Abstract
Objective
To investigate the relationship of novelty seeking traits (NS) with midbrain dopamine (DA) receptors and acyl ghrelin levels (AG) in normal weight (NW) and obese females. NS predict addictive behaviors and are hypothesized to contribute to eating behaviors. In healthy, NS are negatively associated with DA receptors in the substantia nigra (SN). We hypothesized that obesity would influence the regulation of NS by DA signaling and AG.
Design and Methods
We used PET scanning to measure DA type 2/type 3 receptor (D2/D3R) binding potential (BPND) in the SN. Participants completed Tridimensional Personality Questionnaire-Novelty-Seeking Scale (TPQ-NS) and AG were measured.
Results
In 8 NW and 19 obese (BMI 22 vs 38 kg/m2), TPQ-NS (16 vs15) and SN D2/D3R BPND (2.48vs2.66) were similar, while AG higher (256vs60, p<0.01), respectively. D2/D3R BPND and TPQ-NS had a negative relationship in NW (r=−0.7) but not in obese (p>0.10). AG and TPQ-NS were positively correlated in NW (r=0.9) but not in obese (p>0.10). D2R BPND and AG were negatively correlated in NW (r=−0.8) but positively in obese (r=0.6).
Conclusion
Obese do not maintain posited regulatory relationships for NS to either midbrain D2/D3R availability or AG present in NW. Also opposite relationships exist for NW and obese between SN D2/D3R availability and AG. The altered regulation of NS in obesity needs to be further explored.
Keywords: novelty seeking, dopamine receptor, ghrelin, neuroendocrine
Introduction
Central dopamine (DA) signaling is essential to the regulation of food intake and obesity related behaviors. In particular the mesolimbic dopamine (DA) pathway, which originates in the midbrain, serves to drive behaviors necessary for food consumption by integrating homeostatic signals and modulating the rewarding and motivational value of food (1). DA neurons fire when predicting food reward or when exposed to an unexpected food reward. Highly palatable food stimulates some of the same reward pathways that are activated by drugs of abuse. This has led to the hypothesis that disruption in DA signaling, similar to that present in addiction, may underlie unrestrained food intake in obesity (2).
Novelty seeking traits (NS) are characterized by a heightened excitatory response to novel stimuli (3) and are a predictor of drug abuse risk. NS may also influence aspects of appetitive stimuli (4) and are hypothesized to be involved in obesity (5). Using a radioligand that binds DA type 2 class receptors (both D2R and D3R), Zald and colleagues demonstrated that in the region of the substantia nigra (SN) D2/D3R binding potential (BPND) is negatively correlated with NS traits (6) and impulsivity (7). These midbrain receptors serve as autoreceptors providing inhibitory regulation of DA release, and in rodents with increased NS traits the inhibitory function of these receptors is diminished (8), thus leading to impaired inhibitory control. In obese rodents, directly knocking out dorsal striatal DA D2R, which are downstream of the SN dopaminergic neurons, causes compulsive eating behaviors (9). Indeed, we and others using ligands that bind to both D2R and D3R have previously reported that D2/D3R signaling in human obesity is altered (10–12).
We previously demonstrated that D2/D3R BPND in the striatum is negatively associated with fasting acyl ghrelin levels in a cohort of normal weight and obese females (10). Acyl ghrelin is a neuroendocrine hormone that is produced from the peptide ghrelin, which is secreted by the stomach before meals. It is the primary orexigenic signal driving food intake.. Acyl ghrelin is the active ligand for the growth hormone secretagogue receptor (GHSR). The GHSR is abundant in the hypothalamus and has been identified on midbrain dopaminergic neurons (13). Ghrelin exposure to the midbrain neurons of the ventral tegmental area (VTA) causes neuronal activation and DA release in the downstream nucleus accumbens, a region fundamental for sensing reward and pleasure (14). Ghrelin is essential for sensing reward from both sugar and high fat foods (15), and also drugs of abuse (16). Hansson et al demonstrated that ghrelin exposure to midbrain DA neurons increases novelty seeking behaviors in rodents, while such behaviors are diminished by blockade of the GHSR. As ghrelin secretion is related to eating patterns, its regulation of NS furthers the hypothesis that NS may be involved in obesity.
In the current study we hypothesized that obesity would influence the midbrain dopaminergic regulation of NS. Based on the work of Hansson et al, it was suspected that ghrelin would also be relevant to NS and midbrain DA receptors. Lastly, we hypothesized that increased NS would occur in subjects with binge eating, particularly in the obese. Obese and normal weight females completed the Novelty Seeking Scale from the Tridimensional Personality Questionnaire (TPQ-NS), as a measure of NS (17), the binge eating scale (BES) (18), as a continuous measure of binge eating behaviors, and PET imaging with [18F]fallypride to measure DA D2/D3R BPND in the SN, which has previously been shown to be associated with NS in healthy humans.
Methods and procedures
Participants
Protocol approval was obtained from the Vanderbilt University Institutional Review Board and all participants gave written informed consent. The majority of the participants in this study were included in a previous analysis and publication (10), which includes a detailed description of recruitment. Briefly, screening evaluation included laboratory testing and a comprehensive interview and exam to recruit healthy, weight stable individuals without a concerning history for significant systemic, metabolic, or psychiatric disease including substance abuse. No participant was being treated for psychiatric illness or with centrally acting medications, and all participants had scores <20 on the Beck Depression Inventory-II (BDI-II) (19). At screening and before the PET scans, females capable of childbearing underwent serum pregnancy testing.
General Study Protocol
Participants underwent baseline structural magnetic resonance imaging (MRI) to co-register with the PET images. Two days prior to and on the day of the PET study participants were asked to refrain from exercising, drinking alcohol, and to restrict coffee to ≤ 8 ounces daily. On the day of the PET scan participants ate breakfast, then consumed a small meal just before 10:00am and drank water only thereafter. Participants arrived approximately 2 hours before the PET scan and completed the TPQ-NS (17), the BES (18), and a blood sample was taken to measure fasting neuroendocrine hormones. PET scans were started at approximately 1830 hours and finished 3.5 hours later.
Neuroimaging
MRI structural scans of the brain were obtained for co-registration purposes. Thin section T1 weighted images were done on either a 1.5T (GE; General Electric, 1.2–1.4 mm slice thickness, in plane voxel size of 1 × 1 mm) or a 3T MRI scanner (Philips Intera Achieva, 1 mm slice thickness, in plane voxel size of 1 × 1 mm). PET scans with the D2/ D3 receptor radioligand [18F] fallypride were performed on a GE DTSE scanner with a 3-dimensional emission acquisition and a CT scan for attenuation correction, which has a reconstructed resolution of 2.34 mm in plane, approximately 5mm axially, and provides 47 planes over a 30 cm axial field of view. Serial PET scans were obtained over 3.5 hours. The first scan sequence (70 minutes) was initiated with a bolus injection over 15 seconds to deliver 5.0mCi of [18F] fallypride (specific activity >2,000 Ci/mmol). The second and third scan sequences started at 85 and 150 minutes, lasting 50 and 60 minutes, respectively, with 15 minute breaks between scan sequences.
Imaging Analysis
PET imaging analyses were completed as previously described by our group (20). For this study we completed regions of interest (ROI) analysis for the SN. The serial PET scans were co-registered to each other and to the thin section T1-weighted MRI scans and were co-registered using a mutual information rigid body algorithm. Images were reoriented to the anterior commissure-posterior commissure line. The full reference region method was used to calculate regional DA D2/D3R BPND (21) with the cerebellum as the reference region. The SN was delineated bilaterally and the BPND from right and left averaged for analysis as our group has previously shown limited laterality effects in this region both in obese (22) and non-obese subjects (20).
Acyl Ghrelin Assay
Samples were collected for plasma acyl ghrelin into tubes containing the serine protease inhibitor pefabloc sc (4-amidinophenyl-methanesulfonyl fluoride, Roche Applied Science, Germany). Plasma was then acidified with 1N hydrochloric acid (50 µl/ml plasma). Acyl ghrelin concentrations were determined by RIA (Linco Research, Inc. St. Charles, Mo) and run in duplicate.
Statistics
The Mann-Whitney U test was used for descriptive statistics including comparing demographics and outcome measures between normal weight and obese females. Data are reported as median (25%, 75% interquartile range) in the tables and medians in the text. Tests of normality were run for TPQ-NS, BES, SN BPND, and acyl ghrelin. Spearman’s rank correlation coefficient was used as a non-parametric measure of statistical dependence due to the presence of non-normal distributions of key variables. Analyses were completed using SPSS Statistics Version 20.
Results (Table 1, Figures 1, 2, and 3)
Table 1.
Demographics and primary outcomes by weight category represented by median and interquartile range.
| Normal weight (n=8) |
Obese (n=19) |
p-value | |
|---|---|---|---|
| Age (y) | 38(36,51) | 38(33,41) | 0.473 |
| Weight (kg) | 61(52,65) | 104(96,118) | <0.001 |
| BMI (kg/m2) | 22(20,25) | 38(35,42) | <0.001 |
| TPQ-NS | 16(10,17) | 15(10,18) | 0.938 |
| BES | 3(2,10) | 10(8,17) | 0.018 |
| SN D2R BPND | 2.48(2.35,2.57) | 2.66(2.43,2.89) | 0.159 |
| Acyl ghrelin (pg/ml) | 256(159,332) | 60(34,134) | <0.001 |
Figure 1.
Linear representations of the correlations between SN D2/D3R binding potential and novelty seeking traits (determined by TPQ-NS) in normal weight (▲, panel A) (r=−0.711, p=0.048) and obese (●, panel B) (non-significant) participants.
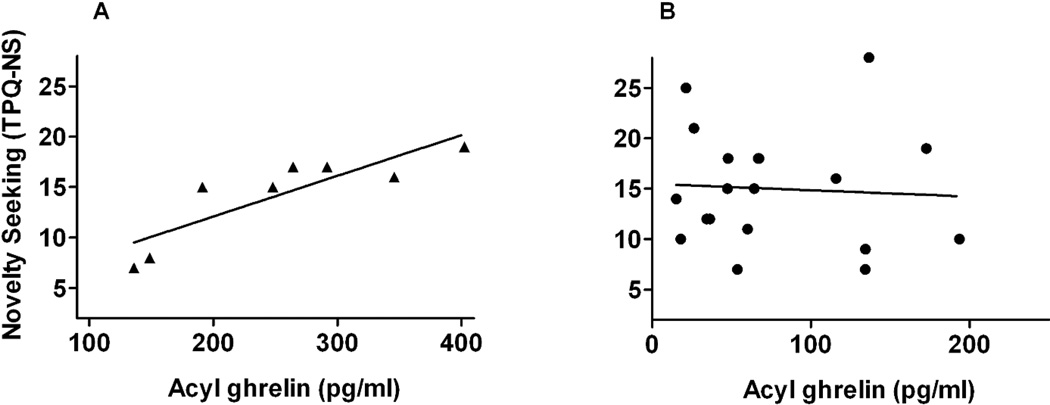
Figure 2.
Linear representations of the correlations between fasting acyl ghrelin levels and novelty seeking traits (determined by TPQ-NS) in normal weight (▲, panel A) (r=0.916, p=0.001) and obese (●, panel B) (non-significant) participants.
Figure 3.
Linear representations of the correlations between fasting acyl ghrelin levels and SN D2/D3R binding potential (BPND) in normal weight (▲, panel A) (r= −0.786, p=0.021) and obese (●, panel B) (r= 0.551, p=0.015) participants.
Participants included 8 normal weight and 19 obese (61kg vs 104kg) females who were similar in age. All were right handed except 2 of the obese participants. Normal weight and obese (16 vs15) had similar TPQ-NS scores while obese had significantly (p=0.018) higher BES scores than the normal weight (10 vs 3, respectively). SN DA D2R BPND were also similar between normal weight and obese (p>0.01).
By Kolmogorov-Smirnov test for normality, neither TPQ-NS scores (p=0.017) nor BES (p=0.020) were normally distributed in the normal weight group, as a significant test indicates a poor fit. In obese, acyl ghrelin levels were not normally distributed (p=0.003) but all other measures were normally distributed. In the normal weight group, we confirmed the previously reported negative relationship between TPQ-NS scores and SN DA D2/D3R BPND (r=−0.711, p=0.048) (Figure 1A). However, the relationship was not significant in the whole cohort (p> 0.10) nor in the obese (r=−0.04, p> 0.8) (Figure 1B). Based on the work of Hansson et al (23), who reported that in rodents ghrelin exposure to midbrain DA neurons led to greater novelty seeking, we investigated the relationship of acyl ghrelin levels to NS. Fasting acyl ghrelin levels were positively associated with TPQ-NS scores in the normal weight participants (r=0.916, p=0.001) but a significant relationship did not occur in the obese (r=−0.03, p> 0.8) (Figure 2A and 2B) nor the cohort as a whole (p> 0.10). Based on these findings, we sought to examine whether a direct relationship occurred between acyl ghrelin levels and SN D2/D3R BPND. SN D2R BPND and acyl ghrelin levels were significantly associated in both normal weight (r= −0.786, 0.021) and obese (r=0.551, p=0.015) but the relationships were in opposite directions (Figure 3A and 3B). There were no significant correlations between TPQ-NS with BES: whole cohort (p>0 .10), normal weight, (p> 0.10), and obese (p>0.10).
Discussion
In our normal weight females, we confirmed that D2/D3R BPND in the substantia nigra is negatively associated with novelty seeking traits as previously demonstrated in a younger, larger cohort of males and females (6), and supported by preclinical observations. However, in obese participants this relationship was not maintained. Obese participants also lacked the relationship of increased novelty seeking occurring with increased acyl ghrelin that was present in normal weight participants. Lastly, the relationship of acyl ghrelin levels to midbrain DA receptors in obese is opposite of that which occurs in normal weight. These findings suggest that DA functioning is altered in obesity, such that the normal relationship between midbrain DA signaling and individual differences in personality no longer hold and that alterations in neuroendocrine regulation may be involved.
We identified novel human relationships for acyl ghrelin with novelty seeking. In normal weight participants, novelty seeking was positively associated with fasting acyl ghrelin levels, which converges with preclinical findings in rodents (23). In the obese participants there was not a significant relationship of novelty seeking and acyl ghrelin levels, revealing that the neuroendocrine regulation of novelty seeking differs for obese and healthy weight.. While two single nucleotide polymorphisms (SNPs) in the GHSR gene have been associated with novelty seeking in humans (23), a relationship with obesity has not been identified (24). Further work investigating these SNPs impact on novelty seeking in obesity may be insightful. Interestingly, heterodimerzation of GHSR and D2R occurs in the hypothalamus and is necessary for D2R mediated anorexia (25). Heterodimerzation of GHSR and D2R has not been identified in other brain regions. A consideration for future investigation is whether heterodimerzation of GHSR and D2-like receptors occurs in the midbrain and if so whether this is influenced by obesity. Ghrelin resistance also occurs in DIO (26), and is another potential contributor to variation between obese and normal weight.
We found that that acyl ghrelin levels are directly related to SN D2/D3R availability, however, the direction of the relationship is opposite in normal weight and obese individuals, negative and positive respectively. Given that D2 receptors in the midbrain are primarily autoreceptors, the data in normal weight participants provides a convergent picture in which acyl ghrelin, midbrain D2/D3 receptor availability, and NS are each related. Acyl ghrelin enhances DA cell firing properties (27), and a reduction in autoreceptor control will similarly enhance DA release when stimulated (8)., This enhancement in DA excitability and release is expected to cause novelty seeking behaviors. To our knowledge, no previous studies have examined whether acyl ghrelin can alter expression of autoreceptors, but the present data suggests this possibility. The possibility of enhanced DA release in individuals with higher ghrelin levels is also consistent with our previous report that fasting acyl ghrelin levels are negatively associated with D2/D3R BPND throughout the striatum in normal weight and obese. If higher acyl ghrelin leads to greater DA release at the time of scanning, it may lower estimates of striatal binding potential, due to competition between [18F]fallypride and endogenous DA, consistent with our past interpretation of a ghrelin striatal dopamine link.
That the obese participants demonstrated an opposite pattern of relations between fasting acyl ghrelin levels and SN D2-like receptor availability, suggests that in obesity the midbrain DA system is altered such that the anticipated interaction with ghrelin is compromised. Because binding potential is multi-determined, ghrelin and other hormones related to obesity and feeding could influence different aspects of midbrain receptor availability. For instance, higher binding potential can reflect either larger numbers of DA neurons, or higher levels of D2/D3 receptors expression per DA neuron. Receptor availability can also be influenced by differences in somatodendritic DA release, which is essential for feedback inhibition and previously shown to be regulated by the hypothalamic stress hormone corticotropin-releasing factor (28), supporting the complexities of integration of information from both homeostatic and midbrain pathways. Another consideration is that [18F]fallypride binds at both D2 and D3 receptors. Recently it was suggested that variation in D3R density may explain the conflicting reports of striatal D2-like availability in obesity that were determined with D2/D3R radioligands (29). The midbrain has high levels of D3R (30), and thus our reported associations could specifically reflect D3R density. Whereas D2R provide the most potent inhibitory influence on DA neurons (31, 32), D3R also play a role in regulating DA neuron activity (33, 34). For instance, it has been suggested that D3R play a specific role in regulating basal DA levels through mechanisms that appear distinct from the intracellular pathways used by D2R (35). If D2R or D3R are differentially modulated by hormones related to feeding and obesity, and only one of these receptor types is linked to novelty seeking, then the ability to observe relations between combined D2/D3R binding in the SN may disappear. Interestingly, emerging data on D3R suggests that it may have some specific roles in feeding and addiction. SN D3R binding is increased in methamphetamine polydrug users and believed to contribute to the compulsive phenotype (36). Systemic antagonism of D3R is associated with reduced reactivity to drugs and food cues in rodents (37), and to food cues in overweight and obese individuals (38), but studies examining the relation of midbrain D3 receptors to obesity and feeding are lacking. Similarly, distinctions between D2R and D3R could help explain why the relationship of [18F]fallypride binding in the SN with acyl ghrelin is opposite in obese compared to normal weight.
We had hypothesized that binge eating would be a relevant food related correlate to novelty seeking behaviors but did not find an association between the two. Binge eating behaviors are reported to occur in as high as 64% of those seeking weight loss surgery. While we did not find a significant relationship, in a cohort of nearly 600 obese patients seeking medical treatment for obesity, BES scores were positively associated with novelty seeking (39). We found similar novelty seeking scores for obese and normal weight, but Sullivan et al in a larger cohort found that obese participants had ~20% higher TPQ-NS scores compared to normal weight. They also reported that lower baseline TPQ-NS predicted better weight loss and concluded that participants with higher novelty seeking possibly eat excessively out of boredom (40). Due to of the expense of PET imaging, our sample is naturally smaller. Additionally, our sample was limited to women, which does minimize any gender related confounds in the study. Thus, we may simply be underpowered to detect relations with BES, obesity and TPQ-NS. It is also worth noting that the TPQ-NS primarily addresses novelty seeking traits to nonfood stimuli which does limit the ability to draw conclusions related to eating behaviors. From our work we cannot conclude that novelty seeking and binge eating have a relevant relationship in obesity, but do propose that future investigations should consider food specific novelty seeking behaviors.
There are various limitations when interpreting the existing data. We focused on NS and the SN based on; 1) the previous human literature (6) in which the correlated voxels were highly consistent with the location of the SN, and 2) the spatial resolution of PET scanning, which limits the ability to dissociate binding potential in the VTA and SN. As scanning methodology advances, improved resolution may allow investigations delineating DA receptors in the SN and VTA, enhancing the understanding as to how these specific regions influence NS. Our participants only completed the NS portion of TPQ, but TPQ also allows measurement of reward dependence and harm avoidance (17), which likely have relevant relationships to DA signaling and obesity. Participants were studied in a fasted state which influences ghrelin levels; therefore postprandial measurements would allow a more complete understanding of neuroendocrine regulation of NS. The obese group had a larger range of variation for SN D2/D3R binding compared to the normal weight and future studies that include sub-group analyses may be insightful. The fasting acyl ghrelin levels were significantly lower in obese compared to normal weight, which others have reported (26). These very low levels potentially make the obese vulnerable to different regulators of NS, thus contributing to the loss of relationship that was present in our normal weight. Lastly, conclusions can only be related to females. Future studies will need to take these limitations into account.
In summary, in normal weight females the relationships of midbrain dopaminergic D2/D3 receptors and ghrelin levels to novelty seeking are consistent with preclinical models while neither relationship is maintained in obesity. This supports that the dopaminergic regulation of novelty seeking is compromised in obesity and may be influenced by changes in neuroendocrine hormones. In addition, we present evidence supporting a role for ghrelin in midbrain DA signaling and reward related behaviors. Hence, further work is necessary to understand the behaviors that drive and perpetuate obesity including in the area of neuroendocrine regulation of reward signaling.
What is already known about this subject?
Midbrain DA neurons regulate eating behaviors and have receptors for appetitive hormones including ghrelin.
D2/D3R availability in the region of the SN is negatively associated with NS traits in healthy humans.
Ghrelin exposure to midbrain DA neurons increases novelty seeking behaviors in rodents.
What this study adds?
D2/D3R availability in the SN had no association with NS traits in obese females but this relationship was confirmed in normal weight females of similar age.
Acyl ghrelin levels were positively associated with NS traits in the normal weight but no relationship occurred in obese.
D2/D3R availability in the SN and acyl ghrelin levels were negatively and positively associated in normal weight and obese females, respectively. To our knowledge this is the first human study to investigate the relationship of ghrelin and midbrain D2/D3R availability.
Acknowledgements
J.P.D. and N.N.A. conceived of experiments and obtained funding. J.P.D., S.W.S., R.M.K., R.L.C., and P.M.S. carried out experiments. J.P.D. oversaw all experiments and data analysis. All authors were involved in writing the paper and had final approval of the submitted and published versions.
This study was supported by National Institutes of Health (NIH) grants UL1-RR-024975 from the National Center for Research Resources (Vanderbilt Clinical and Translational Science Award), DK-20593 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK; Vanderbilt Diabetes Research and Training Award), DK-058404 from NIDDK (Vanderbilt Digestive Disease Research Center), K12- ESO15855 from the National Institute of Environmental Health Sciences (Vanderbilt Environmental Health Science Scholars Program) to J.P.D., and DK-70860 from the NIDDK to N.N.A.
Footnotes
Competing interests: the authors have no competing interests
References
- 1.Wise RA. Role of brain dopamine in food reward and reinforcement. Philosophical transactions of the Royal Society of London. 2006;361:1149–1158. doi: 10.1098/rstb.2006.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Volkow ND, Wang GJ, Baler RD. Reward, dopamine and the control of food intake: implications for obesity. Trends Cogn Sci. 2011;15:37–46. doi: 10.1016/j.tics.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cloninger CR. A unified biosocial theory of personality and its role in the development of anxiety states. Psychiatr Dev. 1986;4:167–226. [PubMed] [Google Scholar]
- 4.Klebaur JE, Bevins RA, Segar TM, Bardo MT. Individual differences in behavioral responses to novelty and amphetamine self-administration in male and female rats. Behav Pharmacol. 2001;12:267–275. doi: 10.1097/00008877-200107000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Stice E, Spoor S, Ng J, Zald DH. Relation of obesity to consummatory and anticipatory food reward. Physiology & Behavior. 2009;97:551–560. doi: 10.1016/j.physbeh.2009.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zald DH, Cowan RL, Riccardi P, Baldwin RM, Ansari MS, Li R, et al. Midbrain dopamine receptor availability is inversely associated with novelty-seeking traits in humans. J Neurosci. 2008;28:14372–14378. doi: 10.1523/JNEUROSCI.2423-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buckholtz JW, Treadway MT, Cowan RL, Woodward ND, Benning SD, Li R, et al. Mesolimbic dopamine reward system hypersensitivity in individuals with psychopathic traits. Nat Neurosci. 2010;13:419–421. doi: 10.1038/nn.2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marinelli M, White FJ. Enhanced vulnerability to cocaine self-administration is associated with elevated impulse activity of midbrain dopamine neurons. J Neurosci. 2000;20:8876–8885. doi: 10.1523/JNEUROSCI.20-23-08876.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson PM, Kenny PJ. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat Neurosci. 2010;13:635–641. doi: 10.1038/nn.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dunn JP, Kessler RM, Feurer ID, Volkow ND, Patterson BW, Ansari MS, et al. Relationship of dopamine type 2 receptor binding potential with fasting neuroendocrine hormones and insulin sensitivity in human obesity. Diabetes care. 2012;35:1105–1111. doi: 10.2337/dc11-2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang GJ, Volkow ND, Logan J, Pappas NR, Wong CT, Zhu W, et al. Brain dopamine and obesity. Lancet. 2001;357:354–357. doi: 10.1016/s0140-6736(00)03643-6. [DOI] [PubMed] [Google Scholar]
- 12.de Weijer BA, van de Giessen E, van Amelsvoort TA, Boot E, Braak B, Janssen IM, et al. Lower striatal dopamine D2/3 receptor availability in obese compared with non-obese subjects. EJNMMI Res. 2012;1:37. doi: 10.1186/2191-219X-1-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Castaneda TR, Tong J, Datta R, Culler M, Tschop MH. Ghrelin in the regulation of body weight and metabolism. Front Neuroendocrinol. 2010;31:44–60. doi: 10.1016/j.yfrne.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 14.Abizaid A. Ghrelin and Dopamine: New Insights on the Peripheral Regulation of Appetite. Journal of Neuroendocrinology. 2009;21:787–793. doi: 10.1111/j.1365-2826.2009.01896.x. [DOI] [PubMed] [Google Scholar]
- 15.Menzies JR, Skibicka KP, Leng G, Dickson SL. Ghrelin, reward and motivation. Endocr Dev. 25:101–111. doi: 10.1159/000346058. [DOI] [PubMed] [Google Scholar]
- 16.Jerlhag E, Egecioglu E, Dickson S, Engel J. Ghrelin receptor antagonism attenuates cocaine- and amphetamine-induced locomotor stimulation, accumbal dopamine release, and conditioned place preference. Psychopharmacology. 2010;211:415–422. doi: 10.1007/s00213-010-1907-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cloninger CR. The tridemensional personality questionnaire. Version IV. St. Louis, MO: Washington University School of Medicine; 1987. [Google Scholar]
- 18.Gormally J, Black S, Daston S, Rardin D. The assessment of binge eating severity among obese persons. Addict Behav. 1982;7:47–55. doi: 10.1016/0306-4603(82)90024-7. [DOI] [PubMed] [Google Scholar]
- 19.Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. Journal of personality assessment. 1996;67:588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- 20.Kessler RM, Woodward ND, Riccardi P, Li R, Ansari MS, Anderson S, et al. Dopamine D2 Receptor Levels in Striatum, Thalamus, Substantia Nigra, Limbic Regions, and Cortex in Schizophrenic Subjects. Biological psychiatry. 2009;65:1024–1031. doi: 10.1016/j.biopsych.2008.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lammertsma AA, Bench CJ, Hume SP, Osman S, Gunn K, Brooks DJ, et al. Comparison of methods for analysis of clinical [11C]raclopride studies. J Cereb Blood Flow Metab. 1996;16:42–52. doi: 10.1097/00004647-199601000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Dunn JP, Cowan RL, Volkow ND, Feurer ID, Li R, Williams DB, et al. Decreased dopamine type 2 receptor availability after bariatric surgery: preliminary findings. Brain research. 2010;1350:123–130. doi: 10.1016/j.brainres.2010.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hansson C, Shirazi RH, Naslund J, Vogel H, Neuber C, Holm G, et al. Ghrelin influences novelty seeking behavior in rodents and men. PLoS One. 2012;7:e50409. doi: 10.1371/journal.pone.0050409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gueorguiev M, Lecoeur C, Meyre D, Benzinou M, Mein CA, Hinney A, et al. Association studies on ghrelin and ghrelin receptor gene polymorphisms with obesity. Obesity (Silver Spring, Md. 2009;17:745–754. doi: 10.1038/oby.2008.589. [DOI] [PubMed] [Google Scholar]
- 25.Kern A, Albarran-Zeckler R, Walsh HE, Smith RG. Apo-ghrelin receptor forms heteromers with DRD2 in hypothalamic neurons and is essential for anorexigenic effects of DRD2 agonism. Neuron. 2012;73:317–332. doi: 10.1016/j.neuron.2011.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Briggs DI, Lockie SH, Wu Q, Lemus MB, Stark R, Andrews ZB. Calorie-restricted weight loss reverses high-fat diet-induced ghrelin resistance, which contributes to rebound weight gain in a ghrelin-dependent manner. Endocrinology. 154:709–717. doi: 10.1210/en.2012-1421. [DOI] [PubMed] [Google Scholar]
- 27.Abizaid A, Liu ZW, Andrews ZB, Shanabrough M, Borok E, Elsworth JD, et al. Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. J Clin Invest. 2006;116:3229–3239. doi: 10.1172/JCI29867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim Y, Park MK, Chung S. Regulation of somatodendritic dopamine release by corticotropin-releasing factor via the inhibition of voltage-operated Ca2+ channels. Neurosci Lett. 2009;465:31–35. doi: 10.1016/j.neulet.2009.08.066. [DOI] [PubMed] [Google Scholar]
- 29.Eisenstein SA, Antenor-Dorsey JA, Gredysa DM, Koller JM, Bihun EC, Ranck SA, et al. A comparison of D2 receptor specific binding in obese and normal-weight individuals using PET with (N-[C]methyl)benperidol. Synapse. doi: 10.1002/syn.21680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rabiner EA, Slifstein M, Nobrega J, Plisson C, Huiban M, Raymond R, et al. In vivo quantification of regional dopamine-D3 receptor binding potential of (+)-PHNO: Studies in non-human primates and transgenic mice. Synapse. 2009;63:782–793. doi: 10.1002/syn.20658. [DOI] [PubMed] [Google Scholar]
- 31.Usiello A, Baik JH, Rouge-Pont F, Picetti R, Dierich A, LeMeur M, et al. Distinct functions of the two isoforms of dopamine D2 receptors. Nature. 2000;408:199–203. doi: 10.1038/35041572. [DOI] [PubMed] [Google Scholar]
- 32.Centonze D, Usiello A, Gubellini P, Pisani A, Borrelli E, Bernardi G, et al. Dopamine D2 receptor-mediated inhibition of dopaminergic neurons in mice lacking D2L receptors. Neuropsychopharmacology. 2002;27:723–726. doi: 10.1016/S0893-133X(02)00367-6. [DOI] [PubMed] [Google Scholar]
- 33.Levant B. The D3 dopamine receptor: neurobiology and potential clinical relevance. Pharmacol Rev. 1997;49:231–252. [PubMed] [Google Scholar]
- 34.Tepper JM, Sun BC, Martin LP, Creese I. Functional roles of dopamine D2 and D3 autoreceptors on nigrostriatal neurons analyzed by antisense knockdown in vivo. J Neurosci. 1997;17:2519–2530. doi: 10.1523/JNEUROSCI.17-07-02519.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Le Foll B, Diaz J, Sokoloff P. Neuroadaptations to hyperdopaminergia in dopamine D3 receptor-deficient mice. Life Sci. 2005;76:1281–1296. doi: 10.1016/j.lfs.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 36.Boileau I, Payer D, Houle S, Behzadi A, Rusjan PM, Tong J, et al. Higher binding of the dopamine D3 receptor-preferring ligand [11C]-(+)-propyl-hexahydro-naphtho-oxazin in methamphetamine polydrug users: a positron emission tomography study. J Neurosci. 2012;32:1353–1359. doi: 10.1523/JNEUROSCI.4371-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thanos PK, Michaelides M, Ho CW, Wang GJ, Newman AH, Heidbreder CA, et al. The effects of two highly selective dopamine D3 receptor antagonists (SB-277011A and NGB-2904) on food self-administration in a rodent model of obesity. Pharmacol Biochem Behav. 2008;89:499–507. doi: 10.1016/j.pbb.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mogg K, Bradley BP, O'Neill B, Bani M, Merlo-Pich E, Koch A, et al. Effect of dopamine D(3) receptor antagonism on approach responses to food cues in overweight and obese individuals. Behav Pharmacol. 2012;23:603–608. doi: 10.1097/FBP.0b013e3283566a4a. [DOI] [PubMed] [Google Scholar]
- 39.Dalle Grave R, Calugi S, Marchesini G, Beck-Peccoz P, Bosello O, Compare A, et al. Personality features of obese women in relation to binge eating and night eating. Psychiatry Res. doi: 10.1016/j.psychres.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 40.Sullivan S, Cloninger CR, Przybeck TR, Klein S. Personality characteristics in obesity and relationship with successful weight loss. Int J Obes (Lond) 2007;31:669–674. doi: 10.1038/sj.ijo.0803464. [DOI] [PMC free article] [PubMed] [Google Scholar]