Abstract
In 1962, epidermal growth factor (EGF) was discovered by Dr. Stanley Cohen while studying nerve growth factor (NGF). It was soon recognized that EGF is the prototypical member of a family of peptide growth factors that activate the EGF receptors, and that the EGF/EGF receptor signaling pathway plays important roles in proliferation, differentiation and migration of a variety of cell types, especially in epithelial cells. After the basic characterization of EGF function in the first decade or so after its discovery, the studies related to EGF and its signaling pathway have extended to a broad range of investigations concerning its biological and pathophysiological roles in development and in human diseases. In this review, we briefly describe the gene organization and tissue distribution of EGF, with emphasis on its biological and pathological roles in human diseases.
Keywords: Epidermal growth factor, expression, cell proliferation, regeneration, ion transport, cancer
1. Introduction
EGF was discovered by Dr. Stanley Cohen more than half a century ago [1]. He found that injection of a submaxillary gland extract into newborn mice induced precocious eyelid opening and incisor eruption due to a direct stimulation of epidermal growth and keratinization. Consequently, EGF was isolated, purified, and characterized. It is a single-chain polypeptide consisting of 53 amino acids that is derived from the cleavage of a large precursor, prepro-EGF. Urogastrone, an inhibitor of gastric acid secretion, was independently isolated from human urine and was subsequently found to be structurally and functionally identical to mouse EGF and was proven to be human EGF [2,3]. EGF is now known as the prototype of the group I EGF family that also includes transforming growth factor-α (TGF-α), heparin-binding EGF (HB-EGF), amphiregulin, betacellulin, epiregulin and epigen [4,5]. Structurally, they all contain one or more EGF repeats (EGF motif) in their extracellular domain, which is a sequence of 35–40 amino acids spaced by six conserved cysteines in the following pattern: CX7CX3–5CX10–12CXCX5GXRC (C, cysteine; G, glycine; R, arginine; X, other amino acids). One glycine and one arginine in this sequence are also conserved in all EGF-related growth factors but not in proteins that contain EGF motifs without growth factor activity [6]. The six cysteines pair and form three intramolecular disulfide bonds with the following interactions: C1–C3, C2–C4 and C5–C6 (numbered according to their order in the sequence), which are important for maintaining their biological activities [7]. Functionally, these growth factors share the ability to bind the same receptor, the EGF receptor (EGFR, ErbB1), activate its intrinsic tyrosine kinase activity, and couple the receptor to downstream signaling pathways controlling cell proliferation, differentiation, survival, or motility [6,8,9]. Studies of the EGF family /EGFR continue to provide insights into roles for this axis in development, physiology and disease. As for EGF per se, the studies have shifted from its basic characterization to its role in biology, pathology and clinical application in human diseases. Therefore, this review will describe briefly the gene organization and tissue distribution of EGF, with emphasis on its biological and pathological roles in human diseases.
2. Gene organization
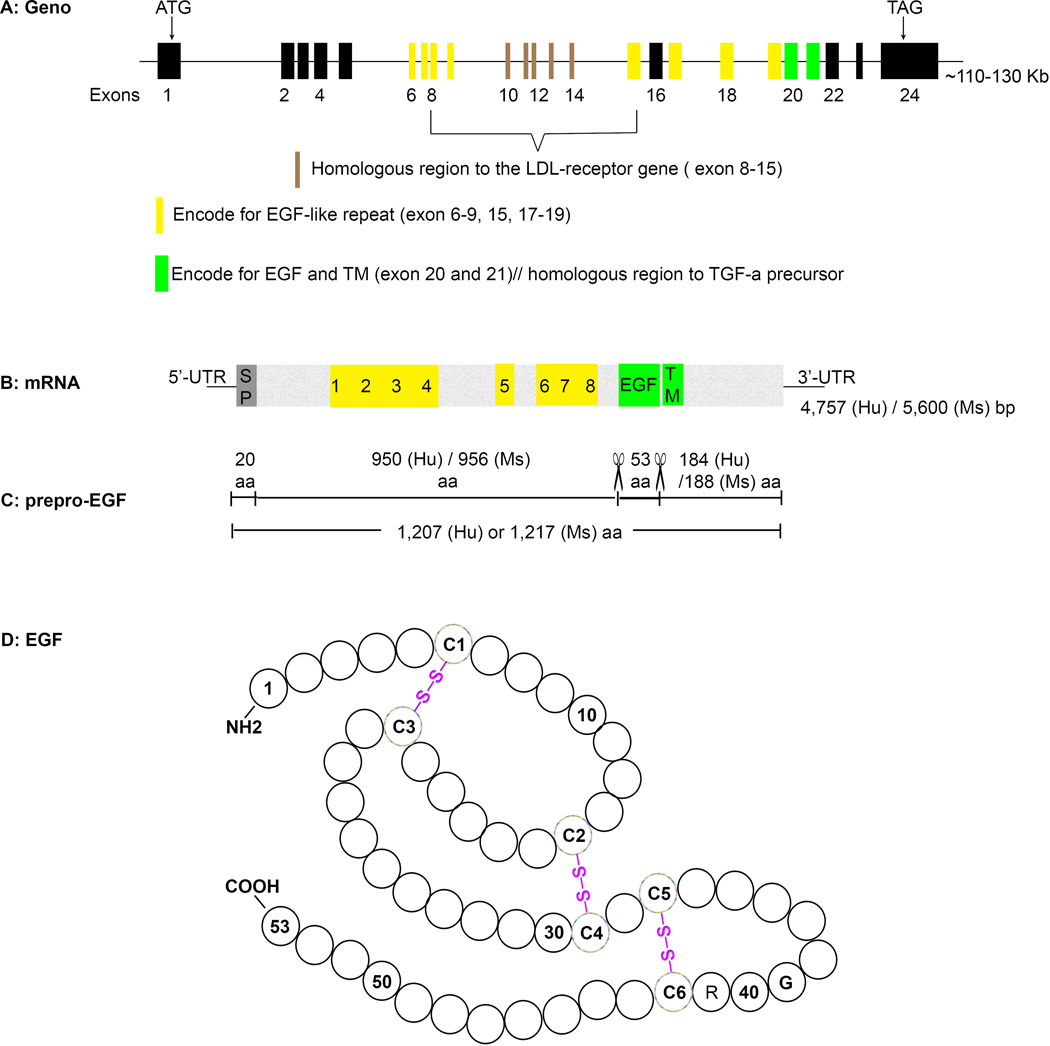
Followed its discovery in mouse salivary glands, genes encoding both mouse and human EGF were cloned and sequenced [10,11]. It was found that EGF is derived from a large precursor, prepro-EGF (Fig. 1). The genes encoding prepro-EGF were mapped to chromosome 4q25–q27 in humans and chromosome 3 (GRCm38) in mice (Table 1). There is 66% homology between these two sequences and both consist of 24 exons. The prepro-EGF gene is a mosaic, as 15 of its exons (exons 6–15, 17–19 and 20–21) encode sequences that are homologous to exon-encoded regions in other proteins. Exons 8–15 are homologous to a region of the low density lipoprotein (LDL) receptor gene. Eight individual cysteine-rich EGF-like repeats (EGF-motif) are encoded by exons 6–9, 15 and 17–19, as introns interrupt the coding sequence and mark the end of each repeat. EGF is encoded by two exons, 20 and 21. In contrast to EGF-like repeats, introns do not mark the end of the EGF-coding region and exon 21, which codes for the COOH-terminal portion of EGF, also encodes the transmembrane (TM) domain of the prepro-EGF. In addition, exons 20 and 21 are also homologous to the TGF-α and transmembrane domains of the TGF-α percursor gene [11,12]. Therefore, the prepro-EGF gene belongs to three gene families: one that includes proteins that have the EGF-like repeat motif; a growth factor family that includes the TGF-α precursor; and a receptor family that includes the LDL receptor [11].
Fig. 1.
Schematic representation of EGF gene, mRNA and protein. A) The EGF precursor gene has 110–130 kb pairs and 24 exons. Some exons encoded protein segments that are homologuous to sequences in other proteins, like LDL receptor, EGF-like repeats, and TGF-α precursor, are noted. EGF was coded by exon 20 and 21. The start codon ATG lies in the first exon and stop codon TAG the last exon. B) EGF precursor mRNA is 4,757 bp long in human and 5,600 bp in mice. Protein coding regions are highlighted. The positions of signal peptide (SP), EGF-like repeats (1–8, highlighted yellow), EGF, and transmembrane (TM) domain (green) are noted. UTR, untranslated region; Hu: human; Ms: mouse. C) Prepro-EGF protein sequence. It has 1,207 amino acids (aa) in human and 1,217 aa in mouse. The first 20–29 aa functions as signal peptide. Mature EGF (53 aa) is flanked by 950/184 aa in human and 956/188 aa in mice and lies immediately external to the hydrophobic transmembrane domain. Cleavage sites for releasing mature EGF are marked with scissor icons. D) The amino acid sequences of EGF with placement of disulfide bonds. The position of conserved cysteines (C1 to C6), glycine (G), and arginine (R) are specified. Modified from Savage CR, et al. [7].
Table 1.
Characterization of EGF gene, DNA, and protein
| Human | Mouse | |
|---|---|---|
| Location in chromosome | 4q25 | 3, GRCm38 |
| Gene ID | NC_000004.11 | NC_000069.6 |
| DNA size | 130 Kb | 101 Kb |
| Number of Exons | 24 | 24 |
| mRNA ID | NM_001963.4 | NM_010113.3 |
| mRNA size | 5,600 bp | 4,757 bp |
| Protien ID | NP_001954.2 | NP_034243.2 |
| Protein size | 1,207 aa (Mr* 130–160 kDa) | 1,217 aa (Mr 130–160 kDa) |
| Mature EGF size | 53 aa (Mr* 6–8 kDa) | 53 aa (Mr* 6–8 kDa) |
Mr: relative molecular mass
The 4.7 to 5.6 Kb cDNA sequence has a long open reading frame that encodes the prepro-EGF of 1,207 amino acids in human and 1,217 amino acids in mouse. There is 75% homology between the coding regions of the human and mouse cDNA sequences. The homology between the 5’- and 3’-untranslated regions of the two sequences is 66% and 60%, respectively. Prepro-EGF is N-glycosylated and contains two prominent hydrophobic regions, one of which represents the signal peptide and the other that anchors the precursor in the plasma membrane. Mature EGF lies immediately external to the hydrophobic transmembrane domain and can be released from the precursor by cleavage of Arg-Asn and Arg-His bonds at its NH2- and COOH-termini, respectively [13]. In cells that do not cleave this precursor, such as kidney cells, the membrane-bound prepro-EGF may function through paracrine and/or juxtacrine growth control mechanisms [14]. It may also serve as a receptor for as yet unknown ligands.
3. EGF expression
EGF has been detected in a variety of body fluids, such as milk [15–17], saliva [18], urine [19], plasma [18], intestinal fluid [20], amniotic fluid [21], and others [22], which is locally produced and secreted by the lactating breast, submaxillary gland, kidney, Brunner’s glands of the duodenum, and placenta, respectively. Submaxillary gland is the major EGF producing site in mice, where it is synthesized, processed and stored in granules of the tubular duct cells. Consequently, EGF concentrations are high in mouse saliva [18]. Interestingly, only mature and diffusible 6 kDa EGF was detected in those secretory granules, where prepro-EGF is not detectable [23]. The release of EGF into saliva involves exocytosis by fusion of the secretory granule membrane with the apical cellular membrane (exocrine). A small amount of the EGF accumulated in submaxillary glands ends up in the blood (endocrine). The production and secretion of EGF in submaxillary gland are dependent on androgen levels and sympathetic system status. The concentration of EGF in submaxillary gland is1000 times higher in adult male mice than that in female mice (1000 ng/mg vs 70 ng/mg wet tissue) [24,25]. EGF concentration in submaxillary gland is low in newborn or immature male mice and gradually increases that parallels the androgen levels [22,24]. On the other hand, the androgen-dependent EGF contents in the submaxillary gland do not reflect its level in the plasma, which is indicated by the observations that there are no significant differences in plasma EGF levels (about 1ng/ml) between adult male and female mice [26]. The release of EGF from this site is highly regulated and may be achieved at least in part through activation of adrenergic receptors expressed by submaxillary glands [27]. Adrenergic stimulation such as phenylephrine injection or emotional stress will dramatically increase EGF levels in the saliva and blood [28,29].
Unlike mice, EGF concentrations in salivary gland and saliva are much lower in humans [30] and rats [31]. In humans, kidney is the predominant source for EGF production and urine contains high levels of EGF [19], although the highest EGF concentration was detected in the prostate fluids [32]. There are several features of kidney EGF production: 1) EGF accumulates as prepro-EGF associated with the apical membrane of epithelial cells. The fully mature 6 kDa EGF is not detectable [33]. 2) EGF-containing peptides of varying molecular masses, from 6 kDa to 160 kDa, can be detected in urine, which indicates that EGF can be released from membrane-anchored prepro-EGF [19,34]. 3) There is a sexual dimorphism of EGF concentrations in both kidney and urine, with higher EGF levels in females. It is not affected by the menstrual cycle, oral contraceptives, or postmenopausal estrogen therapy [35]. However, urine EGF concentrations increase during pregnancy, reaching peak levels at 19–22 gestational weeks [36].
Similar to other members in the EGF family, EGF detected in biological and pathological states exhibits different levels of expression [37–39]. Its expression levels also vary at different stages of development [40–42]. In newborn rat kidneys, positive EGF immunostaining was observed in the proximal tubules and decreased slowly with age. In adult kidneys, high concentrations of prepro-EGF mRNA are found in thick ascending limb of Henle’s loop and distal convoluted tubules [14,40].
4. Animal models
To understand the developmental, physiological, and pathological roles of EGF, transgenic mice with either EGF overexpression [43–47] or deletion [48] have been developed.
4.1 EGF overexpression
4.1.1. Transgenic mice with widespread human EGF expression
By insertion of human EGF cDNA under the beta actin promoter, transgenic mice with human EGF widespread expression were generated. Those transgenic mice showed low birth weight and stunted growth, which was associated with altered chondrocyte development in the growth plate and abnormal osteoblast accumulation in the endosteum and periosteum. The mechanism involved a reduction of serum insulin-like growth factor-binding protein-3 (IGFBP3). In addition, adult male transgenic mice were sterile and exhibited hypospermatogenesis. Interestingly, no signs of tumor formation were found in these transgenic animals [45,49].
4.1.2. Transgenic mice with mouse EGF overexpression
To generate transgenic mice with mouse EGF overexpression, full-length mouse EGF cDNA was inserted under a ubiquitous transcription promoter (cytomegalovirus). As a result, markedly increased levels of EGF mRNA and protein expression were detected in various tissues of the transgenic mice [43,44]. In line with the transgenic mice with human EGF overexpression, transgenic mice with mouse EGF overexpression also showed stunted growth, which was much more severe in homozygous than heterozygous progenies. In addition, those transgenic mice exhibited: 1) Hair follicle deficits and thin fur that could be rescued by inhibiting the EGFR signaling by crossing with waved-2 mice, which harbor a point mutation in EGFR that greatly diminishes its tyrosine kinase activity [50]; 2) Hypersensitivity to psychostimulants, such as cocaine, which may due to the neurotrophic action of EGF on dopamine neurons; 3) Behavioral deficits relevant to schizophrenia [43]. In humans, EGF A61G polymorphism in the 5’ untranslated region (UTR), a single nucleotide substitution (G to A) at position 61 of the EGF gene that results in increased EGF expression (G/G > G/A > A/A) has been associated with lower birth weight and fetal growth restriction in individuals from Western Europe [51,52]. In several meta-analysis, A61G polymorphism was correlated with cancer development in individuals carrying the G alleles, which led to the highest EGF expression [51,53–56].
4.1.3. Transgenic mice with tissue-specific mouse EGF overexpression
Compared to global gene overexpression, transgenic mice with tissue-targeted gene overexpression more directly test the effect of a candidate factor on that particular tissue without directly affecting other organ systems. For example, studies have shown that systemic administration of EGF attenuates intestinal tissue damage and improves mortality in a variety of animal models of noninfectious inflammation and intestinal injury, and work on targeted-transgenic mice made it clear that those healing effects of EGF are directly through the intestine. In studies to test the direct role of EGF on intestinal injury, transgenic mice with EGF overexpression exclusively in enterocytes were generated by ligating murine preproEGF cDNA to the rat intestinal fatty acid-binding protein (I-FABP) promoter [47]. These transgenic mice had improved post-resection adaptation compared to wild-type mice. The transgenic mice appeared phenotypically normal albeit with slightly shorter intestines in the mouse line with the greatest production of EGF. The enterocyte-specific overexpression of EGF also conferred a survival advantage in mice subjected to septic peritonitis. The beneficial effects of EGF were intestine-specific and were associated with prevention of peritonitis-induced intestinal hyper-permeability via a claudin-2-mediated mechanism [46]. The major limitation of those studies was lack of examination of phospho-EGFR levels.
4.2. EGF null mice
EGF null mice were generated by deletion of EGF exon 20. In contrast to EGFR null mice, which exhibit several dermal, gastrointestinal, pulmonary, renal and neurological abnormalities, EGF null mice display no overt phenotype and no gross or histological abnormalities in lung, kidney and gastrointestinal tract, which may indicate overlapping or compensatory functions among EGF family members [57].
5. EGF biological functions
Both prepro-EGF and EGF have been documented to be biologically active by binding to its receptor, EGFR, in a variety of tissues [58]. EGFR, also known as ErbB1 or HER1, is a type I transmembrane receptor tyrosine kinase (RTK) that belongs to the EGFR/ErbB superfamily, which includes three other RTKs: ErbB2/Neu/HER2, ErbB3/HER3, and ErbB4/HER4. Upon ligand binding, EGFR forms homodimers with another EGFR or heterodimers with other EGFR/ErbB family members. The receptor dimerization is a critical step for the activation of intrinsic tyrosine kinases and autophosphorylation of the c-terminal specific tyrosine-containing residues that serve as docking sites for a variety of signaling molecules harboring Src homology 2 (SH2) or phosphotyrosine binding (PTB) motifs, whose recruitments lead to the activation of Ras/Raf/MEK/ERK, JAK/STAT, PI3K/AKT/mTOR, and PLCγ/PKC signaling pathways that affect cell proliferation, differentiation, and apoptosis and consequently regulate many physiological processes, such as organ development, growth, regeneration, ion transportation, etc. [4,14,59–61].
5.1. EGF in embryo development
As a mitogenic growth factor, EGF plays an important role in embryo development from as early as pre-implantation [62,63]. After fertilization, the one cell egg undergoes a series of cleavage divisions, progressing through 2-cell, 4-cell, 8-cell, 16-cell, mulberry-shaped 20- to 30-cell mass (morula) to the formation of blastocyst, which is composed of trophoblast and inner cell mass (embryonic stem cells, ESC) that eventually develop into placenta and embryo respectively. Accumulated evidence suggests that growth factors are necessary components in early embryonic development in vivo and in vitro [64]. Among these growth factors, EGF has been shown to promote pre-implantation embryo growth [62,65,66], as well as trophoblast invasion and post-implantation embryo growth [67,68]. In an in vitro fertilized (IVF) porcine embryo developmental study, the rate of blastocyst formation from either 2-cell or morula stage was significantly improved by EGF addition with the minimal addition of re-crystallized bovine serum albumin in culture media. However, EGF alone was not able to elicit any stimulatory effects on embryo development without protein supplementation [62]. Similarly, EGF treatment significantly increased the blastocyst formation rate, the total number of cells per blastocyst, the cell ratio of the inner cell mass and the trophectoderm, and EGFR protein expression in cloned mouse embryos, and these effects were enhanced when EGF and TGF-α were combined [63]. In pregnant mice, reduction of maternal EGF by sialoadenectomy results in growth restriction of embryos [69]. Those studies indicated that EGF plays an important synergistic effect with other growth factors in embryonic development. In addition, EGF produced by uterine tissues and macrophages can enhance trophoblast outgrowth and regulate urokinase plasminogen activator (uPA) and matrix metalloproteinase 9 (MMP-9) expression [70,71]. PA and MMPs have been implicated in mammalian gametogenesis [72], ovulation [73], fertilization [74], early development and embryo implantation [75].
Consistently, the role of EGFR on embryonic and placental development is much more prominent. EGFR expression has been detected in the apical blastomere membrane of the 4-cell stage mouse embryo [62,76]. In EGFR null mice, placentas have fewer proliferative trophoblasts than wild-type and exhibit strain-specific defects in the spongiotrophoblast and labyrinth layers that can result in mid-gestational embryonic lethality [77–79]. Moreover, increasing the levels of EGFR signaling by using hypermorphic EGFR (Dsk5) allele results in larger placental size with a more prominent spongiotrophoblast layer and increased expression of glycogen cell-specific genes [80]. This study also demonstrated that mice with increased levels of EGFR signalling exhibit an extensive level of genetic background-dependent phenotypic variability, and EGFR expressed in the uterine stroma may play an underappreciated role in preparation of the uterus for embryo implantation [80]. These differences between the relative importance of EGFR and EGF per se once again illustrates that it is sometimes difficult to ascribe biological functions solely to one EGF family member because of redundancy and compensation of expression and activity.
5.2. EGF in tissue regeneration
Stem cells have emerged as one of the fundamental underpinnings in tissue biology and in regenerative medicine [81]. In addition to embryonic stem cells, stem/progenitor cells have been found in various adult tissues, such as skin, blood, gut, heart, brain, etc. [82,83], where they not only replace differentiated cells during normal tissue turnover (homeostasis), but are also capable of massive lineage expansion following injury or transformation (tissue repair). Stem cells are undifferentiated biological cells with a high potential for proliferation and the capacity for self-renewal with retention of multipotency. Therefore, stem cells hold great promise in regenerative medicine and tissue engineering. However, due to their low numbers in a tissue, either in vivo or in vitro expansions without biasing future differentiation for optimal utility are often needed. Studies have shown that in vivo stem cells are maintained and regulated by the local tissue microenvironments surrounding the stem cells, called stem cell niches [84–86]. Yet, precisely how a core niche program is regulated to selectively control the behavior of distinct stem cell populations remains poorly understood [83]. Over the last decade, considerable progress has been made in identification of microenvironmental factors favoring the growth and expansion of the stem cell pool [87]. Among them, EGF has emerged as a powerful regulator of stem cells in different tissues, such as neural stem/progenitor cells [88,89], neural crest stem cells [90], germline stem cells [91], cardiac stem cells [87], bone marrow multipotential stromal cells (MSCs) [92], brain tumor stem cells [93], mouse embryonic stem cells [94], gut stem cells [83,95], keratinocyte stem cells [96], and multipotent stromal cells in the heart [97].
A stage-specific impact of EGF signaling pathway on the stem cell activity has been proposed recently [91]. Studies of the division frequency of germline stem cells (GSCs) in testes of Drosophila melanogaster indicated that GSC division frequency is under genetic control of the highly conserved EGF signaling pathway. When EGF signaling was attenuated, a two-fold increase in the percentage of GSCs in mitotic division was detected. Interestingly, EGF attenuation specifically increased the GSC division frequency in adult testes, but not in larval testes, which indicates an inhibitory effect of EGF on stem cell division in adult testes. This stage-specific requirement for EGF may reflect the different functions of GSCs in immature versus mature tissues. In larval testes, entire germline cell populations from GSC may be required, while in adult testes GSCs may be only used to replenish differentiated cells when needed. In larval testes, nutrient availability and cell growth may be the primary factors governing the frequency of GSC divisions. Conversely, soon after eclosion, Drosophila males reach sexual maturity, and spermatogenesis may rely on EGF-mediated signaling to regulate GSC divisions.
EGF may also impose dose-dependent effects on stem cell function [88,92]. Human neural progenitor cell (hNPC) cultures treated with 100 ng/ml EGF showed significantly increased growth rates compared with traditional level of 20 ng/ml. Interestingly, this was through increased survival of dividing cells rather than increased proliferation and was associated with prolonged activation of ErbB2 and phosphorylated Akt. Furthermore, high EGF levels selectively protect a large proportion of elongated radial glial-like cells within the growing neurospheres, which maintain the capacity to generate neurons upon differentiation. Therefore, the exact concentrations of growth factors added to growing stem and progenitor cell culture systems should be carefully evaluated to maintain specific populations of dividing cells [88].
Different forms of EGF, soluble versus surface-tethered, may affect stem cell expansion differently [92,98,99]. Soluble EGF was shown to augment adult bone marrow multipotential stromal cell (MSC) proliferation while preserving early progenitors within the MSC population, and thus did not induce differentiation. However, the tethered form of EGF was shown to promote osteogenic differentiation. Soluble EGF was also shown to increase paracrine secretion of other growth factors from MSC, including vascular endothelial growth factor (VEGF) and hepatocyte growth factor (HGF). Thus, soluble EGF can be used not only to expand MSC in vitro, but also to enhance paracrine secretion through drug-releasing MSC-encapsulated scaffolds in vivo. Tethered EGF may be utilized to direct MSC towards osteogenic lineage both in vitro and in vivo [92].
Finally, EGF has been shown to impose synergistic effects on stem cell expansion with other growth factors in combined therapy or through EGF-initiated upregulation of other growth factors, such as VEGF, HGF, HB-EGF, etc. [63,92,100]. On the other hand, EGF may also play distinct roles compared to other growth factors on certain kinds of stem cell expansion [90]. For example, in a study to investigate the effect of microenvironmental factors on quail trunk neural crest (NC) stem cell development, EGF was found to induce differentiation of NC to neuronal and melanocytic phenotypes (neurogenesis and melanogenesis), while fibroblast growth factor 2 (FGF2) promoted NC differentiation to Schwann cells (gliogenesis). In the presence of both EGF and FGF2, the neuronal differentiation predominated. These findings suggest that these two growth factors may play distinct roles in the fate decision of NC progenitors and in the development of the peripheral nervous system [90].
5.3 EGF in ion transport
A number of studies have shown that EGF and its related growth factors are involved in regulation of various epithelial ion channels such as epithelial sodium channel (ENaC) [101], Na+/K+/2Cl− co-transporter (NKCC1) [102], transmembrane protein 16A (TMEM16A, Ca2+-dependent Cl− channel) [103], calcium-activated K+ channels (KCa3.1) [104], transient receptor potential melastatin 6 (TRPM6), and cation-nonselective transient receptor potential channel 5 (TRPC5) [105], that govern ion homeostasis of Na+, K+, Cl− or Mg2+, in tissues like kidney, intestine, colon, and lung. The role of EGF on sodium and magnesium transport has been shown to be of particular physiological importance.
5.3.1. EGF and sodium transport
Sodium transport and homeostasis in the kidney connecting segment and collecting duct is mediated by the apical sodium-selective channel, ENaC, and the intracellular gradient driving cellular entry of sodium is dependent upon the Na+/K+-ATPase on the baslolateral membrane of the cell. ENaC is also located in the apical membrane of polarized epithelial cells of lung, and colon. Dysfunction or aberrant regulation of this channel is associated with a spectrum of diseases, such as hypertension, polycystic kidney disease and cystic fibrosis [106–108]. Various stimuli, including hormones and growth factors, modulate ENaC and fine-tune Na+ absorption. EGF has been known for almost two decades to regulate sodium transport. However, several investigations have reported contradictory results indicating opposite effects of EGF on ENaC activity and sodium transport. EGF appears to affect sodium transport and ENaC in a context-dependent manner: it increases sodium absorption in the airways [109] and intestine [110], but decreases sodium transportation in the renal collecting ducts (CDs) [111,112]. The CDs are the final sites for renal regulation of Na+ and water balance. Within the CD, Na+ diffuses from the lumen into principal cells through ENaC and is extruded at the basolateral membrane in exchange for uptake of K+ by the Na+/K+-ATPase. In the kidney, EGF expression can be found in the thick ascending limb of Henle’s loop and distal convoluted tubules (DCTs) [113]. Studies have shown that EGF and its related growth factors TGF-α, HB-EGF, and amphiregulin have a biphasic effect on sodium absorption in cultured murine mpkCCDc14 principal cells [114]. Basolateral application of the EGF family growth factors to polarized mpkCCDc14 principal cells grown on permeable supports acutely increases Na+ reabsorption, whereas chronic treatment of the monolayers with EGF and its related growth factors leads to significant inhibition of ENaC-mediated transport.
Studies have shown that infusion of high EGF levels decreased renal ENaC activity, prevented the development of hypertension, and attenuated glomerular and renal tubular damage. Conversely, deficiency of renal cortical EGF increases ENaC activity and contributes to salt-sensitive hypertension. Therefore, clinical application of EGF receptor inhibition either with antibodies such as Cetuximab or small molecule tyrosine kinase inhibitors may increase the risk of renal injury, especially in patients with a predisposition for salt-sensitive hypertension [106].
5.3.2. EGF and magnesium transportation
Magnesium is a versatile electrolyte known to be involved in many cellular processes. It functions as a cofactor in energy metabolism, nucleotide and protein synthesis, and as a regulator of Na+, K+, and Ca2+ channels. To maintain these cellular functions, both plasma and cellular Mg2+ concentrations have to be tightly controlled [115–117]. The systemic balance of Mg2+ and its intracellular concentration are determined by intestinal absorption and renal reabsorption/excretion. Under physiologic conditions, about 10% of filtered Mg2+ is excreted. The DCT is responsible for reabsorbing only 5–10% of the filtered Mg2+, but is critical for fine-tuning Mg2+ reabsorption to determine the final urinary Mg2+ concentration and thus plays a key role in the regulation of Mg2+ homeostasis [115,118]. For this reabsorption to occur, active transcellular Mg2+ transport requires passive Mg2+ entry across the luminal membrane. Recent studies have shown that TRPM6, an Mg2+-permeable channel that is expressed in the luminal membrane of the intestinal epithelium and the DCT, is a likely candidate for influx of Mg2+ across the luminal membrane [119]. Inactivating mutations of TRPM6 both impair gut absorption of Mg2+ and produce renal wasting [115].
EGF has been identified as a novel autocrine/paracrine magnesiotropic hormone that stimulates Mg2+ reabsorption in the DCT via engagement of its receptor on the basolateral membrane of DCT cells and subsequent activation of the Mg2+ channel TRPM6 in the apical surface [116]. By use of whole-genome linkage analysis and a subsequent candidate gene approach, EGF was shown to be the affected gene in patients with autosomal recessive isolated renal hypomagnesemia. As noted above, the EGF gene is highly expressed along the DCT [113], an important site for regulating urinary magnesium excretion. By activating the EGFR receptor, EGF stimulates the trafficking of TRPM6 channels to the luminal membrane, increasing the reabsorption of Mg2+ through TRPM6 [120]. A point mutation in prepro-EGF that retains EGF on the apical but not the basolateral membrane, or inhibition of EGFR by anti-EGFR antibodies, Cetuximab, or by EGFR tyrosine kinase inhibitors, such as Erlotinib, were shown to lead to suppressed activity of TRPM6 and renal Mg2+ wasting [116,121]. In this regard, use of Cetuximab in patients with head, neck or metastasis colorectal cancer is associated with a high incidence of symptomatic hypomagnesemia [122]. Erlotinib-injected mice also failed to reduce fractional excretion of Mg2+ in response to a decreased serum Mg2+ concentration [121]. In addition, simultaneous TRPM6 and EGF mRNA downregulation were seen in the rat kidneys with cisplatin - or cyclosporin A (CsA) - induced hypomagnesemia, which might indicate that EGF also influences TRPM6 mRNA synthesis [117,123]. EGF-mediated stimulation of TRPM6 may occur via signaling through Src kinases and the small GTPase Rac1, thereby redistributing endomembrane TRPM6 to the plasma membrane [120].
6. EGF clinical relevance
Through binding and activating EGFR, EGF itself or combined with other growth factors triggers many biological responses, including cell proliferation, differentiation and migration, which supporting a regulative role for the EGF/EGFR signaling in normal development [20] as well as pathophysiological events such as tissue repair including ulcer/wound healing [124–127], tissue repair after ischemia/reperfusion injury [128–130], etc. Exogenous EGF administration has shown beneficial antiapoptotic and antioxidant effects and has been found to reduce the tissue injury caused by IRI in different organs, such as heart [129], intestine [128], and kidney [130].
6.1. EGF and ulcer/wound healing
As for ulcer/wound healing, the effect of EGF may vary according to the course of the wound and the routes or forms of EGF being applied. First, the healing action of EGF appears to differ in acute versus chronic wounds. Based on ex vivo research, EGF is upregulated after acute wounding injury, resulting in increased expression of keratins K6 and K16, thereby enhancing re-epithelialization and increasing the tensile strength in wounds [131]. Conversely, downregulation of EGF and its receptor as well as a mislocalization of EGF receptor in the cytoplasm of keratinocytes instead of the membrane are seen in chronic wounds. This probably contributes to an inhibition of epithelialization. Exogenous EGF is readily degraded in the chronic wound environment, which limits its effect on the chronic wound healing process. Secondly, the effect of EGF is reduced due to its poor transdermal permeability and biological stability if applied topically to the wounds. Many studies have suggested that the local concentration of EGF needs to be sufficiently high and prolonged for effective wound healing [126,132,133]. Therefore, different drug delivery systems that are able to protect and stabilize the protein have been investigated, including hydrogels, sponges, polymeric pellets, nanofibers, microspheres, a biomimetic delivery system that incorporated EGF with biocompatible components such as hyaluronic acid, collagen, vitronectin or liposome, and more recently, a poloxamer gel formulation of recombinant low-molecular-weight protamine conjugated EGF (rLMWP-EGF) by conjugating a highly positively charged rLMWP to the N-terminal of EGF [134–140]. The latter has shown better healing effects on burn injuries [139]. Thirdly, the effect of EGF on wound healing is likely dose-dependent [132,141,142]. The higher dose of EGF has been shown to achieve higher healing rates and shorter time to heal than the lower dose. This brings up safety concerns, as repeated application of EGF can induce hyperplasia and hypertrophy of skin keratinocytes and fibroblasts, as well as promote angiogenesis, which may predispose to cancer development, especially in patients who are immune-incompetent [143]. However, the clinical data so far have shown that EGF treatments have been well tolerated, and no significant adverse reactions have been observed [141].
6.2. EGF and cancer
Increased EGFR activity due to more EGF synthesis, EGFR overexpression, and/or EGFR mutation, has been detected in a variety of tumors including glioblastoma (GBM), non-small cell lung cancer (NSCLC), head and neck, breast, colorectal, ovarian, prostate and pancreatic cancers, etc. [144]. The expression levels of EGF and EGFR are correlated with progressive tumor growth and metastasis [59,145–147] through: 1) increasing tumor cell proliferation and migration through EGFR-Ras/Raf/MEK/ERK and EGFR-PI3K/AKT pathways [146]; 2) localization of EGFR to the nucleus to promote cell proliferation through its tyrosine kinase activity or by acting as a transcriptional regulator [144,146,148]; 3) dysregulation of autophage activity [149,150]; 4) stimulation by EGF of the expression of several matrix metalloproteinases (MMPs, such as MMP1 and MMP9) that facilitate cancer invasion and metastasis; and/or 5) EGF-mediated decrease in the abundance of microRNAs that restrain oncogenic transcription factors [151]. Therefore, specific EGFR inhibition is one of the key targets for cancer therapy. The three most common agents are: 1) monoclonal anti-EGFR antibodies (mAbs), such as cetuximab and panitumumab, that inhibit ligand binding; 2) small molecule receptor tyrosine kinase inhibitors (TKIs), such as gefitinib, erlotinib, and lapatinib, that block the activation and phosphoraylation of EGFR; 3) anti-EGFR vaccines that elicit an immune response against EGFR-expressing tumor cells. For example, CDX-110 is a peptide vaccine that induces anti-tumor immune responses to EGFR variant III (EGFRvIII) positive cells [152]. Recently, many other agents such as antisense oligonucleotides, microRNA, affibodies, and nanobodies have been investigated, and some of them have begun to show efficacy in targeting and inhibiting EGFR [144,153].
7. Conclusion
EGF is widely expressed in the body and plays a fundamental role in development, tissue regeneration and ion transport, which occurs either by EGF itself or synergistically with other members of EGF family through binding/activating their receptor, EGFR. It is a pivotal factor in the healing cascade, and has been widely used clinically to accelerate ulcer/wound repair. Several carriers or delivery systems aimed to retard EGF degradation and facilitate EGF to the targeted area have been developed and dramatically improved its healing efficiency. On the other hand, dysregulation of EGF/EGFR axis has been linked to different pathogenesis and diseases and cancer development. Therefore, personal history and family genetic background should be considered when using EGF as a treatment therapy.
Highlights.
Submaxillary gland and kidney are predominant source of EGF production.
EGF/EGFR signaling promotes embryonic development and stem cell regeneration and regulates ion transport.
EGF plays pivotal role in ulcer/wound healing.
Dysregulation of EGF expression may contribute to the cancer development.
Acknowledgements
This review cannot include all the published EGF work and the authors would like to apologize for not being able to cite all of the primary literatures. This work is supported by National Institutes of Health Grants DK38226, DK51265, DK62794, DK095785 (R.C.H) and Grants from the Department of Veterans Affairs (R.C.H).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors declare no competing financial interests.
References
- 1.Cohen S. Isolation of a mouse submaxillary gland protein accelerating incisor eruption and eyelid opening in the new-born animal. J Biol Chem. 1962;237:1555–1562. [PubMed] [Google Scholar]
- 2.Cohen S, Carpenter G. Human epidermal growth factor: isolation and chemical and biological properties. Proc Natl Acad Sci U S A. 1975;72:1317–1321. doi: 10.1073/pnas.72.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gregory H. Isolation and structure of urogastrone and its relationship to epidermal growth factor. Nature. 1975;257:325–327. doi: 10.1038/257325a0. [DOI] [PubMed] [Google Scholar]
- 4.Harris RC, Chung E, Coffey RJ. EGF receptor ligands. Exp Cell Res. 2003;284:2–13. doi: 10.1016/s0014-4827(02)00105-2. [DOI] [PubMed] [Google Scholar]
- 5.Schneider MR, Wolf E. The epidermal growth factor receptor ligands at a glance. J Cell Physiol. 2009;218:460–466. doi: 10.1002/jcp.21635. [DOI] [PubMed] [Google Scholar]
- 6.Massague J, Pandiella A. Membrane-anchored growth factors. Annu Rev Biochem. 1993;62:515–541. doi: 10.1146/annurev.bi.62.070193.002503. [DOI] [PubMed] [Google Scholar]
- 7.Savage CR, Jr, Hash JH, Cohen S. Epidermal growth factor. Location of disulfide bonds. J Biol Chem. 1973;248:7669–7672. [PubMed] [Google Scholar]
- 8.Carpenter G. Receptors for epidermal growth factor and other polypeptide mitogens. Annu Rev Biochem. 1987;56:881–914. doi: 10.1146/annurev.bi.56.070187.004313. [DOI] [PubMed] [Google Scholar]
- 9.Zeineldin R, Hudson LG. Epithelial cell migration in response to epidermal growth factor. Methods Mol Biol. 2006;327:147–158. doi: 10.1385/1-59745-012-X:147. [DOI] [PubMed] [Google Scholar]
- 10.Gray A, Dull TJ, Ullrich A. Nucleotide sequence of epidermal growth factor cDNA predicts a 128,000-molecular weight protein precursor. Nature. 1983;303:722–725. doi: 10.1038/303722a0. [DOI] [PubMed] [Google Scholar]
- 11.Bell GI, Fong NM, Stempien MM, Wormsted MA, Caput D, Ku LL, et al. Human epidermal growth factor precursor: cDNA sequence, expression in vitro and gene organization. Nucleic Acids Res. 1986;14:8427–8446. doi: 10.1093/nar/14.21.8427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Derynck R, Roberts AB, Winkler ME, Chen EY, Goeddel DV. Human transforming growth factor-alpha: precursor structure and expression in E. coli. Cell. 1984;38:287–297. doi: 10.1016/0092-8674(84)90550-6. [DOI] [PubMed] [Google Scholar]
- 13.Le Gall SM, Auger R, Dreux C, Mauduit P. Regulated cell surface pro-EGF ectodomain shedding is a zinc metalloprotease-dependent process. J Biol Chem. 2003;278:45255–45268. doi: 10.1074/jbc.M307745200. [DOI] [PubMed] [Google Scholar]
- 14.Harris RC. Potential physiologic roles for epidermal growth factor in the kidney. Am J Kidney Dis. 1991;17:627–630. doi: 10.1016/s0272-6386(12)80336-2. [DOI] [PubMed] [Google Scholar]
- 15.Read LC, Upton FM, Francis GL, Wallace JC, Dahlenberg GW, Ballard FJ. Changes in the growth-promoting activity of human milk during lactation. Pediatr Res. 1984;18:133–139. doi: 10.1203/00006450-198402000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Dvorak B. Milk epidermal growth factor and gut protection. J Pediatr. 2010;156:S31–S35. doi: 10.1016/j.jpeds.2009.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nojiri T, Yoshizato T, Fukami T, Obama H, Yagi H, Yotsumoto F, et al. Clinical significance of amphiregulin and epidermal growth factor in colostrum. Arch Gynecol Obstet. 2012;286:643–647. doi: 10.1007/s00404-012-2365-8. [DOI] [PubMed] [Google Scholar]
- 18.Carpenter G, Cohen S. Epidermal growth factor. Annu Rev Biochem. 1979;48:193–216. doi: 10.1146/annurev.bi.48.070179.001205. [DOI] [PubMed] [Google Scholar]
- 19.Fisher DA, Salido EC, Barajas L. Epidermal growth factor and the kidney. Annu Rev Physiol. 1989;51:67–80. doi: 10.1146/annurev.ph.51.030189.000435. [DOI] [PubMed] [Google Scholar]
- 20.Nair RR, Warner BB, Warner BW. Role of epidermal growth factor and other growth factors in the prevention of necrotizing enterocolitis. Semin Perinatol. 2008;32:107–113. doi: 10.1053/j.semperi.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 21.Hofmann GE, Abramowicz JS. Epidermal growth factor (EGF) concentrations in amniotic fluid and maternal urine during pregnancy. Acta Obstet Gynecol Scand. 1990;69:217–221. doi: 10.3109/00016349009028683. [DOI] [PubMed] [Google Scholar]
- 22.Fisher DA, Lakshmanan J. Metabolism and effects of epidermal growth factor and related growth factors in mammals. Endocr Rev. 1990;11:418–442. doi: 10.1210/edrv-11-3-418. [DOI] [PubMed] [Google Scholar]
- 23.Scott J, Patterson S, Rall L, Bell GI, Crawford R, Penschow J, et al. The structure and biosynthesis of epidermal growth factor precursor. J Cell Sci Suppl. 1985;3:19–28. doi: 10.1242/jcs.1985.supplement_3.3. [DOI] [PubMed] [Google Scholar]
- 24.Byyny RL, Orth DN, Cohen S. Radioimmunoassay of epidermal growth factor. Endocrinology. 1972;90:1261–1266. doi: 10.1210/endo-90-5-1261. [DOI] [PubMed] [Google Scholar]
- 25.Gresik EW, Barka T. Immunocytochemical localization of epidermal growth factor during the postnatal development of the submandibular gland of the mouse. Am J Anat. 1978;151:1–9. doi: 10.1002/aja.1001510102. [DOI] [PubMed] [Google Scholar]
- 26.Byyny RL, Orth DN, Cohen S, Doyne ES. Epidermal growth factor: effects of androgens and adrenergic agents. Endocrinology. 1974;95:776–782. doi: 10.1210/endo-95-3-776. [DOI] [PubMed] [Google Scholar]
- 27.Pointon SE, Banerjee SP. Alpha- and beta-adrenergic receptors of the rat salivary gland. Elevation after chemical sympathectomy. Biochim Biophys Acta. 1979;584:231–241. doi: 10.1016/0304-4165(79)90267-8. [DOI] [PubMed] [Google Scholar]
- 28.Camprecios G, Navarro M, Soley M, Ramirez I. Acute and chronic adrenergic stimulation of submandibular salivary glands. Effects on the endocrine function of epidermal growth factor in mice. Growth Factors. 2009;27:300–308. doi: 10.1080/08977190903137736. [DOI] [PubMed] [Google Scholar]
- 29.Grau M, Rodriguez C, Soley M, Ramirez I. Relationship between epidermal growth factor in mouse submandibular glands, plasma, and bile: effects of catecholamines and fasting. Endocrinology. 1994;135:1854–1862. doi: 10.1210/endo.135.5.7956907. [DOI] [PubMed] [Google Scholar]
- 30.Hirata Y, Orth DN. Epidermal growth factor (urogastrone) in human tissues. J Clin Endocrinol Metab. 1979;48:667–672. doi: 10.1210/jcem-48-4-667. [DOI] [PubMed] [Google Scholar]
- 31.Sakamoto K, Oka Y, Uda K, Chiba K, Waki Y, Suzuki W, et al. Rat epidermal growth factors: purification and tissue content. Endocrinol Jpn. 1989;36:289–297. doi: 10.1507/endocrj1954.36.289. [DOI] [PubMed] [Google Scholar]
- 32.Gann PH, Klein KG, Chatterton RT, Ellman AE, Grayhack JT, Nadler RB, et al. Growth factors in expressed prostatic fluid from men with prostate cancer, BPH, and clinically normal prostates. Prostate. 1999;40:248–255. doi: 10.1002/(sici)1097-0045(19990901)40:4<248::aid-pros6>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 33.Rall LB, Scott J, Bell GI, Crawford RJ, Penschow JD, Niall HD, et al. Mouse prepro-epidermal growth factor synthesis by the kidney and other tissues. Nature. 1985;313:228–231. doi: 10.1038/313228a0. [DOI] [PubMed] [Google Scholar]
- 34.Parries G, Chen K, Misono KS, Cohen S. The human urinary epidermal growth factor (EGF) precursor. Isolation of a biologically active 160-kilodalton heparin-binding pro-EGF with a truncated carboxyl terminus. J Biol Chem. 1995;270:27954–27960. doi: 10.1074/jbc.270.46.27954. [DOI] [PubMed] [Google Scholar]
- 35.Mattila AL. Human urinary epidermal growth factor: effects of age, sex and female endocrine status. Life Sci. 1986;39:1879–1884. doi: 10.1016/0024-3205(86)90298-5. [DOI] [PubMed] [Google Scholar]
- 36.Watanabe H. Epidermal growth factor in urine of pregnant women and in amniotic fluid throughout pregnancy. Gynecol Endocrinol. 1990;4:43–50. doi: 10.3109/09513599009030690. [DOI] [PubMed] [Google Scholar]
- 37.Okada T, Omoto-Kitao M, Mukamoto M, Nakamura J, Mino M, Kondo T, et al. Compensatory renal growth in uninephrectomized immature rats: proliferative activity and epidermal growth factor. J Vet Med Sci. 2010;72:975–980. doi: 10.1292/jvms.09-0496. [DOI] [PubMed] [Google Scholar]
- 38.Zeng F, Singh AB, Harris RC. The role of the EGF family of ligands and receptors in renal development, physiology and pathophysiology. Exp Cell Res. 2009;315:602–610. doi: 10.1016/j.yexcr.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Staruschenko A, Palygin O, Ilatovskaya DV, Pavlov TS. Epidermal growth factors in the kidney and relationship to hypertension. Am J Physiol Renal Physiol. 2013;305:F12–F20. doi: 10.1152/ajprenal.00112.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raaberg L, Nexo E, Damsgaard Mikkelsen J, Seier Poulsen S. Immunohistochemical localisation and developmental aspects of epidermal growth factor in the rat. Histochemistry. 1988;89:351–356. doi: 10.1007/BF00500636. [DOI] [PubMed] [Google Scholar]
- 41.Goodyer PR, Fata J, Mulligan L, Fischer D, Fagan R, Guyda HJ, et al. Expression of transforming growth factor-alpha and epidermal growth factor receptor in human fetal kidneys. Mol Cell Endocrinol. 1991;77:199–206. doi: 10.1016/0303-7207(91)90075-4. [DOI] [PubMed] [Google Scholar]
- 42.Gattone VH, 2nd, Sherman DA, Hinton DA, Niu FW, Topham RT, Klein RM. Epidermal growth factor in the neonatal mouse salivary gland and kidney. Biol Neonate. 1992;61:54–67. doi: 10.1159/000243531. [DOI] [PubMed] [Google Scholar]
- 43.Eda T, Mizuno M, Araki K, Iwakura Y, Namba H, Sotoyama H, et al. Neurobehavioral deficits of epidermal growth factor-overexpressing transgenic mice: impact on dopamine metabolism. Neurosci Lett. 2013;547:21–25. doi: 10.1016/j.neulet.2013.04.055. [DOI] [PubMed] [Google Scholar]
- 44.Mak KK, Chan SY. Epidermal growth factor as a biologic switch in hair growth cycle. J Biol Chem. 2003;278:26120–26126. doi: 10.1074/jbc.M212082200. [DOI] [PubMed] [Google Scholar]
- 45.Wong RW, Kwan RW, Mak PH, Mak KK, Sham MH, Chan SY. Overexpression of epidermal growth factor induced hypospermatogenesis in transgenic mice. J Biol Chem. 2000;275:18297–18301. doi: 10.1074/jbc.M001965200. [DOI] [PubMed] [Google Scholar]
- 46.Clark JA, Gan H, Samocha AJ, Fox AC, Buchman TG, Coopersmith CM. Enterocyte-specific epidermal growth factor prevents barrier dysfunction and improves mortality in murine peritonitis. Am J Physiol Gastrointest Liver Physiol. 2009;297:G471–G479. doi: 10.1152/ajpgi.00012.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Erwin CR, Helmrath MA, Shin CE, Falcone RA, Jr, Stern LE, Warner BW. Intestinal overexpression of EGF in transgenic mice enhances adaptation after small bowel resection. Am J Physiol. 1999;277:G533–G540. doi: 10.1152/ajpgi.1999.277.3.G533. [DOI] [PubMed] [Google Scholar]
- 48.Troyer KL, Luetteke NC, Saxon ML, Qiu TH, Xian CJ, Lee DC. Growth retardation, duodenal lesions, and aberrant ileum architecture in triple null mice lacking EGF, amphiregulin, and TGF-alpha. Gastroenterology. 2001;121:68–78. doi: 10.1053/gast.2001.25478. [DOI] [PubMed] [Google Scholar]
- 49.Chan SY, Wong RW. Expression of epidermal growth factor in transgenic mice causes growth retardation. J Biol Chem. 2000;275:38693–38698. doi: 10.1074/jbc.M004189200. [DOI] [PubMed] [Google Scholar]
- 50.Luetteke NC, Phillips HK, Qiu TH, Copeland NG, Earp HS, Jenkins NA, et al. The mouse waved-2 phenotype results from a point mutation in the EGF receptor tyrosine kinase. Genes Dev. 1994;8:399–413. doi: 10.1101/gad.8.4.399. [DOI] [PubMed] [Google Scholar]
- 51.Shahbazi M, Pravica V, Nasreen N, Fakhoury H, Fryer AA, Strange RC, et al. Association between functional polymorphism in EGF gene and malignant melanoma. Lancet. 2002;359:397–401. doi: 10.1016/S0140-6736(02)07600-6. [DOI] [PubMed] [Google Scholar]
- 52.Dissanayake VH, Tower C, Broderick A, Stocker LJ, Seneviratne HR, Jayasekara RW, et al. Polymorphism in the epidermal growth factor gene is associated with birthweight in Sinhalese and white Western Europeans. Mol Hum Reprod. 2007;13:425–429. doi: 10.1093/molehr/gam011. [DOI] [PubMed] [Google Scholar]
- 53.Tanabe KK, Lemoine A, Finkelstein DM, Kawasaki H, Fujii T, Chung RT, et al. Epidermal growth factor gene functional polymorphism and the risk of hepatocellular carcinoma in patients with cirrhosis. JAMA. 2008;299:53–60. doi: 10.1001/jama.2007.65. [DOI] [PubMed] [Google Scholar]
- 54.Yang Z, Wu Q, Shi Y, Nie Y, Wu K, Fan D. Epidermal growth factor 61A>G polymorphism is associated with risk of hepatocellular carcinoma: a meta-analysis. Genet Test Mol Biomarkers. 2012;16:1086–1091. doi: 10.1089/gtmb.2012.0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Araujo AP, Ribeiro R, Pereira D, Pinto D, Sousa B, Catarino R, et al. Ovarian cancer and genetic susceptibility: association of A61G polymorphism in the EGF gene. Exp Biol Med (Maywood) 2009;234:241–245. doi: 10.3181/0805-RM-146. [DOI] [PubMed] [Google Scholar]
- 56.Li TF, Ren KW, Liu PF. Meta-analysis of epidermal growth factor polymorphisms and cancer risk: involving 9,779 cases and 15,932 controls. DNA Cell Biol. 2012;31:568–574. doi: 10.1089/dna.2011.1394. [DOI] [PubMed] [Google Scholar]
- 57.Luetteke NC, Qiu TH, Fenton SE, Troyer KL, Riedel RF, Chang A, et al. Targeted inactivation of the EGF and amphiregulin genes reveals distinct roles for EGF receptor ligands in mouse mammary gland development. Development. 1999;126:2739–2750. doi: 10.1242/dev.126.12.2739. [DOI] [PubMed] [Google Scholar]
- 58.Kwan RW, Wong RW, Chan SY. Expression of full length or truncated epidermal growth factor precursor transforms NIH3T3 fibroblasts. Int J Oncol. 1999;15:281–284. doi: 10.3892/ijo.15.2.281. [DOI] [PubMed] [Google Scholar]
- 59.Galvez-Contreras AY, Quinones-Hinojosa A, Gonzalez-Perez O. The role of EGFR and ErbB family related proteins in the oligodendrocyte specification in germinal niches of the adult mammalian brain. Front Cell Neurosci. 2013;7:258. doi: 10.3389/fncel.2013.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chong CR, Janne PA. The quest to overcome resistance to EGFR-targeted therapies in cancer. Nat Med. 2013;19:1389–1400. doi: 10.1038/nm.3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Carpenter G, Cohen S. Epidermal growth factor. J Biol Chem. 1990;265:7709–7712. [PubMed] [Google Scholar]
- 62.Wei Z, Park KW, Day BN, Prather RS. Effect of epidermal growth factor on preimplantation development and its receptor expression in porcine embryos. Mol Reprod Dev. 2001;60:457–462. doi: 10.1002/mrd.1110. [DOI] [PubMed] [Google Scholar]
- 63.Dadi TD, Li MW, Lloyd KC. EGF and TGF-alpha supplementation enhances development of cloned mouse embryos. Cloning Stem Cells. 2007;9:315–326. doi: 10.1089/clo.2006.0040. [DOI] [PubMed] [Google Scholar]
- 64.Schultz GA, Heyner S. Growth factors in preimplantation mammalian embryos. Oxf Rev Reprod Biol. 1993;15:43–81. [PubMed] [Google Scholar]
- 65.Paria BC, Dey SK. Preimplantation embryo development in vitro: cooperative interactions among embryos and role of growth factors. Proc Natl Acad Sci U S A. 1990;87:4756–4760. doi: 10.1073/pnas.87.12.4756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Grazul-Bilska AT, Choi JT, Bilski JJ, Weigl RM, Kirsch JD, Kraft KC, et al. Effects of epidermal growth factor on early embryonic development after in vitro fertilization of oocytes collected from ewes treated with follicle stimulating hormone. Theriogenology. 2003;59:1449–1457. doi: 10.1016/s0093-691x(02)01192-5. [DOI] [PubMed] [Google Scholar]
- 67.Nielsen LL, Werb Z, Pedersen RA. Induction of c-fos transcripts in early postimplantation mouse embryos by TGF-alpha, EGF, PDGF, and FGF. Mol Reprod Dev. 1991;29:227–237. doi: 10.1002/mrd.1080290304. [DOI] [PubMed] [Google Scholar]
- 68.Haimovici F, Anderson DJ. Effects of growth factors and growth factor-extracellular matrix interactions on mouse trophoblast outgrowth in vitro. Biology of reproduction. 1993;49:124–130. doi: 10.1095/biolreprod49.1.124. [DOI] [PubMed] [Google Scholar]
- 69.Kamei Y, Tsutsumi O, Yamakawa A, Oka Y, Taketani Y, Imaki J. Maternal epidermal growth factor deficiency causes fetal hypoglycemia and intrauterine growth retardation in mice: possible involvement of placental glucose transporter GLUT3 expression. Endocrinology. 1999;140:4236–4243. doi: 10.1210/endo.140.9.6993. [DOI] [PubMed] [Google Scholar]
- 70.Martinez-Hernandez MG, Baiza-Gutman LA, Castillo-Trapala A, Armant DR. Regulation of proteinases during mouse peri-implantation development: urokinase-type plasminogen activator expression and cross talk with matrix metalloproteinase 9. Reproduction. 2011;141:227–239. doi: 10.1530/REP-10-0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Aflalo ED, Sod-Moriah UA, Potashnik G, Har-Vardi I. EGF increases expression and activity of PAs in preimplantation rat embryos and their implantation rate. Reprod Biol Endocrinol. 2007;5:4. doi: 10.1186/1477-7827-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Huarte J, Belin D, Vassalli JD. Plasminogen activator in mouse and rat oocytes: induction during meiotic maturation. Cell. 1985;43:551–558. doi: 10.1016/0092-8674(85)90184-9. [DOI] [PubMed] [Google Scholar]
- 73.Tsafriri A, Bicsak TA, Cajander SB, Ny T, Hsueh AJ. Suppression of ovulation rate by antibodies to tissue-type plasminogen activator and alpha 2-antiplasmin. Endocrinology. 1989;124:415–421. doi: 10.1210/endo-124-1-415. [DOI] [PubMed] [Google Scholar]
- 74.Huarte J, Vassalli JD, Belin D, Sakkas D. Involvement of the plasminogen activator/plasmin proteolytic cascade in fertilization. Dev Biol. 1993;157:539–546. doi: 10.1006/dbio.1993.1156. [DOI] [PubMed] [Google Scholar]
- 75.Sappino AP, Huarte J, Belin D, Vassalli JD. Plasminogen activators in tissue remodeling and invasion: mRNA localization in mouse ovaries and implanting embryos. J Cell Biol. 1989;109:2471–2479. doi: 10.1083/jcb.109.5.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wiley LM, Wu JX, Harari I, Adamson ED. Epidermal growth factor receptor mRNA and protein increase after the four-cell preimplantation stage in murine development. Dev Biol. 1992;149:247–260. doi: 10.1016/0012-1606(92)90282-l. [DOI] [PubMed] [Google Scholar]
- 77.Dackor J, Strunk KE, Wehmeyer MM, Threadgill DW. Altered trophoblast proliferation is insufficient to account for placental dysfunction in Egfr null embryos. Placenta. 2007;28:1211–1218. doi: 10.1016/j.placenta.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dackor J, Caron KM, Threadgill DW. Placental and embryonic growth restriction in mice with reduced function epidermal growth factor receptor alleles. Genetics. 2009;183:207–218. doi: 10.1534/genetics.109.104372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Threadgill DW, Dlugosz AA, Hansen LA, Tennenbaum T, Lichti U, Yee D, et al. Targeted disruption of mouse EGF receptor: effect of genetic background on mutant phenotype. Science. 1995;269:230–234. doi: 10.1126/science.7618084. [DOI] [PubMed] [Google Scholar]
- 80.Dackor J, Li M, Threadgill DW. Placental overgrowth and fertility defects in mice with a hypermorphic allele of epidermal growth factor receptor. Mamm Genome. 2009;20:339–349. doi: 10.1007/s00335-009-9189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lindvall O, Kokaia Z. Stem cells in human neurodegenerative disorders--time for clinical translation? J Clin Invest. 2010;120:29–40. doi: 10.1172/JCI40543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li L, Clevers H. Coexistence of quiescent and active adult stem cells in mammals. Science. 2010;327:542–545. doi: 10.1126/science.1180794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Strand M, Micchelli CA. Regional Control of Drosophila Gut Stem Cell Proliferation: EGF Establishes GSSC Proliferative Set Point & Controls Emergence from Quiescence. PLoS One. 2013;8:e80608. doi: 10.1371/journal.pone.0080608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Medema JP, Vermeulen L. Microenvironmental regulation of stem cells in intestinal homeostasis and cancer. Nature. 2011;474:318–326. doi: 10.1038/nature10212. [DOI] [PubMed] [Google Scholar]
- 85.Fuchs E, Tumbar T, Guasch G. Socializing with the neighbors: stem cells and their niche. Cell. 2004;116:769–778. doi: 10.1016/s0092-8674(04)00255-7. [DOI] [PubMed] [Google Scholar]
- 86.Watt FM, Hogan BL. Out of Eden: stem cells and their niches. Science. 2000;287:1427–1430. doi: 10.1126/science.287.5457.1427. [DOI] [PubMed] [Google Scholar]
- 87.Aghila Rani KG, Kartha CC. Effects of epidermal growth factor on proliferation and migration of cardiosphere-derived cells expanded from adult human heart. Growth Factors. 2010;28:157–165. doi: 10.3109/08977190903512628. [DOI] [PubMed] [Google Scholar]
- 88.Nelson AD, Suzuki M, Svendsen CN. A high concentration of epidermal growth factor increases the growth and survival of neurogenic radial glial cells within human neurosphere cultures. Stem Cells. 2008;26:348–355. doi: 10.1634/stemcells.2007-0299. [DOI] [PubMed] [Google Scholar]
- 89.Suzuki Y, Yanagisawa M, Yagi H, Nakatani Y, Yu RK. Involvement of beta1-integrin up-regulation in basic fibroblast growth factor- and epidermal growth factor-induced proliferation of mouse neuroepithelial cells. J Biol Chem. 2010;285:18443–18451. doi: 10.1074/jbc.M110.114645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Garcez RC, Teixeira BL, Schmitt Sdos S, Alvarez-Silva M, Trentin AG. Epidermal growth factor (EGF) promotes the in vitro differentiation of neural crest cells to neurons and melanocytes. Cellular and molecular neurobiology. 2009;29:1087–1091. doi: 10.1007/s10571-009-9406-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Parrott BB, Hudson A, Brady R, Schulz C. Control of germline stem cell division frequency--a novel, developmentally regulated role for epidermal growth factor signaling. PLoS One. 2012;7:e36460. doi: 10.1371/journal.pone.0036460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tamama K, Kawasaki H, Wells A. Epidermal growth factor (EGF) treatment on multipotential stromal cells (MSCs). Possible enhancement of therapeutic potential of MSC. J Biomed Biotechnol. 2010;2010:795385. doi: 10.1155/2010/795385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Soeda A, Inagaki A, Oka N, Ikegame Y, Aoki H, Yoshimura S, et al. Epidermal growth factor plays a crucial role in mitogenic regulation of human brain tumor stem cells. J Biol Chem. 2008;283:10958–10966. doi: 10.1074/jbc.M704205200. [DOI] [PubMed] [Google Scholar]
- 94.Park JH, Han HJ. Caveolin-1 plays important role in EGF-induced migration and proliferation of mouse embryonic stem cells: involvement of PI3K/Akt and ERK. Am J Physiol Cell Physiol. 2009;297:C935–C944. doi: 10.1152/ajpcell.00121.2009. [DOI] [PubMed] [Google Scholar]
- 95.Suzuki A, Sekiya S, Gunshima E, Fujii S, Taniguchi H. EGF signaling activates proliferation and blocks apoptosis of mouse and human intestinal stem/progenitor cells in long-term monolayer cell culture. Laboratory investigation; a journal of technical methods and pathology. 2010;90:1425–1436. doi: 10.1038/labinvest.2010.150. [DOI] [PubMed] [Google Scholar]
- 96.Nanba D, Toki F, Barrandon Y, Higashiyama S. Recent advances in the epidermal growth factor receptor/ligand system biology on skin homeostasis and keratinocyte stem cell regulation. J Dermatol Sci. 2013;72:81–86. doi: 10.1016/j.jdermsci.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 97.Belmadani S, Matrougui K, Kolz C, Pung YF, Palen D, Prockop DJ, et al. Amplification of coronary arteriogenic capacity of multipotent stromal cells by epidermal growth factor. Arterioscler Thromb Vasc Biol. 2009;29:802–808. doi: 10.1161/ATVBAHA.109.186189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kuhl PR, Griffith-Cima LG. Tethered epidermal growth factor as a paradigm for growth factor-induced stimulation from the solid phase. Nat Med. 1996;2:1022–1027. doi: 10.1038/nm0996-1022. [DOI] [PubMed] [Google Scholar]
- 99.Fan VH, Tamama K, Au A, Littrell R, Richardson LB, Wright JW, et al. Tethered epidermal growth factor provides a survival advantage to mesenchymal stem cells. Stem Cells. 2007;25:1241–1251. doi: 10.1634/stemcells.2006-0320. [DOI] [PubMed] [Google Scholar]
- 100.Kerpedjieva SS, Kim DS, Barbeau DJ, Tamama K. EGFR ligands drive multipotential stromal cells to produce multiple growth factors and cytokines via early growth response-1. Stem Cells Dev. 2012;21:2541–2551. doi: 10.1089/scd.2011.0711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zheleznova NN, Wilson PD, Staruschenko A. Epidermal growth factor-mediated proliferation and sodium transport in normal and PKD epithelial cells. Biochim Biophys Acta. 2011;1812:1301–1313. doi: 10.1016/j.bbadis.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.O'Mahony F, Toumi F, Mroz MS, Ferguson G, Keely SJ. Induction of Na+/K+/2Cl− cotransporter expression mediates chronic potentiation of intestinal epithelial Cl− secretion by EGF. Am J Physiol Cell Physiol. 2008;294:C1362–C1370. doi: 10.1152/ajpcell.00256.2007. [DOI] [PubMed] [Google Scholar]
- 103.Mroz MS, Keely SJ. Epidermal growth factor chronically upregulates Ca(2+)-dependent Cl(−) conductance and TMEM16A expression in intestinal epithelial cells. J Physiol. 2012;590:1907–1920. doi: 10.1113/jphysiol.2011.226126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Trinh NT, Prive A, Maille E, Noel J, Brochiero E. EGF and K+ channel activity control normal and cystic fibrosis bronchial epithelia repair. Am J Physiol Lung Cell Mol Physiol. 2008;295:L866–L880. doi: 10.1152/ajplung.90224.2008. [DOI] [PubMed] [Google Scholar]
- 105.Bezzerides VJ, Ramsey IS, Kotecha S, Greka A, Clapham DE. Rapid vesicular translocation and insertion of TRP channels. Nat Cell Biol. 2004;6:709–720. doi: 10.1038/ncb1150. [DOI] [PubMed] [Google Scholar]
- 106.Pavlov TS, Levchenko V, O'Connor PM, Ilatovskaya DV, Palygin O, Mori T, et al. Deficiency of renal cortical EGF increases ENaC activity and contributes to salt-sensitive hypertension. J Am Soc Nephrol. 2013;24:1053–1062. doi: 10.1681/ASN.2012080839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Veizis IE, Cotton CU. Abnormal EGF-dependent regulation of sodium absorption in ARPKD collecting duct cells. Am J Physiol Renal Physiol. 2005;288:F474–F482. doi: 10.1152/ajprenal.00227.2004. [DOI] [PubMed] [Google Scholar]
- 108.Cao L, Owsianik G, Becq F, Nilius B. Chronic exposure to EGF affects trafficking and function of ENaC channel in cystic fibrosis cells. Biochem Biophys Res Commun. 2005;331:503–511. doi: 10.1016/j.bbrc.2005.03.201. [DOI] [PubMed] [Google Scholar]
- 109.Danto SI, Borok Z, Zhang XL, Lopez MZ, Patel P, Crandall ED, et al. Mechanisms of EGF-induced stimulation of sodium reabsorption by alveolar epithelial cells. Am J Physiol. 1998;275:C82–C92. doi: 10.1152/ajpcell.1998.275.1.C82. [DOI] [PubMed] [Google Scholar]
- 110.Khurana S, Nath SK, Levine SA, Bowser JM, Tse CM, Cohen ME, et al. Brush border phosphatidylinositol 3-kinase mediates epidermal growth factor stimulation of intestinal NaCl absorption and Na+/H+ exchange. J Biol Chem. 1996;271:9919–9927. doi: 10.1074/jbc.271.17.9919. [DOI] [PubMed] [Google Scholar]
- 111.Vehaskari VM, Herndon J, Hamm LL. Mechanism of sodium transport inhibition by epidermal growth factor in cortical collecting ducts. Am J Physiol. 1991;261:F896–F903. doi: 10.1152/ajprenal.1991.261.5.F896. [DOI] [PubMed] [Google Scholar]
- 112.Vehaskari VM, Hering-Smith KS, Moskowitz DW, Weiner ID, Hamm LL. Effect of epidermal growth factor on sodium transport in the cortical collecting tubule. Am J Physiol. 1989;256:F803–F809. doi: 10.1152/ajprenal.1989.256.5.F803. [DOI] [PubMed] [Google Scholar]
- 113.Poulsen SS, Nexo E, Olsen PS, Hess J, Kirkegaard P. Immunohistochemical localization of epidermal growth factor in rat and man. Histochemistry. 1986;85:389–394. doi: 10.1007/BF00982668. [DOI] [PubMed] [Google Scholar]
- 114.Levchenko V, Zheleznova NN, Pavlov TS, Vandewalle A, Wilson PD, Staruschenko A. EGF and its related growth factors mediate sodium transport in mpkCCDc14 cells via ErbB2 (neu/HER-2) receptor. J Cell Physiol. 2010;223:252–259. doi: 10.1002/jcp.22033. [DOI] [PubMed] [Google Scholar]
- 115.Muallem S, Moe OW. When EGF is offside, magnesium is wasted. J Clin Invest. 2007;117:2086–2089. doi: 10.1172/JCI33004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Groenestege WM, Thebault S, van der Wijst J, van den Berg D, Janssen R, Tejpar S, et al. Impaired basolateral sorting of pro-EGF causes isolated recessive renal hypomagnesemia. J Clin Invest. 2007;117:2260–2267. doi: 10.1172/JCI31680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ledeganck KJ, Boulet GA, Bogers JJ, Verpooten GA, De Winter BY. The TRPM6/EGF pathway is downregulated in a rat model of cisplatin nephrotoxicity. PLoS One. 2013;8:e57016. doi: 10.1371/journal.pone.0057016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Quamme GA, Dirks JH. Intraluminal and contraluminal magnesium on magnesium and calcium transfer in the rat nephron. Am J Physiol. 1980;238:F187–F198. doi: 10.1152/ajprenal.1980.238.3.F187. [DOI] [PubMed] [Google Scholar]
- 119.Voets T, Nilius B, Hoefs S, van der Kemp AW, Droogmans G, Bindels RJ, et al. TRPM6 forms the Mg2+ influx channel involved in intestinal and renal Mg2+ absorption. J Biol Chem. 2004;279:19–25. doi: 10.1074/jbc.M311201200. [DOI] [PubMed] [Google Scholar]
- 120.Thebault S, Alexander RT, Tiel Groenestege WM, Hoenderop JG, Bindels RJ. EGF increases TRPM6 activity and surface expression. J Am Soc Nephrol. 2009;20:78–85. doi: 10.1681/ASN.2008030327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dimke H, van der Wijst J, Alexander TR, Meijer IM, Mulder GM, van Goor H, et al. Effects of the EGFR Inhibitor Erlotinib on Magnesium Handling. J Am Soc Nephrol. 2010;21:1309–1316. doi: 10.1681/ASN.2009111153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tejpar S, Piessevaux H, Claes K, Piront P, Hoenderop JG, Verslype C, et al. Magnesium wasting associated with epidermal-growth-factor receptor-targeting antibodies in colorectal cancer: a prospective study. Lancet Oncol. 2007;8:387–394. doi: 10.1016/S1470-2045(07)70108-0. [DOI] [PubMed] [Google Scholar]
- 123.Ledeganck KJ, Boulet GA, Horvath CA, Vinckx M, Bogers JJ, Van Den Bossche R, et al. Expression of renal distal tubule transporters TRPM6 and NCC in a rat model of cyclosporine nephrotoxicity and effect of EGF treatment. Am J Physiol Renal Physiol. 2011;301:F486–F493. doi: 10.1152/ajprenal.00116.2011. [DOI] [PubMed] [Google Scholar]
- 124.Mimura Y, Ihn H, Jinnin M, Asano Y, Yamane K, Tamaki K. Epidermal growth factor affects the synthesis and degradation of type I collagen in cultured human dermal fibroblasts. Matrix Biol. 2006;25:202–212. doi: 10.1016/j.matbio.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 125.Marquez EB, De Ortueta D, Royo SB, Martinez-Carpio PA. Epidermal growth factor receptor in corneal damage: update and new insights from recent reports. Cutan Ocul Toxicol. 2011;30:7–14. doi: 10.3109/15569527.2010.498398. [DOI] [PubMed] [Google Scholar]
- 126.Hardwicke J, Schmaljohann D, Boyce D, Thomas D. Epidermal growth factor therapy and wound healing--past, present and future perspectives. Surgeon. 2008;6:172–177. doi: 10.1016/s1479-666x(08)80114-x. [DOI] [PubMed] [Google Scholar]
- 127.Tiaka EK, Papanas N, Manolakis AC, Georgiadis GS. Epidermal growth factor in the treatment of diabetic foot ulcers: an update. Perspect Vasc Surg Endovasc Ther. 2012;24:37–44. doi: 10.1177/1531003512442093. [DOI] [PubMed] [Google Scholar]
- 128.Arda-Pirincci P, Bolkent S. The role of epidermal growth factor in prevention of oxidative injury and apoptosis induced by intestinal ischemia/reperfusion in rats. Acta Histochem. 2013 doi: 10.1016/j.acthis.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 129.Lorita J, Soley M, Ramirez I. Epidermal growth factor protects the heart against low-flow ischemia-induced injury. J Physiol Biochem. 2010;66:55–62. doi: 10.1007/s13105-010-0009-7. [DOI] [PubMed] [Google Scholar]
- 130.Hussein Ael A, Shokeir AA, Sarhan ME, El-Menabawy FR, Abd-Elmoneim HA, El-Nashar EM, et al. Effects of combined erythropoietin and epidermal growth factor on renal ischaemia/reperfusion injury: a randomized experimental controlled study. BJU Int. 2011;107:323–328. doi: 10.1111/j.1464-410X.2010.09328.x. [DOI] [PubMed] [Google Scholar]
- 131.Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008;16:585–601. doi: 10.1111/j.1524-475X.2008.00410.x. [DOI] [PubMed] [Google Scholar]
- 132.Chen RR, Mooney DJ. Polymeric growth factor delivery strategies for tissue engineering. Pharm Res. 2003;20:1103–1112. doi: 10.1023/a:1025034925152. [DOI] [PubMed] [Google Scholar]
- 133.Brown GL, Nanney LB, Griffen J, Cramer AB, Yancey JM, Curtsinger LJ, 3rd, et al. Enhancement of wound healing by topical treatment with epidermal growth factor. N Engl J Med. 1989;321:76–79. doi: 10.1056/NEJM198907133210203. [DOI] [PubMed] [Google Scholar]
- 134.Xie Y, Upton Z, Richards S, Rizzi SC, Leavesley DI. Hyaluronic acid: evaluation as a potential delivery vehicle for vitronectin:growth factor complexes in wound healing applications. J Control Release. 2011;153:225–232. doi: 10.1016/j.jconrel.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 135.Ulubayram K, Nur Cakar A, Korkusuz P, Ertan C, Hasirci N. EGF containing gelatin-based wound dressings. Biomaterials. 2001;22:1345–1356. doi: 10.1016/s0142-9612(00)00287-8. [DOI] [PubMed] [Google Scholar]
- 136.Choi JS, Leong KW, Yoo HS. In vivo wound healing of diabetic ulcers using electrospun nanofibers immobilized with human epidermal growth factor (EGF) Biomaterials. 2008;29:587–596. doi: 10.1016/j.biomaterials.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 137.Alemdaroglu C, Degim Z, Celebi N, Zor F, Ozturk S, Erdogan D. An investigation on burn wound healing in rats with chitosan gel formulation containing epidermal growth factor. Burns. 2006;32:319–327. doi: 10.1016/j.burns.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 138.Yang CH. Evaluation of the release rate of bioactive recombinant human epidermal growth factor from crosslinking collagen sponges. J Mater Sci Mater Med. 2008;19:1433–1440. doi: 10.1007/s10856-007-3249-5. [DOI] [PubMed] [Google Scholar]
- 139.Lee JH, Bae IH, Choi JK, Park JW. Evaluation of a highly skin permeable low-molecular-weight protamine conjugated epidermal growth factor for novel burn wound healing therapy. J Pharm Sci. 2013;102:4109–4120. doi: 10.1002/jps.23725. [DOI] [PubMed] [Google Scholar]
- 140.Marquez L, de Abreu FA, Ferreira CL, Alves GD, Miziara MN, Alves JB. Enhanced bone healing of rat tooth sockets after administration of epidermal growth factor (EGF) carried by liposome. Injury. 2013;44:558–564. doi: 10.1016/j.injury.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 141.Berlanga-Acosta J, Gavilondo-Cowley J, Lopez-Saura P, Gonzalez-Lopez T, Castro-Santana MD, Lopez-Mola E, et al. Epidermal growth factor in clinical practice - a review of its biological actions, clinical indications and safety implications. Int Wound J. 2009;6:331–346. doi: 10.1111/j.1742-481X.2009.00622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Fernandez-Montequin JI, Valenzuela-Silva CM, Diaz OG, Savigne W, Sancho-Soutelo N, Rivero-Fernandez F, et al. Intra-lesional injections of recombinant human epidermal growth factor promote granulation and healing in advanced diabetic foot ulcers: multicenter, randomised, placebo-controlled, double-blind study. Int Wound J. 2009;6:432–443. doi: 10.1111/j.1742-481X.2009.00641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sari Kilicaslan SM, Coskun Cevher S, Gulec Peker EG. Ultrastructural changes in blood vessels in epidermal growth factor treated experimental cutaneous wound model. Pathol Res Pract. 2013;209:710–715. doi: 10.1016/j.prp.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 144.Gomez GG, Wykosky J, Zanca C, Furnari FB, Cavenee WK. Therapeutic resistance in cancer: microRNA regulation of EGFR signaling networks. Cancer Biol Med. 2013;10:192–205. doi: 10.7497/j.issn.2095-3941.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.De Jong KP, Stellema R, Karrenbeld A, Koudstaal J, Gouw AS, Sluiter WJ, et al. Clinical relevance of transforming growth factor alpha, epidermal growth factor receptor, p53, and Ki67 in colorectal liver metastases and corresponding primary tumors. Hepatology. 1998;28:971–979. doi: 10.1002/hep.510280411. [DOI] [PubMed] [Google Scholar]
- 146.Lo HW, Hung MC. Nuclear EGFR signalling network in cancers: linking EGFR pathway to cell cycle progression, nitric oxide pathway and patient survival. Br J Cancer. 2006;94:184–188. doi: 10.1038/sj.bjc.6602941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bracher A, Cardona AS, Tauber S, Fink AM, Steiner A, Pehamberger H, et al. Epidermal growth factor facilitates melanoma lymph node metastasis by influencing tumor lymphangiogenesis. The Journal of investigative dermatology. 2013;133:230–238. doi: 10.1038/jid.2012.272. [DOI] [PubMed] [Google Scholar]
- 148.Wang SC, Hung MC. Nuclear translocation of the epidermal growth factor receptor family membrane tyrosine kinase receptors. Clin Cancer Res. 2009;15:6484–6489. doi: 10.1158/1078-0432.CCR-08-2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Jutten B, Rouschop KM. EGFR signaling and autophagy dependence for growth, survival, and therapy resistance. Cell Cycle. 2014;13:42–51. doi: 10.4161/cc.27518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wei Y, Zou Z, Becker N, Anderson M, Sumpter R, Xiao G, et al. EGFR-mediated Beclin 1 phosphorylation in autophagy suppression, tumor progression, and tumor chemoresistance. Cell. 2013;154:1269–1284. doi: 10.1016/j.cell.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Avraham R, Sas-Chen A, Manor O, Steinfeld I, Shalgi R, Tarcic G, et al. EGF decreases the abundance of microRNAs that restrain oncogenic transcription factors. Sci Signal. 2010;3:ra43. doi: 10.1126/scisignal.2000876. [DOI] [PubMed] [Google Scholar]
- 152.Del Vecchio CA, Li G, Wong AJ. Targeting EGF receptor variant III: tumor-specific peptide vaccination for malignant gliomas. Expert Rev Vaccines. 2012;11:133–144. doi: 10.1586/erv.11.177. [DOI] [PubMed] [Google Scholar]
- 153.Yewale C, Baradia D, Vhora I, Patil S, Misra A. Epidermal growth factor receptor targeting in cancer: a review of trends and strategies. Biomaterials. 2013;34:8690–8707. doi: 10.1016/j.biomaterials.2013.07.100. [DOI] [PubMed] [Google Scholar]