Abstract
Multidrug resistant (MDR) Gram negative bacterial infections are increasing in frequency and are associated with significant financial costs, morbidity and mortality. Current antibiotic therapies are associated with unacceptably poor clinical outcomes and toxicity. Unfortunately, the development of novel antimicrobials is stagnant leaving a significant clinical need for alternative treatments of MDR Gram negative rod infections. Recent preclinical studies have identified Th17 cells as critical mediators of broadly protective adaptive immunity, including protection against MDR infections. Studies of Th17 eliciting antigens, adjuvants and routes of immunization have identified potential vaccine strategies that may confer long-lived adaptive immunity against MDR Gram negative bacterial infections.
Introduction
Gram negative bacteria comprise a diverse group of organisms with various impacts on human health. Clinically, infections caused by Gram negative bacilli or rods (GNRs) manifest as meningitis, pneumonia, urinary tract infections and central venous catheter (CVC) infections amongst others, and currently effective treatment relies on the use of effective antibiotics. Increasingly, GNRs have become resistant to many currently available antibiotics due to inappropriate use of antibiotics [1,2], excessive use of antibiotics in agriculture [3], through person to person spread [4]and transmission of genetic elements that encode resistance between GNR species in these settings and others [4–7]. Some GNR species are resistant to several different classes of antibiotic and though the definition of multidrug resistance (MDR) varies by organism and author most MDR GNRs are resistant to at least three different antibiotic classes (e.g. penicillins, cephalosporins, quinolones, aminoglycosides, carbapenems). The molecular mechanisms of resistance include the acquisition of genes that encode enzymes such as extended-spectrum beta-lactamase (ESBL) and Klebsiella pneumoniae carbapenemase (KPC), which enzymatically inactivate these classes of antibiotics; decreased uptake of antibiotics including porin mutations; efflux pumps that actively transport antibiotic out of the organism, and altered antibiotic targets, such as penicillin binding proteins (PBPs). Often organisms employ more than one resistance mechanism. Infections caused by MDR GNR are increasingly frequent, and the morbidity, mortality and financial costs associated with these infections are unacceptably high [8,9]. The CDC estimates that in the U.S. MDR GNR infection results in approximately 40,000 cases leading to more than 2,800 deaths (CDC 2013 Threat report). Meanwhile, the development of novel antibacterial agents and classes remains stagnant and a cause of significant concern [•10]. This combination of increasing numbers of clinically significant MDR GNR infections coupled with limited new therapeutic options has led practitioners, healthcare organizations and government healthcare agencies to devote considerable resources to the evaluation, prevention and treatment of these infections [11]. Indeed, in his 2014 State of the Union address, President Obama espoused the importance of supporting research focusing on “vaccines that stay ahead of drug-resistant bacteria”.
Carbapenemase-resistant Enterobacteriaciae (CRE), such as Enterobacter species and Klebsiella pneumoniae have been described causing nosocomial outbreaks in hospitals, intensive care units and long-term care facilities, and preventive measures including extensive infection control procedures, outbreak investigation, and antimicrobial stewardship efforts, have had a real but limited impact on the burden of these infections [4]. Other organisms such as Pseudomonas aeruginosa, Stenotrophamonas maltophilia, and Burkholderia species are intrinsically resistant to many classes of antibiotic and can develop resistance while patients are receiving antibiotic therapy. In addition to a major cause of hospital acquired infections, MDR Acinetobacter baumanii has recently been increasingly recognized for causing infections involving traumatic wounds in military personnel [12].
Current Approaches to Multidrug Resistant Gram Negative Infection Treatment
Current treatment for MDR GNR infection centers on antimicrobial therapy. Many novel antibiotic treatment strategies include the use of agents, such as polymyxin derivatives (e.g. colistin), aminoglycosides, quinolones and tigecycline. Clinical efficacy of treatment with these agents has been limited and many studies have not been well-controlled. Further, the associated toxicities (nephrotoxicity, cardiotoxicity) of these agents limit their use given the co-morbidities that MDR GNR infected patients often face. Combination antimicrobial therapy (including the use of carbapenems) has shown significant benefit over monotherapy[13], though this does not obviate the associated drug toxicities. At this time, few clinical trials are underway to evaluate the best management of these devastating infections (clinicaltrials.gov.). Although enhancing infection control practices, improving the reliability of the screening methods and optimizing the usage of antibiotics currently available can address some of the urgent clinical challenges, the best therapeutic approach to MDR GNR organisms has yet to be defined. As the burden of disease caused by MDR GNR infections increases, collaborative efforts aimed at improved infection prevention, research and development of antibiotic alternatives are desperately needed[14].
Immune Responses to MDR GNR infection
Although Enterobacteriaciae (Klebsiella, E. coli and Serratia) can cause infection in young, healthy individuals such as urinary tract infections, liver and lung abscesses, MDR GNRs disproportionately infect chronically ill, healthcare exposed and immunocompromised individuals. Understanding host immune responses against MDR GNR infection is critical to identifying possible immunotherapeutics as an antibiotic alternative. Preclinical studies examining immunity to MDR GNR primarily has involved murine systems to date. Infection by Gram negative organisms are recognized in an innate immune response through the involvement of pattern recognition receptors (LPS, lipoproteins, flagella) that activate Toll-like receptors (TLRs), Nod-like receptors (NLRs), C-type lectin receptors and complement receptors resulting in recruitment of innate immune effector cells including neutrophils, macrophages. In the case of K. pneumoniae, TLR4 is an essential component of innate recognition and controls nearly 70% of the gene expression changes in response to the organisms [15]. In pre-clinical models of infection, adaptive immune responses elicited by GNR infection result in B and T cell memory responses that confer long-term serotype dependent and independent immunity, as summarized in Figure 1[16]. However, a clinical trial of pathogen specific hyperimmune IVIG against Klebsiella and Pseudomonas administered to critically-ill adults failed to significantly improve overall outcomes and the trial terminated due to increased side effects, most commonly fever and hypertension, in the intervention group[17]. Recent preclinical studies examining immunity to GNR infection have identified a critical role for T-cell immunity in host resistance against K. pneumoniae. Moreover, there are emerging potential immunogenic antigens that may serve as vaccine candidates.
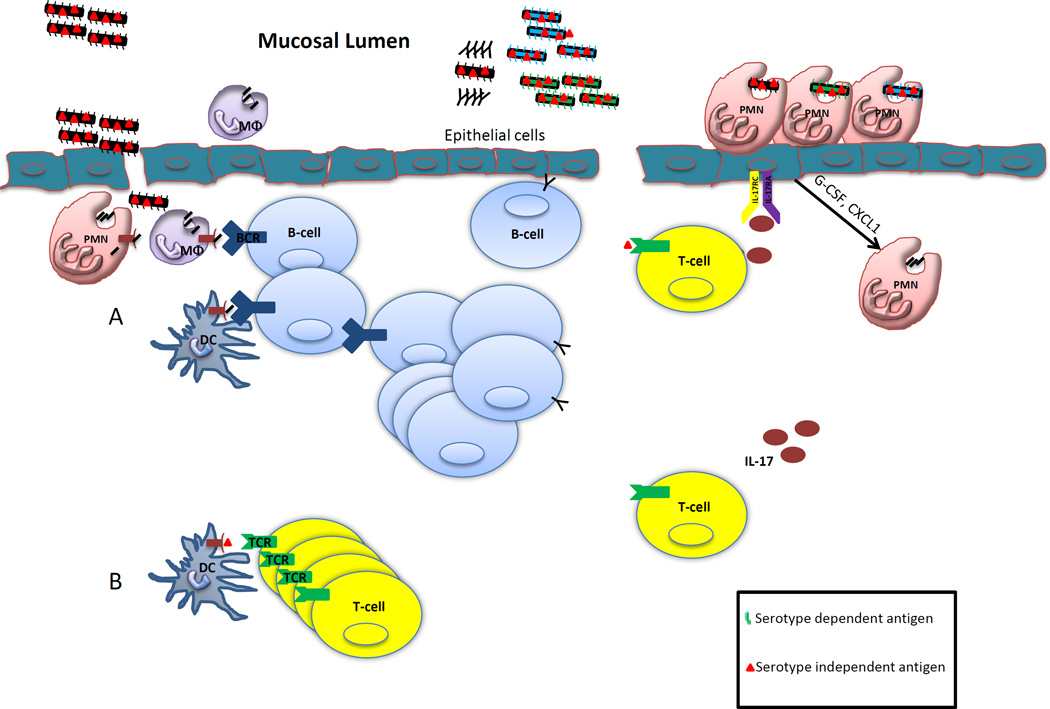
Figure 1. Serotype dependent and independent vaccine immunity.
A) Polysaccharide based vaccines are thought to induce clonally expanded memory B-cells and antibody secreting cells resulting in humoral protection against the vaccine serotypes. Upon challenge by the pathogen, antibodies can be rapidly produced neutralizing the infection, though conferring no protective immunity against heterologous strains. B) Alternatively, following vaccination with certain protein antigens from bacteria, class II MHC restricted Th17 cells can be elicited. Upon challenge by autologous or heterologous serotypes of the bacterial pathogens, Th17 cells secrete IL-17 activating IL-17RA/RC receptors eliciting cytokines and chemoattractants. This results in the rapid recruitment of neutrophils to site of infection which aid in pathogen clearance.
K. pneumoniae infection has been studied extensively in pre-clinical mouse models and many T-cell derived cytokines such as IFN-γ and IL-17 have been shown to be essential in the host defense against pulmonary infection[18]. Since both cytokines can be made by CD4+ T helper cells in an antigen specific manor, a T-cell based vaccine seems to be a promising alternative in addressing the challenges raised by K. pneumoniae infections. Th1 immunity mediates host defense against K. pneumoniae as bacterial clearance can be improved by giving exogenous IL-12 or immunogenic agents such as CpG ODN[19,20]. Both interventions augment IFN-γ production and the enhanced protection is compromised by blockade of IFN-γ, suggesting that Th1 responses elicited by immunization can be beneficial in improving vaccine efficacy against K. pneumoniae. Furthermore, overexpression of IL-17 by adenovirus can promote the clearance of the bacteria suggesting that enhancement of IL-17 can be beneficial in the development of a vaccine against K. pneumoniae[21]. Indeed, immunization with heat killed K. pneumoniae induces a robust Th17 response. Although the immunization also induces substantial titers of antibodies against the immunization serotype of K. pneumoniae, this antibody response was largely directed against the vaccine serotype (serotype 2) with limited cross-reactivity to other clinically important serotypes including serotype 1, and 16, as well as the metallobetalactamase-producing K. pneumoniae strain NDM1. In contrast, the vaccine elicited Th17 response recognized crude antigen preparations from all of these serotypes as well as other Enterobacterciae family members such as E. coli. Th17 cells were thus able to mediate B-cell independent heterologous immunity when mice were immunized with serotype 2 K. pneumoniae but challenged with a serotype 1 isolate[••22]. B-cell independent vaccine-induced immunity against K. pneumonniae requires effective recruitment of neutrophils and signaling via IL-17 receptor C (IL-17RC) signaling as depletion of neutrophils and blockade of IL-17RC signal abolished the vaccine induced protection.
Pseudomonas aeruginosa pneumonia models have similarly identified a significant role for LPS serotype independent T-cell mediated immunity, and this heterologous immunity is IL-17 dependent[23]. Indeed, Th17 stimulating antigens identified by protein library screening effectively confer serotype-independent immunity, potentiated by a Th17 promoting adjuvant[••24]. Though beyond the scope of this article, Streptococcus pneumoniae pneumonia models have similarly identified that Th17 cells mediate serotype-independent immunity that is broadly protective[25]. Indeed, antigen preparation used in these models can elicit IL-17 production from peripheral blood mononuclear cells in pediatric and adult populations in from developed and developing countries, suggesting that Th17 cells mediate natural S. pneumoniae immune protection[26].
Other models of GNR infection suggest that Th17 responses play a critical role in immune protection. Innate intestinal Th17 cells mediate protection against Citrobacter and Salmonella infection[27]. Intestinal infection with Shigella flexneri elicits a robust T cell response to initial infection associated with IFNγ, IL-17 and IL-22 production. Upon re-challenge Th17 cells mediate bacterial clearance and improved survival[28]. Control of Acinetobacter baumanii infection is neutrophil dependent, though the role of IL-17 and Th17 cells has not been adequately investigated [29].
Taken together, these data demonstrate the critical role of Th17 mediated immunity and feasibility to develop Th17 based vaccine against GNRs. The advantage of these Th17 based vaccines is that the protection is broad, includes MDR strains and is not restricted to specific serotypes. However, the requirements of propter neutrophil function and IL-17R signaling will possibly limit these vaccine strategies to patients without neutropenia or defects in IL-17/IL-17R function.
MDR GNR Vaccine Design
As Th17 cells confer serotype-independent immunity against heterologous infection, these cells likely recognize conserved antigens common among enterobacteriaciae family members. One group of antigens recognized by Th17 cells are outer membrane proteins of K. pneumoniae, a group of proteins that are highly conserved among Klebsiella species, and the recognition of these antigens was highly dependent on MHC class II, suggesting that these proteins can be well defined and serve as antigen candidates for clinical translation. Vaccination with purified outer membrane proteins of K. pneumoniae also elicits a strong Th17 response and provides heterologous protection against a broad spectrum of different strains including the newly described metallo-beta-lactamase 1 producing strain [22]. Another group of antigens include the machinery of the type 3 secretion system, such as the Pseudomonas aeruginosa PopB[24].
In this model, Th17 elicited responses were potentiated by the adjuvant curdlan. Adjuvants compounds have commonly been employed to augment the immune responses against immunizing antigens and improve the efficacy of the vaccines. Aluminum-based adjuvants are most commonly used in most FDA approved vaccines (CDC). Though the precise mechanism by which alum functions as an adjuvant remains unclear, it has been shown prime Th2 responses and promote antibody production as well as inflammasome[30,31]. To achieve better protection using Th17-based vaccines, many mucosal adjuvants have been tested for the ability of inducing antigen specific Th17 cells. Cholera toxin is a robust Th17 inducing mucosal adjuvant by augmenting the secretion of IL-1 by dendritic cells in a cAMP dependent mechanism [32]. Heat labile enterotoxin from E. coli can also induce robust mucosal Th17 responses through activation of inflammasome and IL-1 and IL-23 produciton from dendritic cells[33]. Toll-like receptor ligands such as monophospho lipid A (MPL)- trehalose dimycolate (TDM) can also induce a mixed Th1 and Th17 responses [34] and serve as a promising adjuvants for vaccine against MDR GNR. While robust pathogen specific T helper responses are highly desired and can be achieved by using highly immunogenic antigen with effective adjuvants the safety of such vaccines must be carefully examined in preclinical models. Indeed, Th17 inducing adjuvants, in the absence of antigen, were associated with increased lung pathology, morbidity and mortality in an influenza murine model[•35]. However, less toxic modified adjuvants, are being actively being studied to bridge this gap in vaccine design[36,37]. Ideal antigen candidates should be a conserved antigen among different serotypes to elicit a broad response against multiple species while an ideal adjuvant should maximize the antigen specific adaptive immune response with limited or no toxicity to the host.
Route of administration must be an additional consideration in MDR GNR vaccine design. As most pathogens access to the body via mucous membranes, it is not surprising that mucosal immunization is highly effective at inducing long-term B and T cell memory[38–40]. Th17 cells are long-lived effector memory cells in mucosal tissues[41]. Pre-clinical models demonstrating Th17 mediated protection and the development of mucosa-associated lymphoid tissue (MALT) have employed intranasal and oral antigen immunization[22,24,42]. The immune responses induced by nasal delivery are usually highly robust and confer effective protection. Moreover, the injection delivery method can be costly and requires trained personnel for delivery. In contrast, the ease of administration of mucosal vaccines may confer a higher rate of compliance. It must be noted however that mucosal vaccination necessarily must maintain immunogenicity as vaccine antigens and adjuvants are exposed to mucosal enzymes in the in the gastrointestinal and respiratory tract before generating the immune responses. The protection efficacy of oral delivery will be a major challenge for the development Th17 based oral vaccines.
Conclusions and Perspectives
Multidrug resistant Gram negative bacilli are responsible for a variety of nosocomial and community acquired infections. The incidence of MDR infections has increased worldwide, and the morbidity, mortality and financial costs are significant. Considerable resources focused on evaluation and prevention have had limited success in controlling the spread and impact of MDR GNR infection. Current antibiotic therapies are associated with poor outcomes, considerable toxicity and are largely unproven. Unfortunately, few new antibiotic agents are under development, and novel prevention and treatment strategies are imperative. The few clinical trials addressing MDR infection focus entirely on antimicrobials, but no MDR GNR vaccine trials are currently underway.
Immune mechanisms underlying defense against GNR infection suggest that innate and adaptive responses including antibody, B and T cell responses are critical to long lasting immunity. Strategies to identify Th17 stimulating immunogenic proteins have identified potential vaccine candidates such as highly conserved outer membrane proteins and virulence factors. Such pre-clinical vaccine models show that Th17 mediated immunity can confer broad protection from MDR GNR infection. Ongoing investigation into specific factors mediating immunity against Gram negative bacteria can identify future vaccine targets, adjuvants and techniques. Clinical translation of these findings has the potential to provide a novel strategy for protection against multidrug resistant Gram negative infection.
Highlights.
Multidrug resistant Gram negative infections are increasingly frequent largely due to inappropriate antibiotic usage.
Current therapies for MDR GNR infection are associated with poor outcomes, and few novel antibiotics are on the horizon.
Adaptive immune responses protect against GNR infection. Antibody confers autologous immunity; Th17 cells confer heterologous immunity.
Th17 vaccine antigens include highly conserved pathogenic molecules.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and Recommended Reading
•of particular interest
••of outstanding interest
- 1.Barnett ML, Linder JA. Antibiotic prescribing to adults with sore throat in the United States, 1997–2010. JAMA Intern Med. 2014;174:138–140. doi: 10.1001/jamainternmed.2013.11673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hersh AL, Shapiro DJ, Pavia AT, Shah SS. Antibiotic prescribing in ambulatory pediatrics in the United States. Pediatrics. 2011;128:1053–1061. doi: 10.1542/peds.2011-1337. [DOI] [PubMed] [Google Scholar]
- 3.Harrison EM, Paterson GK, Holden MT, Larsen J, Stegger M, Larsen AR, Petersen A, Skov RL, Christensen JM, Bak Zeuthen A, et al. Whole genome sequencing identifies zoonotic transmission of MRSA isolates with the novel mecA homologue mecC. EMBO Mol Med. 2013;5:509–515. doi: 10.1002/emmm.201202413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Snitkin ES, Zelazny AM, Thomas PJ, Stock F, Group NCSP, Henderson DK, Palmore TN, Segre JA. Tracking a hospital outbreak of carbapenem-resistant Klebsiella pneumoniae with whole-genome sequencing. Sci Transl Med. 2012;4 doi: 10.1126/scitranslmed.3004129. 148ra116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cuzon G, Naas T, Truong H, Villegas MV, Wisell KT, Carmeli Y, Gales AC, Venezia SN, Quinn JP, Nordmann P. Worldwide diversity of Klebsiella pneumoniae that produce beta-lactamase blaKPC-2 gene. Emerg Infect Dis. 2010;16:1349–1356. doi: 10.3201/eid1609.091389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Z, Zhang XX, Huang K, Miao Y, Shi P, Liu B, Long C, Li A. Metagenomic profiling of antibiotic resistance genes and mobile genetic elements in a tannery wastewater treatment plant. PLoS One. 2013;8:e76079. doi: 10.1371/journal.pone.0076079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Disease C, Prevention. Vital signs: carbapenem-resistant Enterobacteriaceae. MMWR Morb Mortal Wkly Rep. 2013;62:165–170. [PMC free article] [PubMed] [Google Scholar]
- 8.Roberts RR, Hota B, Ahmad I, Scott RD, 2nd, Foster SD, Abbasi F, Schabowski S, Kampe LM, Ciavarella GG, Supino M, et al. Hospital and societal costs of antimicrobial-resistant infections in a Chicago teaching hospital: implications for antibiotic stewardship. Clin Infect Dis. 2009;49:1175–1184. doi: 10.1086/605630. [DOI] [PubMed] [Google Scholar]
- 9.Sievert DM, Ricks P, Edwards JR, Schneider A, Patel J, Srinivasan A, Kallen A, Limbago B, Fridkin S. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009–2010. Infect Control Hosp Epidemiol. 2013;34:1–14. doi: 10.1086/668770. [DOI] [PubMed] [Google Scholar]
- 10. Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, Scheld M, Spellberg B, Bartlett J. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis. 2009;48:1–12. doi: 10.1086/595011. •This report by the IDSA summarizes the increasing burden of multidrug resistant infections, the state of clincal antimicrobial discovery and identifies barriers to antibiotic development.
- 11.Tacconelli E, Cataldo MA, Dancer SJ, De Angelis G, Falcone M, Frank U, Kahlmeter G, Pan A, Petrosillo N, Rodriguez-Bano J, et al. ESCMID guidelines for the management of the infection control measures to reduce transmission of multidrug-resistant Gram-negative bacteria in hospitalized patients. Clin Microbiol Infect. 2014;20(Suppl 1):1–55. doi: 10.1111/1469-0691.12427. [DOI] [PubMed] [Google Scholar]
- 12.Davis KA, Moran KA, McAllister CK, Gray PJ. Multidrug-resistant Acinetobacter extremity infections in soldiers. Emerg Infect Dis. 2005;11:1218–1224. doi: 10.3201/1108.050103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qureshi ZA, Paterson DL, Potoski BA, Kilayko MC, Sandovsky G, Sordillo E, Polsky B, Adams-Haduch JM, Doi Y. Treatment outcome of bacteremia due to KPC-producing Klebsiella pneumoniae: superiority of combination antimicrobial regimens. Antimicrob Agents Chemother. 2012;56:2108–2113. doi: 10.1128/AAC.06268-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stanton TB. A call for antibiotic alternatives research. Trends Microbiol. 2013;21:111–113. doi: 10.1016/j.tim.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 15.Schurr JR, Young E, Byrne P, Steele C, Shellito JE, Kolls JK. Central role of toll-like receptor 4 signaling and host defense in experimental pneumonia caused by Gram-negative bacteria. Infect Immun. 2005;73:532–545. doi: 10.1128/IAI.73.1.532-545.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yates JL, Racine R, McBride KM, Winslow GM. T cell-dependent IgM memory B cells generated during bacterial infection are required for IgG responses to antigen challenge. J Immunol. 2013;191:1240–1249. doi: 10.4049/jimmunol.1300062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Donta ST, Peduzzi P, Cross AS, Sadoff J, Haakenson C, Cryz SJ, Jr., Kauffman C, Bradley S, Gafford G, Elliston D, et al. Immunoprophylaxis against klebsiella and pseudomonas aeruginosa infections. The Federal Hyperimmune Immunoglobulin Trial Study Group. J Infect Dis. 1996;174:537–543. doi: 10.1093/infdis/174.3.537. [DOI] [PubMed] [Google Scholar]
- 18.Moore TA, Perry ML, Getsoian AG, Newstead MW, Standiford TJ. Divergent role of gamma interferon in a murine model of pulmonary versus systemic Klebsiella pneumoniae infection. Infect Immun. 2002;70:6310–6318. doi: 10.1128/IAI.70.11.6310-6318.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deng JC, Moore TA, Newstead MW, Zeng X, Krieg AM, Standiford TJ. CpG oligodeoxynucleotides stimulate protective innate immunity against pulmonary Klebsiella infection. J Immunol. 2004;173:5148–5155. doi: 10.4049/jimmunol.173.8.5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greenberger MJ, Kunkel SL, Strieter RM, Lukacs NW, Bramson J, Gauldie J, Graham FL, Hitt M, Danforth JM, Standiford TJ. IL-12 gene therapy protects mice in lethal Klebsiella pneumonia. J Immunol. 1996;157:3006–3012. [PubMed] [Google Scholar]
- 21.Ye P, Garvey PB, Zhang P, Nelson S, Bagby G, Summer WR, Schwarzenberger P, Shellito JE, Kolls JK. Interleukin-17 and lung host defense against Klebsiella pneumoniae infection. Am J Respir Cell Mol Biol. 2001;25:335–340. doi: 10.1165/ajrcmb.25.3.4424. [DOI] [PubMed] [Google Scholar]
- 22. Chen K, McAleer JP, Lin Y, Paterson DL, Zheng M, Alcorn JF, Weaver CT, Kolls JK. Th17 cells mediate clade-specific, serotype-independent mucosal immunity. Immunity. 2011;35:997–1009. doi: 10.1016/j.immuni.2011.10.018. ••This study demonstrates the critical role of Th17 cells in mediating heterologous immunity in a Klebsiella pneumoniae model, including multidrug resistant strains.
- 23.Priebe GP, Walsh RL, Cederroth TA, Kamei A, Coutinho-Sledge YS, Goldberg JB, Pier GB. IL-17 is a critical component of vaccine-induced protection against lung infection by lipopolysaccharide-heterologous strains of Pseudomonas aeruginosa. J Immunol. 2008;181:4965–4975. doi: 10.4049/jimmunol.181.7.4965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wu W, Huang J, Duan B, Traficante DC, Hong H, Risech M, Lory S, Priebe GP. Th17-stimulating protein vaccines confer protection against Pseudomonas aeruginosa pneumonia. Am J Respir Crit Care Med. 2012;186:420–427. doi: 10.1164/rccm.201202-0182OC. ••This study illustrates a novel Th17 antigen discovery technique and validates the findings in a Pseudomonas aeruginosa model.
- 25.Moffitt KL, Gierahn TM, Lu YJ, Gouveia P, Alderson M, Flechtner JB, Higgins DE, Malley R. T(H)17-based vaccine design for prevention of Streptococcus pneumoniae colonization. Cell Host Microbe. 2011;9:158–165. doi: 10.1016/j.chom.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lundgren A, Bhuiyan TR, Novak D, Kaim J, Reske A, Lu YJ, Qadri F, Malley R. Characterization of Th17 responses to Streptococcus pneumoniae in humans: comparisons between adults and children in a developed and a developing country. Vaccine. 2012;30:3897–3907. doi: 10.1016/j.vaccine.2012.03.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geddes K, Rubino SJ, Magalhaes JG, Streutker C, Le Bourhis L, Cho JH, Robertson SJ, Kim CJ, Kaul R, Philpott DJ, et al. Identification of an innate T helper type 17 response to intestinal bacterial pathogens. Nat Med. 2011;17:837–844. doi: 10.1038/nm.2391. [DOI] [PubMed] [Google Scholar]
- 28.Sellge G, Magalhaes JG, Konradt C, Fritz JH, Salgado-Pabon W, Eberl G, Bandeira A, Di Santo JP, Sansonetti PJ, Phalipon A. Th17 cells are the dominant T cell subtype primed by Shigella flexneri mediating protective immunity. J Immunol. 2010;184:2076–2085. doi: 10.4049/jimmunol.0900978. [DOI] [PubMed] [Google Scholar]
- 29.Breslow JM, Meissler JJ, Jr., Hartzell RR, Spence PB, Truant A, Gaughan J, Eisenstein TK. Innate immune responses to systemic Acinetobacter baumannii infection in mice: neutrophils, but not interleukin-17, mediate host resistance. Infect Immun. 2011;79:3317–3327. doi: 10.1128/IAI.00069-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar P, Chen K, Kolls JK. Th17 cell based vaccines in mucosal immunity. Curr Opin Immunol. 2013;25:373–380. doi: 10.1016/j.coi.2013.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kool M, Soullie T, van Nimwegen M, Willart MA, Muskens F, Jung S, Hoogsteden HC, Hammad H, Lambrecht BN. Alum adjuvant boosts adaptive immunity by inducing uric acid and activating inflammatory dendritic cells. J Exp Med. 2008;205:869–882. doi: 10.1084/jem.20071087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Datta SK, Sabet M, Nguyen KP, Valdez PA, Gonzalez-Navajas JM, Islam S, Mihajlov I, Fierer J, Insel PA, Webster NJ, et al. Mucosal adjuvant activity of cholera toxin requires Th17 cells and protects against inhalation anthrax. Proc Natl Acad Sci U S A. 2010;107:10638–10643. doi: 10.1073/pnas.1002348107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brereton CF, Sutton CE, Ross PJ, Iwakura Y, Pizza M, Rappuoli R, Lavelle EC, Mills KH. Escherichia coli heat-labile enterotoxin promotes protective Th17 responses against infection by driving innate IL-1 and IL-23 production. J Immunol. 2011;186:5896–5906. doi: 10.4049/jimmunol.1003789. [DOI] [PubMed] [Google Scholar]
- 34.Vitoriano-Souza J, Moreira N, Teixeira-Carvalho A, Carneiro CM, Siqueira FA, Vieira PM, Giunchetti RC, Moura SA, Fujiwara RT, Melo MN, et al. Cell recruitment and cytokines in skin mice sensitized with the vaccine adjuvants: saponin, incomplete Freund's adjuvant, and monophosphoryl lipid A. PLoS One. 2012;7:e40745. doi: 10.1371/journal.pone.0040745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gopal R, Rangel-Moreno J, Fallert Junecko BA, Mallon DJ, Chen K, Pociask DA, Connell TD, Reinhart TA, Alcorn JF, Ross TM, et al. Mucosal pre-exposure to th17-inducing adjuvants exacerbates pathology after influenza infection. Am J Pathol. 2014;184:55–63. doi: 10.1016/j.ajpath.2013.09.012. •This study identifies toxicity of Th17 inducing adjuvants in the absence of antigen leading to worsened pathology and increased morbidity in an influenza model.
- 36.Summerton NA, Welch RW, Bondoc L, Yang HH, Pleune B, Ramachandran N, Harris AM, Bland D, Jackson WJ, Park S, et al. Toward the development of a stable, freeze-dried formulation of Helicobacter pylori killed whole cell vaccine adjuvanted with a novel mutant of Escherichia coli heat-labile toxin. Vaccine. 2010;28:1404–1411. doi: 10.1016/j.vaccine.2009.10.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lycke N, Bemark M. Mucosal adjuvants and long-term memory development with special focus on CTA1-DD and other ADP-ribosylating toxins. Mucosal Immunol. 2010;3:556–566. doi: 10.1038/mi.2010.54. [DOI] [PubMed] [Google Scholar]
- 38.Brandtzaeg P. Induction of secretory immunity and memory at mucosal surfaces. Vaccine. 2007;25:5467–5484. doi: 10.1016/j.vaccine.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 39.Sheridan BS, Lefrancois L. Regional and mucosal memory T cells. Nat Immunol. 2011;12:485–491. doi: 10.1038/ni.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lycke N. Recent progress in mucosal vaccine development: potential and limitations. Nat Rev Immunol. 2012;12:592–605. doi: 10.1038/nri3251. [DOI] [PubMed] [Google Scholar]
- 41.Kryczek I, Zhao E, Liu Y, Wang Y, Vatan L, Szeliga W, Moyer J, Klimczak A, Lange A, Zou W. Human TH17 cells are long-lived effector memory cells. Sci Transl Med. 2011;3 doi: 10.1126/scitranslmed.3002949. 104ra100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rangel-Moreno J, Carragher DM, de la Luz Garcia-Hernandez M, Hwang JY, Kusser K, Hartson L, Kolls JK, Khader SA, Randall TD. The development of inducible bronchus-associated lymphoid tissue depends on IL-17. Nat Immunol. 2011;12:639–646. doi: 10.1038/ni.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]