5 (.4) | 1.7 (.4) |
| IL-6 (ng/dL) | 9.5 (4.5) | 9.1 (2.2) | 8.6 (4.7) | 11.1 (5.2) |
| TNF-a (ng/dL) | 6.5 (1.9) | 6.7 (1.4) | 6.4 (2) | 6.5 (2.1) |
Values are presented as means with standard deviation. BMI=body mass index, WC=waist circumference, ALT=alanine transferase, AST=aspartate transferase, GGT=gamma-glutamyl-transpeptidase, HOMA-IR=homeostatic model of insulin resistance, CRP=c-reactive protein, TNF α=tumor necrosis factor alpha, IL-6=interleukin six
Overweight/obese children had a higher mean adipocyte area than the lean participants (2742±800.8 cm2 vs. 1924.2±364.9 cm2; p=0.03, respectively) and quantity of CLS per cm2 in adipose tissue was positively correlated with BMI and WC in all subjects (r=0.43, p=0.01 and r=0.41, p=0.01, respectively). Fasting plasma insulin and HOMA-IR values increased from lean to overweight to obese, however significantly higher fasting plasma insulin (22.8±15.4 vs.14.2±7.7 µU/L; p=0.03) and HOMA-IR (4.6±3.3 vs. 2.8±1.5;p=0.03) were only observed in obese vs. overweight participants. There were no significant differences in any other insulin or glucose parameters by obesity status.
Mean scores for liver steatosis (1.8±0.6 vs. 2.0±0.6 vs. 2.2±0.7), ballooning (1.1±0.6 vs. 1.2±0.5 vs. 1.5±0.6) and NAS (4.4±1.5 vs. 4.6±1.2 vs. 5.2±1.3) increased as a function of obesity (lean vs. overweight vs. obese, respectively), but these trends did not reach significance. Measures of liver disease were not associated with any glucose or insulin parameters.
Subcutaneous WAT morphology
Of the 33 participants, 14 had one or more CLS present in SAT sections (Table 2). Microscopy identified clear clinical differences in adipocyte cell size, CLS presence and fibrosis by obesity classification (Figure 1). Adipose cell area was significantly positively associated quantity of CLS/cm2 (r=0.4, p=0.01). Obese participants exhibited less SAT collagen than their overweight or lean counterparts (16.7±15.1 vs. 22.1±15.6 vs. 25.0±10.8, respectively), however these values did not significantly differ.
Table 2.
Adipose and Liver Immunohistological Measures
| Adipose Histology | All (n=33) | Lean (n=7) | Overweight (n=16) | Obese (n=10) | |
|---|---|---|---|---|---|
| Macrophage count | 4.9 (3.6) | 4.7 (2.2) | 4.9 (4.1) | 5.1 (3.9) | |
| Macrophages per cm2 | 0.51 (0.35) | 0.78 (0.52) | 0.48 (0.25) | 0.37 (0.28) | |
| CLS (−/+) | 19/14 | 6/1 | 10/6 | 3/7 | |
| CLS per cm2 (among CLS+) | 0.06 (0.05) | 0.07 (NA) | 0.07 (0.06) | 0.05 (0.03) | |
| Adipose Cell Area (µ2) | 2267.1 (704.4) | 1924.2 (364.9) | 2148.1 (648) | 2742 (800.8) | |
| Collagen Ratio | 21.1 (14.5) | 25.1 (10.8) | 22.1 (15.6) | 16.7 (15.1) | |
| Liver Histology | Frequency (%) | ||||
| Fibrosis | 0–1 | 17 (60.6) | 5 (71.4) | 8 (50) | 7 (70) |
| 2–3 | 13 (39.4) | 2 (28.6) | 8 (50) | 3 (30) | |
| Steatosis | 0–1 | 25 (75.7) | 2 (28.6) | 3 (18.8) | 2 (20) |
| ≥2 | 8 (24.2) | 5 (71.4) | 13 (81.2) | 8 (80) | |
| Lobular Inflammation | 0–1 | 15 (44.5) | 3 (42.9) | 9 (56.2) | 3 (30) |
| 2 | 18 (54.5) | 4 (57.1) | 7 (43.8) | 7 (70) | |
| Portal Inflammation | 0–1 | 19 (57.5) | 3 (42.9) | 10 (62.5) | 6 (60) |
| 2 | 14 (42.4) | 4 (57.1) | 6 (37.5) | 4 (40) | |
| Ballooning | 0–1 | 23 (69.7) | 5 (71.4) | 12 (75) | 6 (60) |
| 2 | 10 (30.3) | 2 (28.6) | 4 (25) | 4 (40) | |
| NAS | 0–2 | 1 (3) | 1 (14.3) | 0 (0) | 0 (0) |
| 3–4 | 15 (45.5) | 2 (28.6) | 9 (56) | 4 (40) | |
| ≥5 (NASH) | 17 (51.5) | 4 (57.2) | 7 (44) | 6 (60) | |
Values are presented as means with standard deviation, unless otherwise noted. NASH=non alcoholic steatohepatitis, CLS=crown-like structure, NAS=NAFLD activity score.
Figure 1.
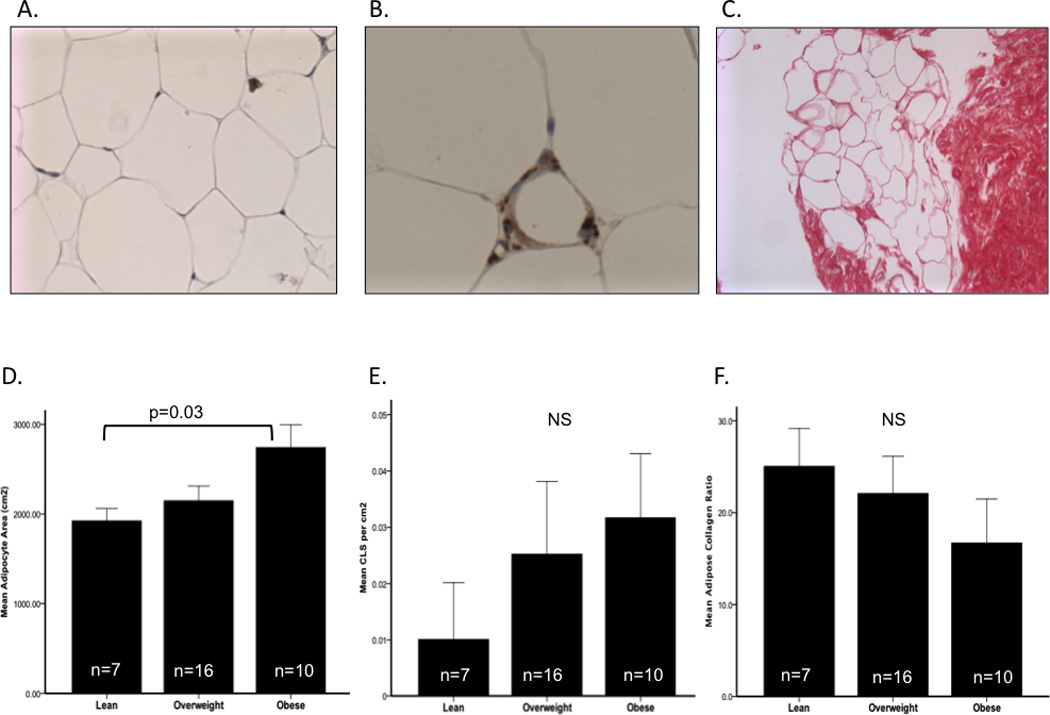
Adipose cell size, CLS presence and collagen ratio as a function of obesity.
Histological analysis of SAT adipose sections (Panel A) at 20x magnification demonstrated a significant increase in adipocyte cell area (µ2) between lean and obese participants (Panel D). CD68 immunihistochemical staining for macrophages identified CLS in 14 of 33 participants (Panel B) and a clinical increase in CLS per cm2 by obesity was observed (Panel E). Picrosirius red staining was used to assess fibrosis in SAT through obtaining a collagen ratio (Panel C). A trend in decreasing collagen ratio as a function of obesity was observed (Panel F). CLS=crown-like structures, SAT= subcutaneous adipose tissue.
Subcutaneous WAT and liver disease
A NAS score of ≥ 5 was present in over 50% of the study participants (Table 2) and histology (18) confirmed NASH with advanced steatosis and fibrosis (Figure 2). Anthropometric and biochemical measures were not significantly different when means were compared by NAS category (0–4 and ≥ 5). Serum pro-inflammatory cytokines (CRP, IL-6 and TNF-α) were not related to adipose inflammation and fibrosis or NAFLD features in this cohort. Serum adiponectin was not related to any aspects of adiposity or markers of subcutaneous WAT inflammation, however mean serum adiponectin was significantly lower in the high-steatosis scored participants (ptrend<0.001) and displayed a highly significant negative correlation with NAS score (r=−0.62, p<0.001) even when controlling for BMI z-score and gender (−0.31, p=0.05). Subjects who had SAT with CLS demonstrated significantly higher liver fibrosis scores (CLS+; 1.7±0.7 vs. CLS-; 1.2±0.7, p=0.04), independent of BMI. Similarly, CLS+ participants had a tendency for greater hepatocyte ballooning (1.4±0.7 vs. 1.0±0.5, p=0.07) compared to participants with no CLS. There were no other statistically significant relationships between indicators of SAT inflammation or fibrosis by NASH status.
Figure 2.
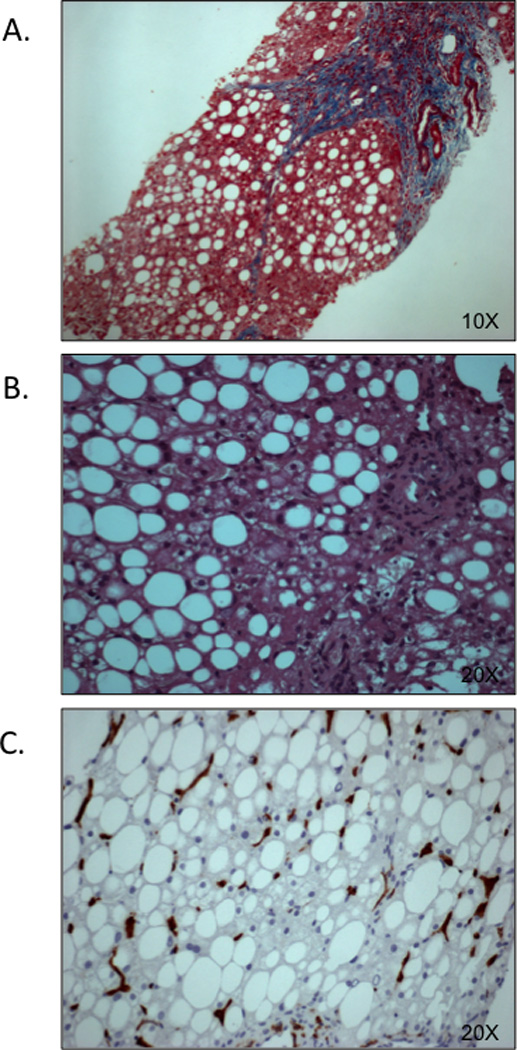
Liver histology: Steatosis, fibrosis and inflammation
Panel A (10× magnification), is a representative image of NASH with steatosis, and fibrosis score F3. Panel B represents a child with NASH (steatosis grade 2, ballooning grade 1, and inflammation grade 1. Panel C depicts steatosis score 2 and hepatic inflammation as evidenced by CD163+ Kupfer cell staining. Images adapted from Alkhouri et al. (18).
Subcutaneous WAT and glucose homeostasis
Adipose collagen accumulation was significantly inversely correlated with insulinogenic index (r=−0.37, p=0.03) and disposition index (DI) (r=−0.48, p=0.006), but not with HOMA or fasting plasma insulin or glucose. The relationship between collagen and DI was maintained (r=−0.31, p=0.05) after adjusting for age, gender and BMI (Figure 3).
Figure 3.
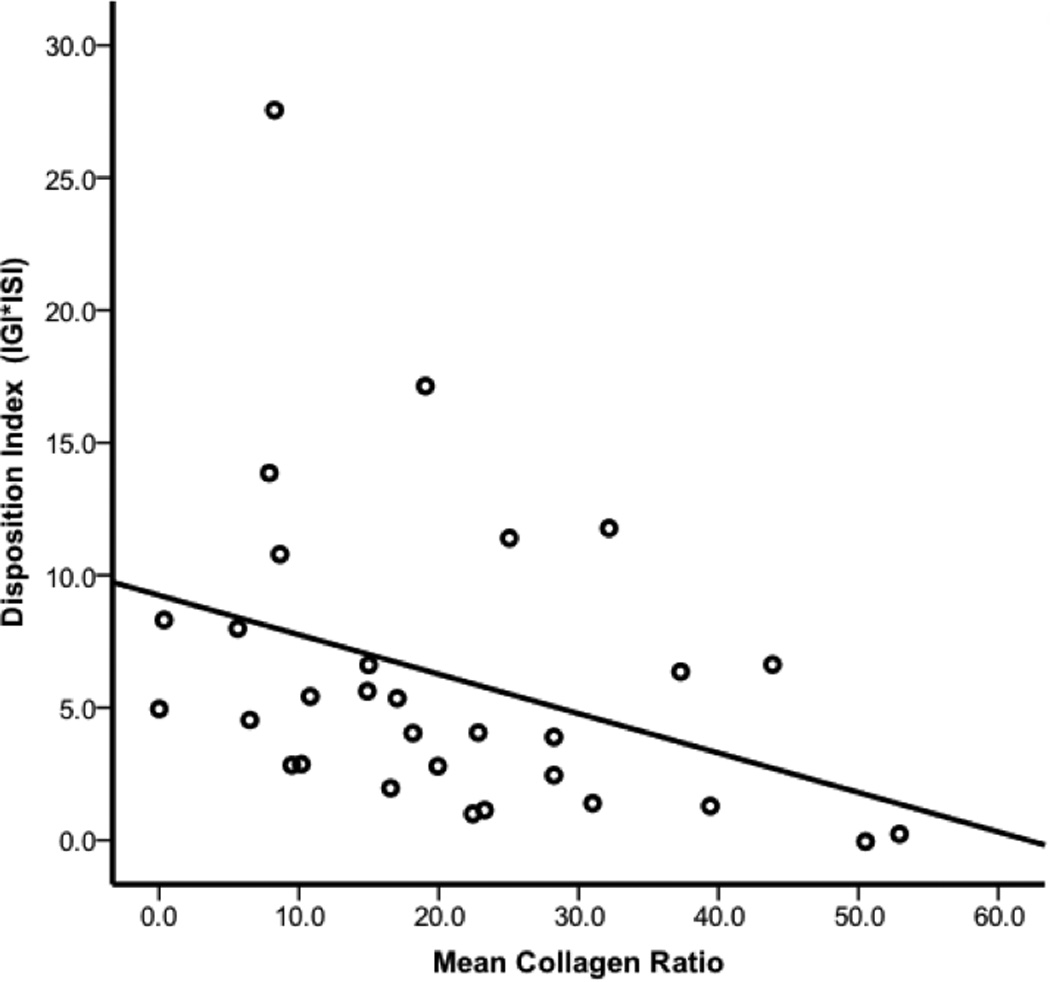
Mean adipose collagen ratio is inversely associated with disposition index Mean adipose collagen ratio is displayed on the x-axis and mean disposition index is on the y-axis. Disposition index decreased as a function of increasing adipose fibrosis. Graphical data are presented as raw, unadjusted values and the correlation (−0.31, p=0.05) persisted after controlling for age, gender and BMI.
Discussion
This is the first study to examine the links between adipose tissue inflammation/fibrosis and liver damage in children with biopsy-proven NAFLD. Although this is a preliminary study in a small sample, we identified several novel findings that merit further investigation: 1) Markers of subcutaneous WAT inflammation were significantly related to liver fibrosis; 2) Fibrosis in SAT was significantly associated with impaired DI, which was supported by clinically higher glucose AUC and lower insulin AUC values observed in patients with higher adipose fibrosis; 3) Obesity was significantly associated with markers of subcutaneous WAT inflammation and obese children tended to have higher qualitative liver disease scores than lean counterparts. These data suggest that increasing obesity is strongly related to both adipose tissue damage and liver damage with some evidence of a link between inflammatory CLS presence in SAT and liver disease.
Our study has established a novel link between subcutaneous adipose inflammation, as indicated by presence of CLS, and the extent of liver fibrosis in children. Nearly half of the children in the present study had CLS in adipose tissue and the number of CLS was positively related to obesity status and adipose cell size. This is consistent with a prior study (17) in which the number of CLS increases with adipocyte hypertrophy, indicating a heightened inflammatory state. However, a recent study in children did not detect CLS in the abdominal adipose tissue of healthy, overweight children (8). Tordjman et al. (29) recently demonstrated that there are no observable differences in macrophage number or CD68 gene expression in SAT by NASH status in adults, however they were able to show highly significant differences by liver disease status in both of these measures when examining deep SAT or VAT, suggesting differential roles for distinct adipose depots on liver disease. Although we were not able to determine whether our adipose samples represented deep SAT vs. SAT, the abundance of CLS is consistent with other work in deep SAT tissue (29). Increased delivery of pro-inflammatory factors from macrophages to the liver along with free fatty acid flux may be one possible link between VAT and liver disease (30), but this observation has yet to be demonstrated in SAT depots (29). Given that CLS+ subjects in our study had significantly greater liver fibrosis scores, our data suggests that markers of subcutaneous WAT inflammation may play a role in modulating inflammation in the liver possibly through an increase in pro-inflammatory factors in the liver. Interestingly, in a study composed of obese adults, subjects with CLS in their SAT had elevated liver fat, as measured by proton magnetic resonance spectroscopy (31), and similar to our study there was no association between adipocyte cell size and steatosis. It is possible that quantitative measures of liver disease, such as liver fat fraction, would demonstrate a stronger relationship to inflammatory SAT measures in children and other adipose regions may be linked to liver disease. We were not able to test these hypotheses directly, as qualitative measures of liver fat were not available, however subsequent studies should include more comprehensive measures of steatosis.
Adipose tissue fibrosis, as indicated by SAT collagen amount, was inversely related to DI, which is the ability to compensate for insulin resistance through increased insulin secretion. Fibrosis and inflammation in adipose tissue have been previously associated with insulin resistance (32) and although SAT fibrosis did not correlate significantly with any other measures of glucose and insulin dynamics in our population, an observation also noted in adults (33), our participants with greater fibrosis had a clinically higher glucose AUC and lower insulin AUC in response to an OGTT. Higher collagen deposition in adipose tissue is thought to inhibit adipose expansion, which can lead to inflammation through the recruitment of macrophages, pointing to a potential relationship between adipose ECM and inflammation (19, 8). Interestingly, a recent study in adults found obese subjects had a higher degree of pericellular adipose fibrosis in both SAT and omental adipose tissue than lean participants (32) with no significant difference in total fibrosis by obesity status. Similarly, in healthy children, obese individuals were found to have significantly less collagen that lean peers and this collagen was inversely associated with both adipose cell size (8). Our findings are consistent with this observation, yet it remains unclear if the amount of fibrosis in the adipose tissue of children is related to a pathology or if it reflects the normal physiological development and growth of adipose tissue (8). This suggests that collagen type as well as adipose region may contribute differentially to the regulation of glucose homeostasis. Thus, it remains possible that the relationships between specific adipose depots, the types of collagen in said depots and glucose homeostasis are very different and modulated to a greater extent by the degree of inflammation in the developing adipose tissues of children than that of adults.
Several important limitations of this study should be mentioned. Our relatively small sample size was useful in identifying preliminary relationships between features of inflamed adipose morphology and liver disease for future exploration, but the small size limited us from finding other expected associations between adipose and liver inflammation. This initial study was limited to Italian children and although some findings have been reported in some studies in children and adults, the links between adipose tissue and liver tissue may vary based on age, maturation status and ethnicity. In this initial study we did not quantify SAT or visceral adipose tissue and our measures of adipose tissue fibrosis were restricted to total rather than subtypes of collagen, which limits the application of our findings to general relationships between SAT and total collagen with liver disease. The surgical biopsy of adipose tissue, when compared to needle-biopsy, is an ideal sampling technique (34). However, because the children in this study were subject to a liver biopsy, we chose to minimize further risk and ethical burden and perform a needle biopsy. Our measures of insulin resistance and DI were based on data from an OGTT rather than intravenous glucose tolerance tests or clamp methods and we did not assay c-peptide, which could be used to further verify insulin resistance, beyond DI, and test for associations with SAT collagen. Despite these limitations, the results of this preliminary study identify novel findings that merit further and more detailed studies that address the present limitations.
In summary, our study has demonstrated a link between adipose tissue inflammation and liver fibrosis, suggesting a role for markers of subcutaneous WAT inflammation in the mediation of liver inflammation. Additionally, higher levels of SAT collagen appear to effect glucose homeostasis indicating that SAT inflammation and fibrosis could be risk factors for insulin resistance and type 2 diabetes. Obesity clearly negatively impacts SAT health in children while increasing risk for NAFLD and NASH. Inflammation and fibrosis in SAT may be involved in promoting hepatic fibrosis and insulin resistance in children with NAFLD, and these relationships require further investigation.
What is already known about this subject?
-
-
Adipose and liver tissues demonstrate similar yet independent relationships between excess fat deposition and the ensuing cell-signalling responses and resultant inflammation.
-
-
The inability of adipose fat mass to expand under fibrotic conditions may force ectopic deposition of fat into other organs such as the liver, which suggests a potential link between inflammation in adipose and hepatic tissues.
-
-
It was recently proposed that fibrotic adipose tissue may be involved in several pathological changes to tissue locally and at a systems level including impaired glucose homeostasis and ectopic fat deposition
What does this study add?
-
-
This is the first study to examine the links between adipose tissue inflammation/fibrosis and liver damage in children with biopsy-proven non-alcoholic fatty liver disease (NAFLD).
-
-
Establishes a novel link between markers of subcutaneous white adipose tissue (WAT) inflammation and liver fibrosis in children.
-
-
Higher levels of collagen in SAT were associated with reduced disposition index independent of BMI, suggesting that adipose tissue fibrosis could be a factor in insulin resistance and type 2 diabetes risk.
Acknowledgmnents
We would like to acknowledge our patients and their families for cooperation in this study. Support for this study was provided by a) Bambino Gesu' Children’s Hospital (design and conduct of the study, data collection), b) The Ruth L. Kirschstein National Research Service Award NIH grant number 2T32ES013678-06 (analysis, interpretation of the data, preparation and review of the manuscript) and c) The Dr. Robert C. and Veronica Atkins Foundation (analysis and interpretation of the data, preparation and review of the manuscript).
Footnotes
The authors declare no conflicts of interest.
Contributor Information
Ryan W Walker, Email: ryan.walker@mssm.edu, Charles Bronfman Institute for Personalized Medicine, Icahn School of Medicine, Mount Sinai, New York, United States.
Hooman Allayee, Email: hallayee@usc.edu, Preventive Medicine, University of Southern California, Los Angeles, United States.
Alessandro Inserra, Email: alessandro.inserra@opbg.net, General Surgery, Bambino Gesu Children Hospital, Rome, Italy.
Rodolfo Fruhwirth, Email: rodolfo.fruhwirth@opbg.net, Interventional Radiology, Bambino Gesu Children Hospital, Rome, Italy.
Anna Alisi, Email: anna.alisi@opbg.net, Liver Research Laboratory, Bambino Gesu Children Hospital, Rome, Italy.
Rita Devito, Email: Rita.devito@opbg.net, Pathology Department, Bambino Gesu Children Hospital, Rome, Italy.
Magalie E Carey, Email: careyme@usc.edu, University of Southern California, Los Angeles, United States.
Frank Sinatra, Email: sinatra@usc.edu, Pediatrics, University of Southern California, Los Angeles, United States.
Michael I Goran, Email: goran@usc.edu, Preventive Medicine, University of Southern California, Los Angeles, United States.
Valerio Nobili, Email: valerio.nobili@opbg.net, Hepatometabolic Unit and Liver Research Laboratory, Bambino Gesu Children Hospital, Rome, Italy.
References
- 1.Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest. 2011 Jun 1;121(6):2111–2117. doi: 10.1172/JCI57132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen Y, et al. Variations in DNA elucidate molecular networks that cause disease. Nature. 2008;452(7186):429–435. doi: 10.1038/nature06757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suganami T, Tanaka M, Ogawa Y. Adipose tissue inflammation and ectopic lipid accumulation. Endocr J. 2012;59(10):849–857. doi: 10.1507/endocrj.ej12-0271. Epub 2012 Aug 9. [DOI] [PubMed] [Google Scholar]
- 4.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002 Apr 18;346(16):1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 5.Finucane MM, Stevens GA, Cowan MJ et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet. 2011;377:557–567. doi: 10.1016/S0140-6736(10)62037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feldstein AE, Charatcharoenwitthaya P, Treeprasertsuk S, Benson JT, Enders FB, Angulo P. The natural history of non-alcoholic fatty liver disease in children: a follow-up study for up to 20 years. Gut. 2009 Nov;58(11):1538–1544. doi: 10.1136/gut.2008.171280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Welsh JA, Karpen S, Vos MB. Increasing Prevalence of Nonalcoholic Fatty Liver Disease Among United States Adolescents, 1988–1994 to 2007–2010. J Pediatr. 2013;162:496–500. doi: 10.1016/j.jpeds.2012.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tam CS, Tordjman J, Divoux A, Baur LA, Clément K. Adipose tissue remodeling in children: the link between collagen deposition and age-related adipocyte growth. J Clin Endocrinol Metab. 2012 Apr;97(4):1320–1327. doi: 10.1210/jc.2011-2806. [DOI] [PubMed] [Google Scholar]
- 9.Alkhouri N, De Vito R, Alisi A, Yerian L, Lopez R, Feldstein AE, Nobili V. Development and validation of a new histological score for pediatric non-alcoholic fatty liver disease. J Hepatol. 2012 Dec;57(6):1312–1318. doi: 10.1016/j.jhep.2012.07.027. Epub 2012 Aug 4. [DOI] [PubMed] [Google Scholar]
- 10.Dunn W, Schwimmer JB. The obesity epidemic and nonalcoholic fatty liver disease in children. Curr Gastroenterol Rep. 2008 Feb;10(1):67–72. doi: 10.1007/s11894-008-0011-1. [DOI] [PubMed] [Google Scholar]
- 11.Kursawe R, et al. Cellularity and adipogenic profile of the abdominal subcutaneous adipose tis- sue from obese adolescents: association with insulin resistance and hepatic steatosis. Diabetes. 2010;59(9):2288–2296. doi: 10.2337/db10-0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ekstedt M, Franzén LE, Mathiesen UL, Thorelius L, Holmqvist M, Bodemar G, Kechagias S. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006 Oct;44(4):865–873. doi: 10.1002/hep.21327. [DOI] [PubMed] [Google Scholar]
- 13.Alisi A, Manco M, Vania A, Nobili V. Pediatric nonalcoholic fatty liver disease in 2009. J Pediatr. 2009 Oct;155(4):469–474. doi: 10.1016/j.jpeds.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 14.Spruss A, Kanuri G, Wagnerberger S, Haub S, Bischoff SC, Bergheim I. Toll-like receptor 4 is involved in the development of fructose-induced hepatic steatosis in mice. Hepatology. 2009 Oct;50(4):1094–1104. doi: 10.1002/hep.23122. [DOI] [PubMed] [Google Scholar]
- 15.Nakajima I, Muroya S, Tanabe R, Chikuni K. Extracellular matrix development during differentiation into adipocytes with a unique increase in type V and VI collagen. Biol Cell. 2002 Jun;94(3):197–203. doi: 10.1016/s0248-4900(02)01189-9. [DOI] [PubMed] [Google Scholar]
- 16.Pasarica M, Gowronska-Kozak B, Burk D, Remedios I, Hymel D, Gimble J, Ravussin E, Bray GA, Smith SR. Adipose tissue collagen VI in obesity. J Clin Endocrinol Metab. 2009 Dec;94(12):5155–5162. doi: 10.1210/jc.2009-0947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, Wang S, Fortier M, Greenberg AS, Obin MS. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J. Lipid Res. 2005;46:2347–2355. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- 18.Duval C, Thissen U, Keshtkar S, Accart B, Stienstra R, Boekschoten MV, Roskams T, Kersten S, Müller M. Adipose tissue dysfunction signals progression of hepatic steatosis towards nonalcoholic steatohepatitis in C57BL/6 mice. Diabetes. 2010 Dec;59(12):3181–3191. doi: 10.2337/db10-0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun, et al. Fibrosis and Adipose Tissue Dysfunction, Cell Metabolism. 2013 doi: 10.1016/j.cmet.2013.06.016. http://dx.doi.org/10.1016/j.cmet.2013.06.016. [DOI] [PMC free article] [PubMed]
- 20.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States. Adv Data. 2000:1–27. [PubMed] [Google Scholar]
- 21.Cole TJ, Bellizzi MC, Flegal KM, et al. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. 2000;320:1240–1243. doi: 10.1136/bmj.320.7244.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 23.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 24.Manco M, Alisi A, Nobili V. Risk of severe liver disease in NAFLD with normal ALT levels: a pediatric report. Hepatology. 2008 Dec;48(6):2087–2088. doi: 10.1002/hep.22631. author reply 2088. [DOI] [PubMed] [Google Scholar]
- 25.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ. Nonalcoholic Steatohepatitis Clinical Research Network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 26.Brunt EM, Kleiner DE, Wilson LA, Belt P, Neuschwander-Tetri BA. Nonalcoholic fatty liver disease (NAFLD) activity score and the histopathologic diagnosis in NAFLD: distinct clinicopathologic meanings. Hepatology. 2011;53:810–820. doi: 10.1002/hep.24127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schindelin Johannes, Arganda-Carreras Ignacio, Frise Erwin, Kaynig Verena, Longair Mark, Pietzsch Tobias, Preibisch Stephan, Rueden Curtis, Saalfeld Stephan, Schmid Benjamin, Tinevez Jean-Yves, White Daniel James, Hartenstein Volker, Eliceiri Kevin, Tomancak Pavel, Cardona Albert. Fiji: an open-source platform for biological-image analysis. Nature Methods. 2012;9(7):676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bedossa P, Lemaigre G, Bacci J, Martin E. Quantitative estimation of the collagen content in normal and pathologic pancreas tissue. Digestion. 1989;44:7–13. doi: 10.1159/000199886. [DOI] [PubMed] [Google Scholar]
- 29.Tordjman J, Divoux A, Prifti E, Poitou C, Pelloux V, Hugol D, Basdevant A, Bouillot JL, Chevallier JM, Bedossa P, Guerre-Millo M, Clement K. Structural and inflammatory heterogeneity in subcutaneous adipose tissue: relation with liver histopathology in morbid obesity. J Hepatol. 2012 May;56(5):1152–1158. doi: 10.1016/j.jhep.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 30.Fontana L, Eagon JC, Trujillo ME, Scherer PE, Klein S. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes. 2007 Apr;56(4):1010–1013. doi: 10.2337/db06-1656. [DOI] [PubMed] [Google Scholar]
- 31.Jansen HJ, Vervoort GM, van der Graaf M, Stienstra R, Tack CJ. Liver fat content is linked to inflammatory changes in subcutaneous adipose tissue in type 2 diabetes patients. Clin Endocrinol (Oxf) 2012 Nov 20; doi: 10.1111/cen.12105. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 32.Divoux A, Tordjman J, Lacasa D, Veyrie N, Hugol D, Aissat A, Basdevant A, Guerre-Millo M, Poitou C, Zucker JD, Bedossa P, Clément K. Fibrosis in human adipose tissue: composition, distribution, and link with lipid metabolism and fat mass loss. Diabetes. 2010 Nov;59(11):2817–2825. doi: 10.2337/db10-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spencer M, Yao-Borengasser A, Unal R, Rasouli N, Gurley CM, Zhu B, Peterson CA, Kern PA. Adipose tissue macrophages in insulin-resistant subjects are associated with collagen VI and fibrosis and demonstrate alternative activation. Am J Physiol Endocrinol Metab. 2010 Dec;299(6):E1016–E1027. doi: 10.1152/ajpendo.00329.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mutch DM, Tordjman J, Pelloux V, Hanczar B, Henegar C, Poitou C, Veyrie N, Zucker JD, Clément K. Needle and surgical biopsy techniques differentially affect adipose tissue gene expression profiles. Am J Clin Nutr. 2009 Jan;89(1):51–57. doi: 10.3945/ajcn.2008.26802. [DOI] [PubMed] [Google Scholar]


